Abstract
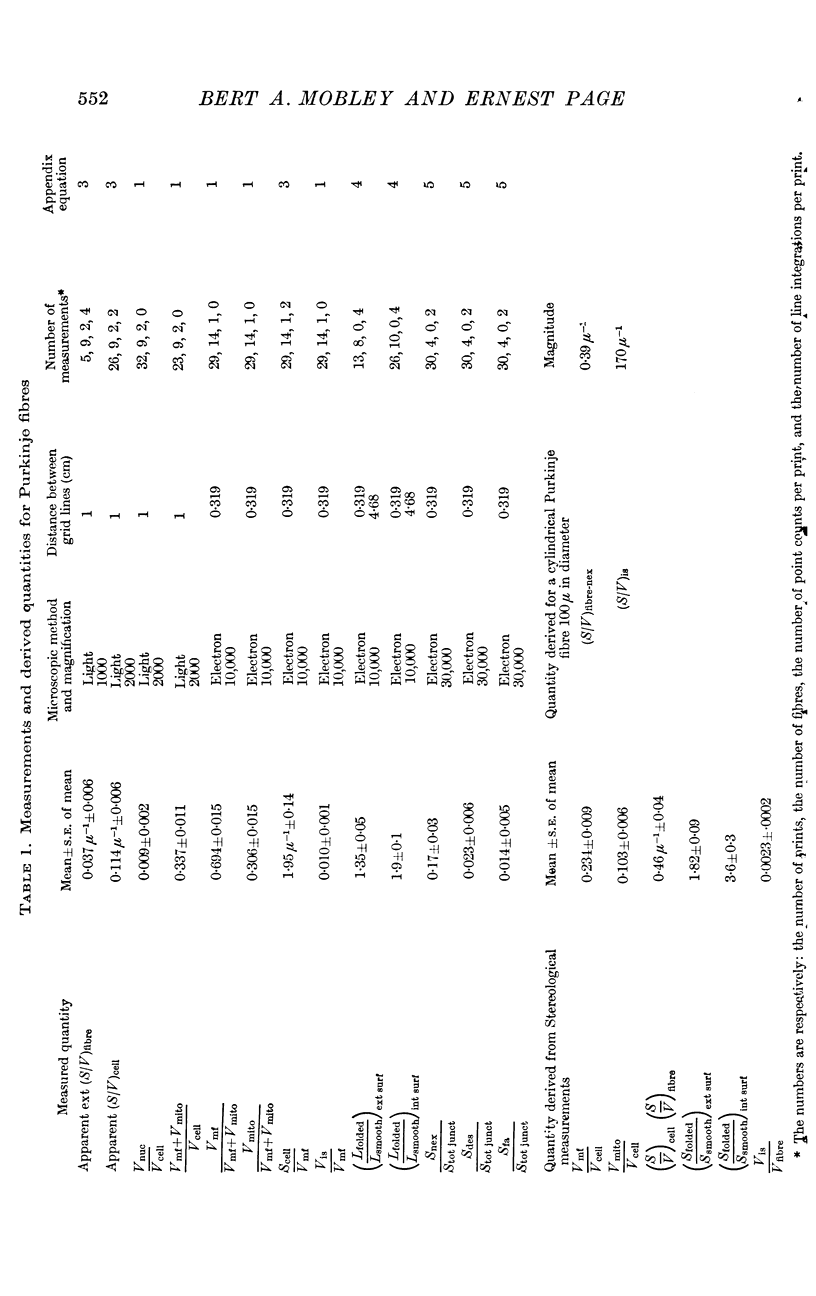
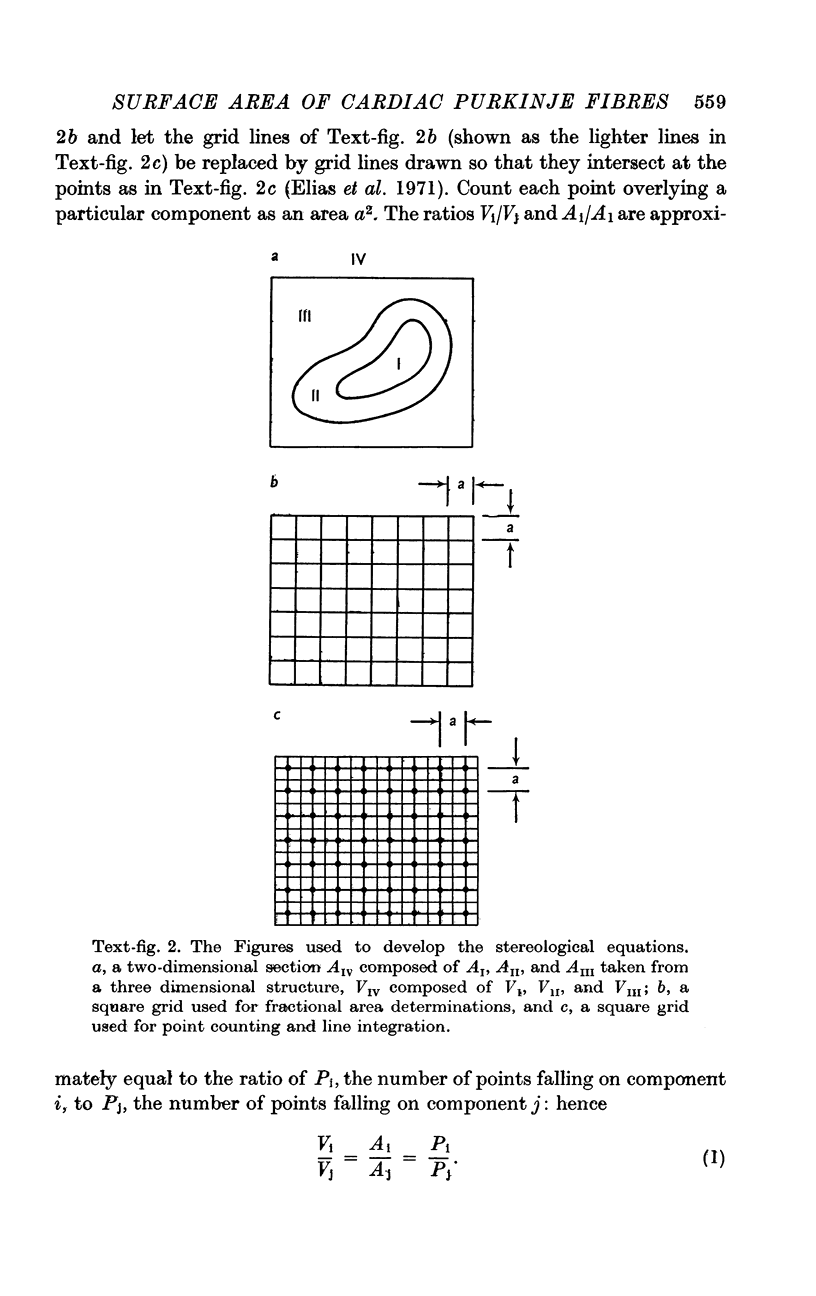
1. Measurements combining the techniques of point counting and line integration were performed on light and electron micrographs of Purkinje fibres from the sheep's heart. The measurements were aimed at determining membrane areas of importance for the cellular electrophysiology of this tissue.
2. The mean volume fractions of the cells occupied by various constituents were: myofibrils, 0·234; mitochondria, 0·103; and nuclei, 0·009. The mean volume fraction of the fibres occupied by the interspaces between the tightly packed cells was 0·0023.
3. The mean fractions of intercellular surface area occupied by junctional specializations were: nexus, 0·17; desmosome, 0·023; and fascia adherens, 0·014.
4. The mean surface to volume ratio of the Purkinje cells and fibres was 0·46 μ-1 which is 11·5 times the value of the surface to volume ratio of a long right circular cylinder 100 μ in diameter.
5. There are two reasons for the increment in the surface to volume ratio of the fibre (when compared to that of a long right circular cylinder 100 μ in diameter): the multicellular composition of the fibres and the extensive folding of the surface of the cells.
6. After correction for the intercellular nexal area the surface to volume ratio of a long cylindrical fibre 100 μ in diameter was 0·39 μ-1, or about 10 times the value for a long right circular cylinder 100 μ in diameter. The surface to volume ratio of the tissue interspaces in the same fibre was 170 μ-1.
7. It was concluded that the total sarcolemmal area in this tissue is great enough so that the specific membrane capacitance could be about 1 μF/cm2 and the specific membrane resistance 20,000 Ω cm2.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARR L., DEWEY M. M., BERGER W. PROPAGATION OF ACTION POTENTIALS AND THE STRUCTURE OF THE NEXUS IN CARDIAC MUSCLE. J Gen Physiol. 1965 May;48:797–823. doi: 10.1085/jgp.48.5.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias H., Hennig A., Schwartz D. E. Stereology: applications to biomedicalresearch. Physiol Rev. 1971 Jan;51(1):158–200. doi: 10.1152/physrev.1971.51.1.158. [DOI] [PubMed] [Google Scholar]
- FALK G., FATT P. LINEAR ELECTRICAL PROPERTIES OF STRIATED MUSCLE FIBRES OBSERVED WITH INTRACELLULAR ELECTRODES. Proc R Soc Lond B Biol Sci. 1964 Apr 14;160:69–123. doi: 10.1098/rspb.1964.0030. [DOI] [PubMed] [Google Scholar]
- Fawcett D. W., McNutt N. S. The ultrastructure of the cat myocardium. I. Ventricular papillary muscle. J Cell Biol. 1969 Jul;42(1):1–45. doi: 10.1083/jcb.42.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
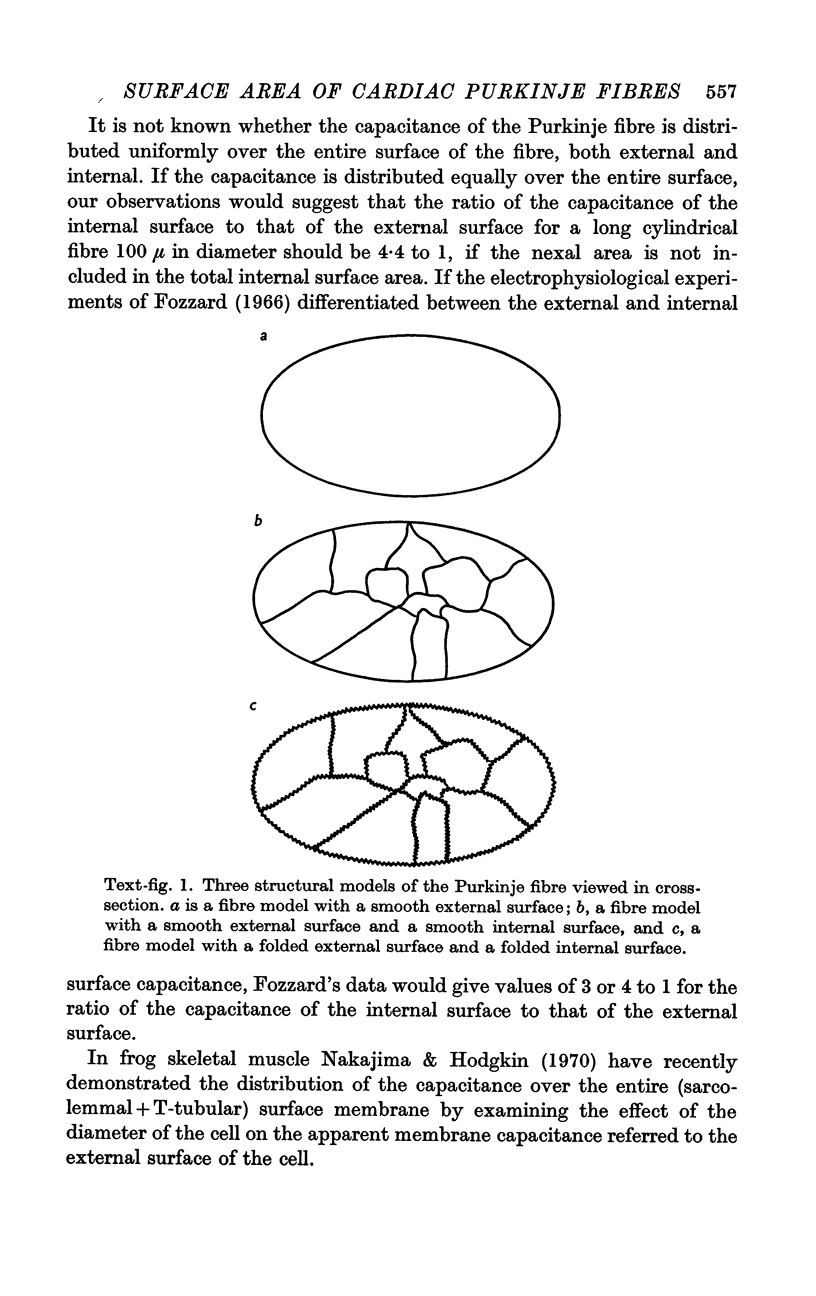
- Fozzard H. A. Membrane capacity of the cardiac Purkinje fibre. J Physiol. 1966 Jan;182(2):255–267. doi: 10.1113/jphysiol.1966.sp007823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freygang W. H., Trautwein W. The structural implications of the linear electrical properties of cardiac Purkinje strands. J Gen Physiol. 1970 Apr;55(4):524–547. doi: 10.1085/jgp.55.4.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNutt N. S., Weinstein R. S. The ultrastructure of the nexus. A correlated thin-section and freeze-cleave study. J Cell Biol. 1970 Dec;47(3):666–688. doi: 10.1083/jcb.47.3.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S., Hodgkin A. L. Effect of diameter on the electrical constants of frog skeletal muscle fibres. Nature. 1970 Sep 5;227(5262):1053–1055. doi: 10.1038/2271053a0. [DOI] [PubMed] [Google Scholar]
- Page E., McCallister L. P., Power B. Sterological measurements of cardiac ultrastructures implicated in excitation-contraction coupling. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1465–1466. doi: 10.1073/pnas.68.7.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page E., Power B., Fozzard H. A., Meddoff D. A. Sarcolemmal evaginations with knob-like or stalked projections in Purkinje fibers of the sheep's heart. J Ultrastruct Res. 1969 Aug;28(3):288–300. doi: 10.1016/s0022-5320(69)90086-0. [DOI] [PubMed] [Google Scholar]
- Peachey L. D. The sarcoplasmic reticulum and transverse tubules of the frog's sartorius. J Cell Biol. 1965 Jun;25(3 Suppl):209–231. doi: 10.1083/jcb.25.3.209. [DOI] [PubMed] [Google Scholar]
- Reedy M. K. Section staining for electron microscopy. Incompatibility of methyl nadic anhydride with permanganates. J Cell Biol. 1965 Jul;26(1):309–311. doi: 10.1083/jcb.26.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel J. P., Karnovsky M. J. Hexagonal array of subunits in intercellular junctions of the mouse heart and liver. J Cell Biol. 1967 Jun;33(3):C7–C12. doi: 10.1083/jcb.33.3.c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer J. R., Johnson E. A. Cardiac muscle. A comparative study of Purkinje fibers and ventricular fibers. J Cell Biol. 1968 Mar;36(3):497–526. doi: 10.1083/jcb.36.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDMANN S. The electrical constants of Purkinje fibres. J Physiol. 1952 Nov;118(3):348–360. doi: 10.1113/jphysiol.1952.sp004799. [DOI] [PMC free article] [PubMed] [Google Scholar]