Abstract
1. If activation heat reflects the operation of the calcium pump it should be independent of actomyosin activity. The semitendinosus preparation affords a technique for removing actomyosin activity since the muscle can be stretched till there is almost no overlap between the filaments.
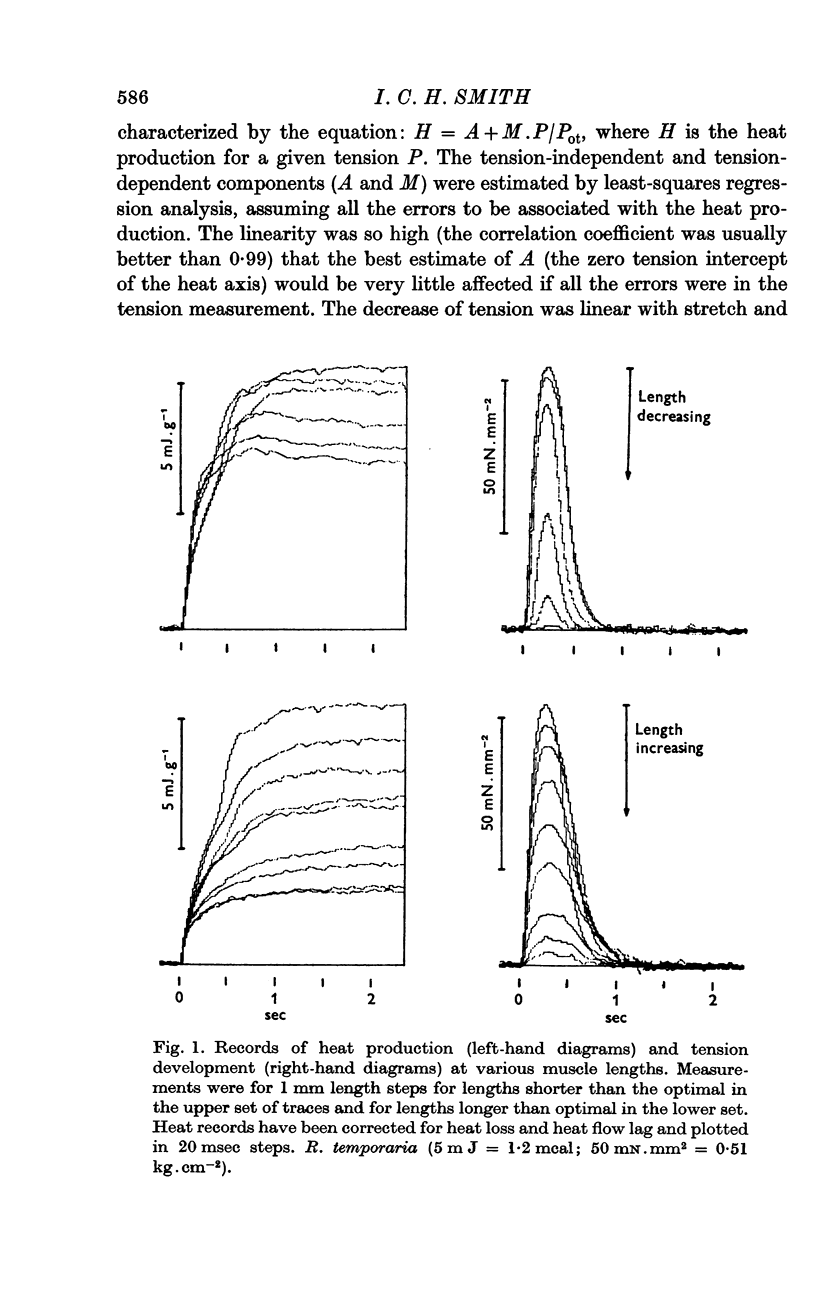
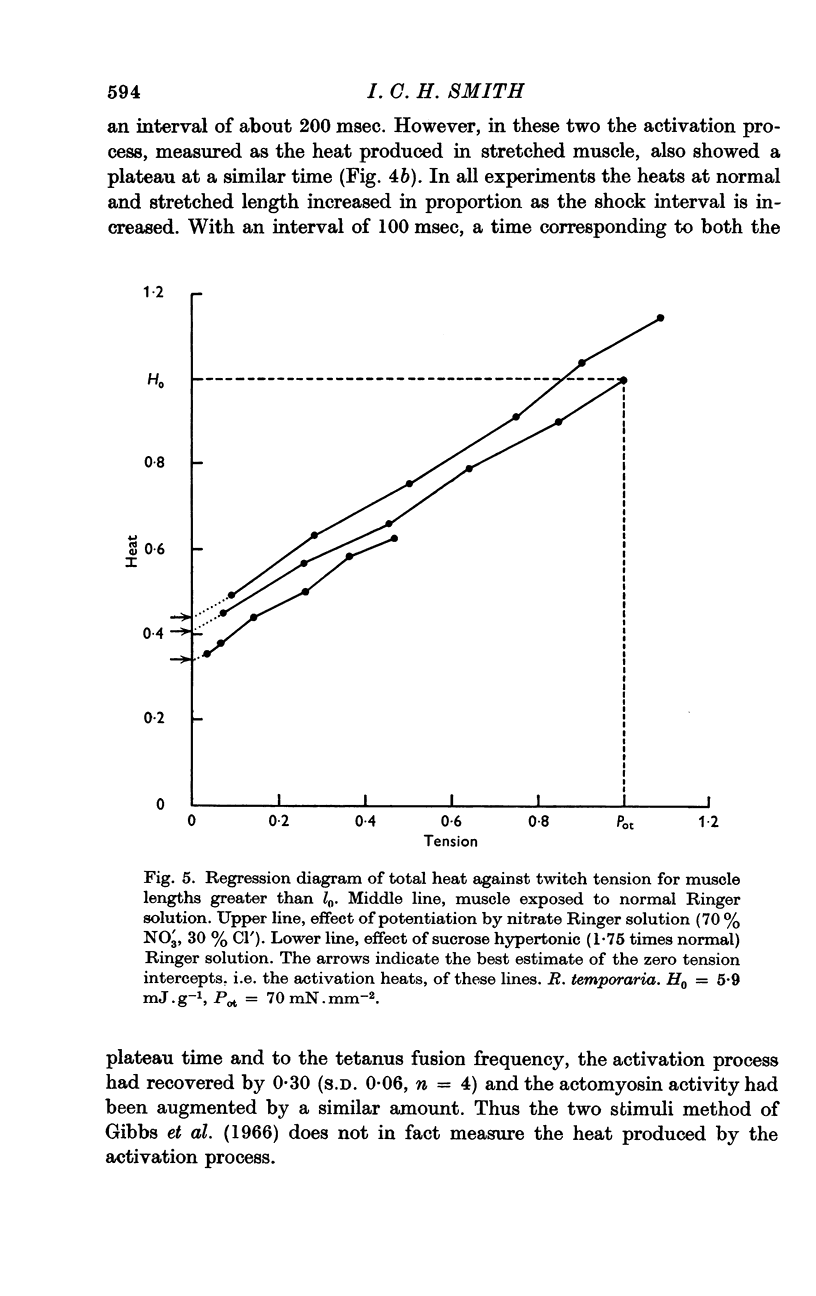
2. Heat production, H, in twitches and tetani of stretched muscle fits the relation H = A+M.P/Pot where P/Pot is the fraction of the optimal tension remaining at the stretched length and A and M are assumed to be the activation dependent and actomyosin dependent heat components.
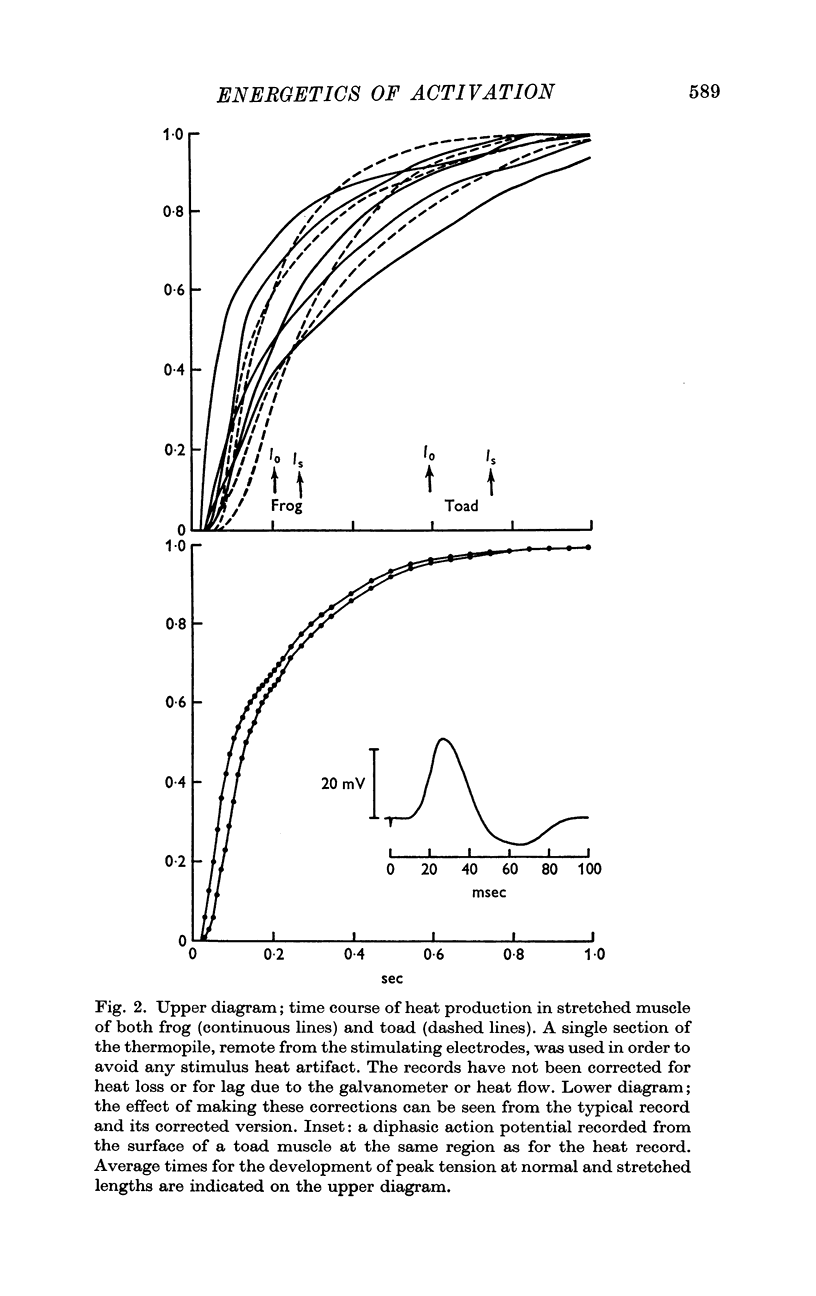
3. For twitches the A component is early and fast and constitutes 0·26 (S.D. 0·09) of the heat production at normal muscle lengths. Its time course is similar in both frog and toad muscle although both M and P are twofold slower in toad muscle. High concentrations of CO2 slow only M and Pot. The A component is associated with a normal recovery heat.
4. The twitch-tetanus tension ratio, after correction for the extra shortening that occurs during a tetanus, does not vary with the degree of muscle stretch: it is thus probable that twitch activation does not vary with muscle stretch.
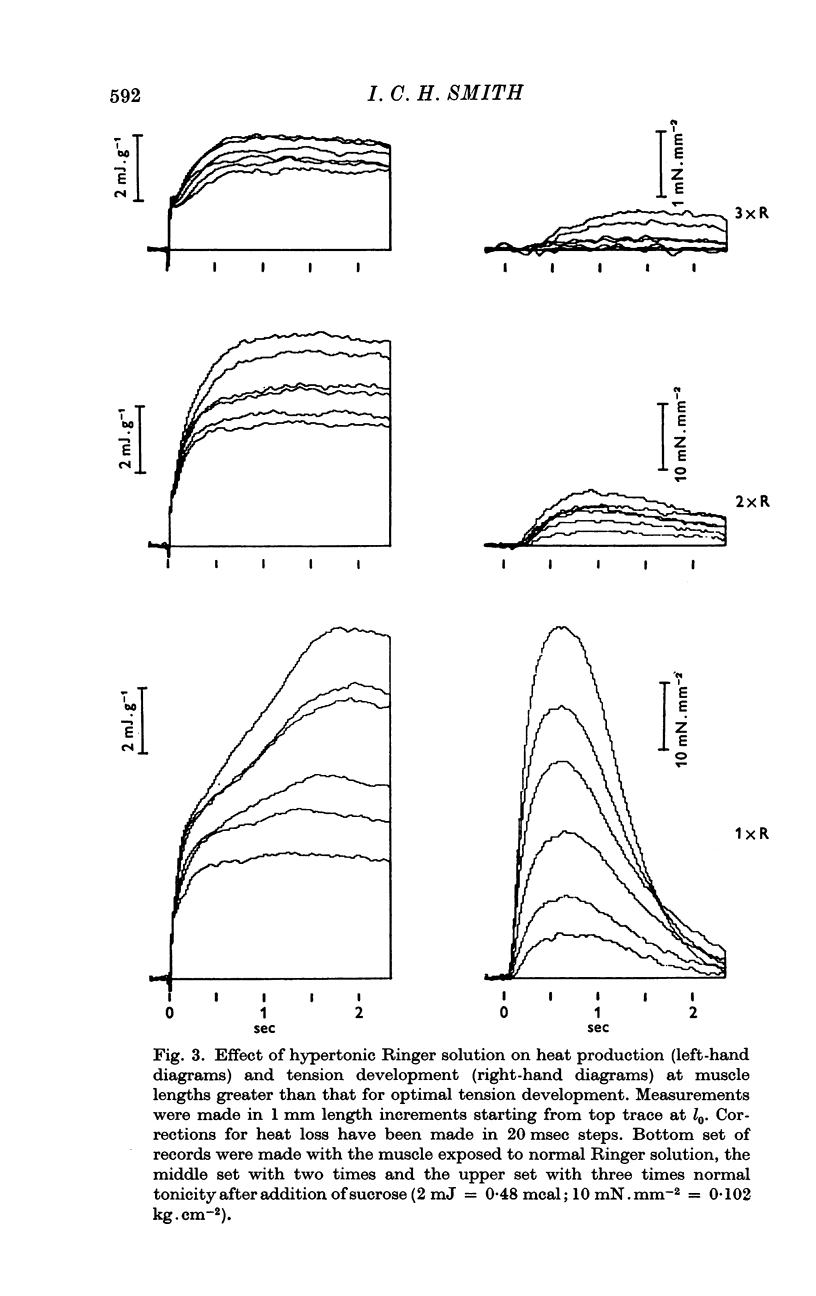
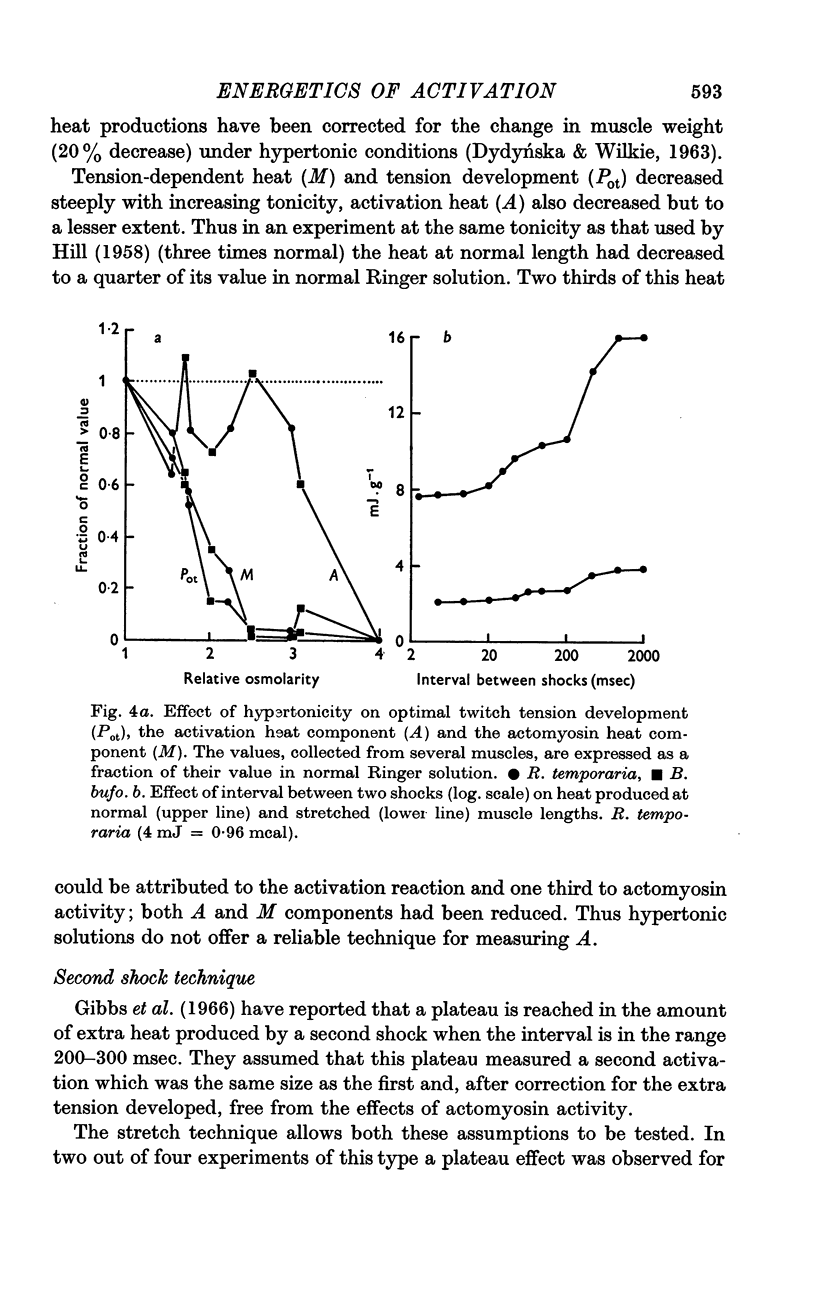
5. Moderately hypertonic Ringer solution reduces M and Pot but not A, but strongly hypertonic solution also reduces A. Zn2+, No3- and second shock potentiation of a twitch increase A, M and Pot in proportion to each other.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley C. C., Ridgway E. B. On the relationships between membrane potential, calcium transient and tension in single barnacle muscle fibres. J Physiol. 1970 Jul;209(1):105–130. doi: 10.1113/jphysiol.1970.sp009158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L. M., Gonzalez-Serratos H., Huxley A. F. Electron microscopy of frog muscle fibres in extreme passive shortening. J Physiol. 1970 Jun;208(2):86P–88P. [PubMed] [Google Scholar]
- CHANCE B. THE ENERGY-LINKED REACTION OF CALCIUM WITH MITOCHONDRIA. J Biol Chem. 1965 Jun;240:2729–2748. [PubMed] [Google Scholar]
- DAVIES R. E. A MOLECULAR THEORY OF MUSCLE CONTRACTION: CALCIUM-DEPENDENT CONTRACTIONS WITH HYDROGEN BOND FORMATION PLUS ATP-DEPENDENT EXTENSIONS OF PART OF THE MYOSIN-ACTIN CROSS-BRIDGES. Nature. 1963 Sep 14;199:1068–1074. doi: 10.1038/1991068a0. [DOI] [PubMed] [Google Scholar]
- DYDYNSKA M., WILKIE D. R. THE OSMOTIC PROPERTIES OF STRIATED MUSCLE FIBERS IN HYPERTONIC SOLUTIONS. J Physiol. 1963 Nov;169:312–329. doi: 10.1113/jphysiol.1963.sp007258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebashi S., Endo M., Otsuki I. Control of muscle contraction. Q Rev Biophys. 1969 Nov;2(4):351–384. doi: 10.1017/s0033583500001190. [DOI] [PubMed] [Google Scholar]
- Fales J. T., Zierler K. L. Relation between length, tension, and heat: frog sartorius muscle, brief tetani. Am J Physiol. 1969 Jan;216(1):70–75. doi: 10.1152/ajplegacy.1969.216.1.70. [DOI] [PubMed] [Google Scholar]
- Gibbs C. L., Ricchiuti N. V., Mommaerts W. F. Activation heat in frog sartorius muscle. J Gen Physiol. 1966 Jan;49(3):517–535. doi: 10.1085/jgp.49.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. M., Huxley A. F., Julian F. J. Tension development in highly stretched vertebrate muscle fibres. J Physiol. 1966 May;184(1):143–169. doi: 10.1113/jphysiol.1966.sp007908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. M., Huxley A. F., Julian F. J. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol. 1966 May;184(1):170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL A. V. The heat of activation and the heat of shortening in a muscle twitch. Proc R Soc Lond B Biol Sci. 1949 Jun 23;136(883):195–211. doi: 10.1098/rspb.1949.0019. [DOI] [PubMed] [Google Scholar]
- HILL A. V. The priority of the heat production in a muscle twitch. Proc R Soc Lond B Biol Sci. 1958 Mar 18;148(932):397–402. doi: 10.1098/rspb.1958.0033. [DOI] [PubMed] [Google Scholar]
- Homsher E., Mommaerts W. F., Ricchiuti N. V., Wallner A. Activation heat, activation metabolism and tension-related heat in frog semitendinosus muscles. J Physiol. 1972 Feb;220(3):601–625. doi: 10.1113/jphysiol.1972.sp009725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JEWELL B. R., WILKIE D. R. An analysis of the mechanical components in frog's striated muscle. J Physiol. 1958 Oct 31;143(3):515–540. doi: 10.1113/jphysiol.1958.sp006075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JEWELL B. R., WILKIE D. R. The mechanical properties of relaxing muscle. J Physiol. 1960 Jun;152:30–47. doi: 10.1113/jphysiol.1960.sp006467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewell B. R., Kretzschmar M., Woledge R. C. Length and tension transducers. J Physiol. 1967 Jul;191(1):10P–12P. [PubMed] [Google Scholar]
- Jöbsis F. F., O'Connor M. J. Calcium release and reabsorption in the sartorius muscle of the toad. Biochem Biophys Res Commun. 1966 Oct 20;25(2):246–252. doi: 10.1016/0006-291x(66)90588-2. [DOI] [PubMed] [Google Scholar]
- Kushmerick M. J., Larson R. E., Davies R. E. The chemical energetics of muscle contraction. I. Activation heat, heat of shortening and ATP utilization for activation-relaxation processes. Proc R Soc Lond B Biol Sci. 1969 Dec 23;174(1036):293–313. doi: 10.1098/rspb.1969.0095. [DOI] [PubMed] [Google Scholar]
- Mommaerts W. F. Energetics of muscular contraction. Physiol Rev. 1969 Jul;49(3):427–508. doi: 10.1152/physrev.1969.49.3.427. [DOI] [PubMed] [Google Scholar]
- Ogawa Y. Some properties of fragmented frog sarcoplasmic reticulum with particular reference to its response to caffeine. J Biochem. 1970 May;67(5):667–683. doi: 10.1093/oxfordjournals.jbchem.a129295. [DOI] [PubMed] [Google Scholar]
- Smith I. C. Heat production in twitches of stretched muscle. J Physiol. 1970 Jun;208(2):71P–72P. [PubMed] [Google Scholar]
- Taylor S. R., Preiser H., Sandow A. Mechanical threshold as a factor in excitation-contraction coupling. J Gen Physiol. 1969 Sep;54(3):352–368. doi: 10.1085/jgp.54.3.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S. R., Rüdel R. Striated muscle fibers: inactivation of contraction induced by shortening. Science. 1970 Feb 6;167(3919):882–884. doi: 10.1126/science.167.3919.882. [DOI] [PubMed] [Google Scholar]
- Walsh T. H., Woledge R. C. Heat production and chemical change in tortoise muscle. J Physiol. 1970 Feb;206(2):457–469. doi: 10.1113/jphysiol.1970.sp009024. [DOI] [PMC free article] [PubMed] [Google Scholar]



