Abstract
1. In urethane/pentobarbitone anaesthetized male rats, the hypothalamus and pituitary stalk were exposed by a transpharyngeal approach. Cells recorded with glass micro-electrodes from the ventral hypothalamus near the bifurcation of the internal carotid artery were identified as supraoptic neurones only when their antidromic action potential evoked at constant latency by stimulation of the pituitary stalk was cancelled by collision with a spontaneously occurring action potential.
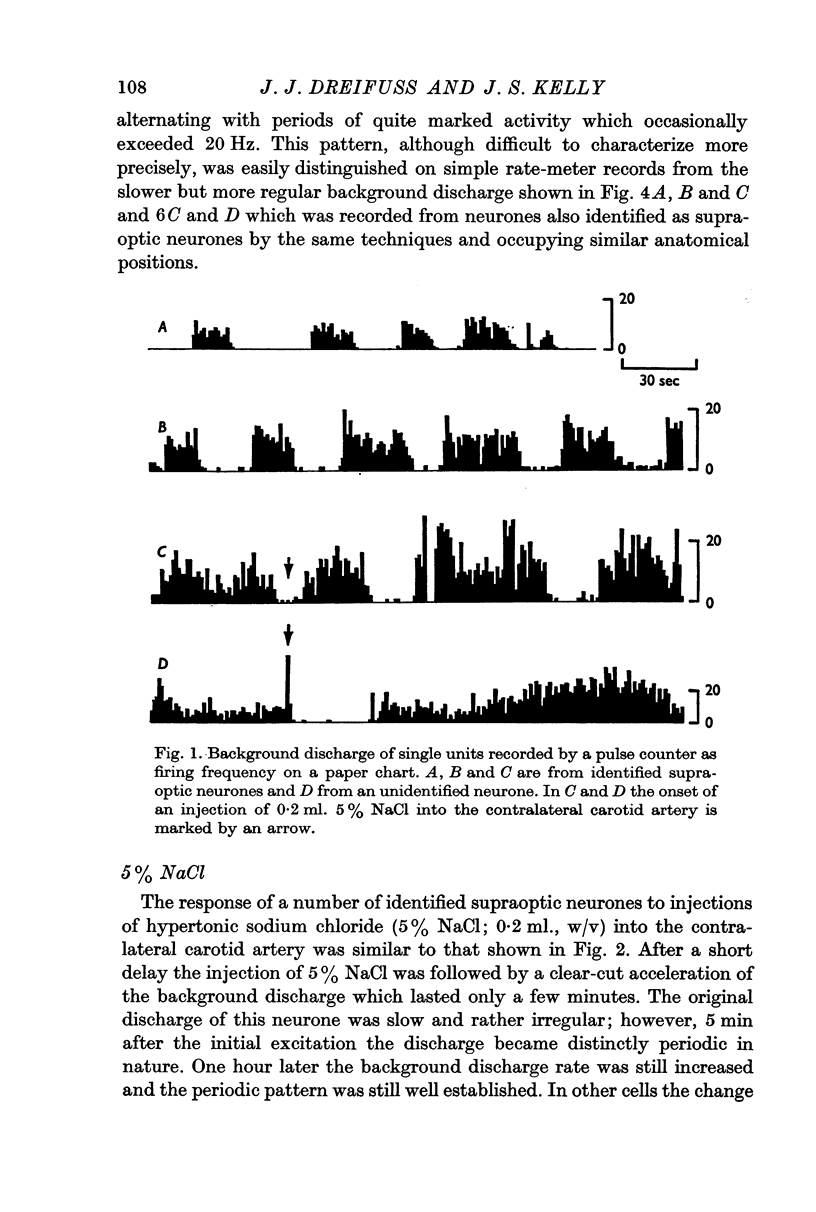
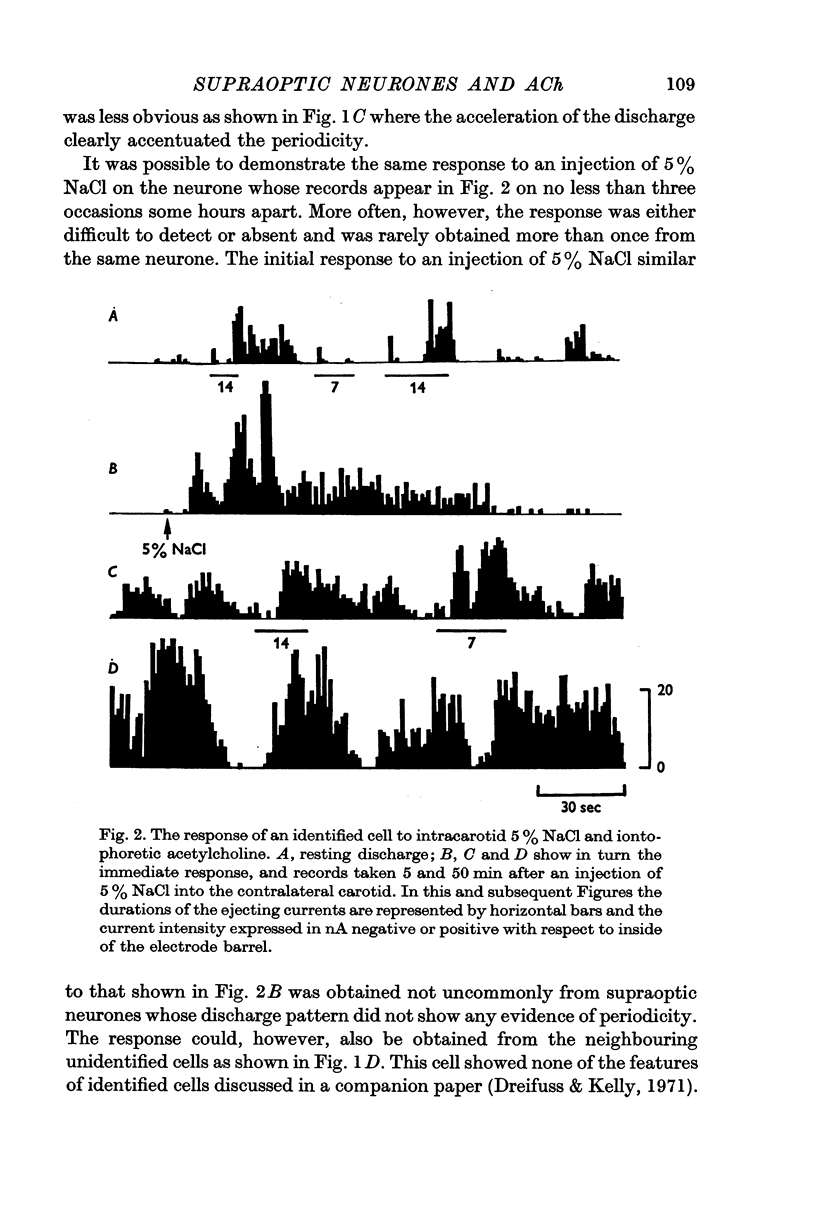
2. More than 80% of the identified cells were spontaneously active. The frequency of firing was often slow (under 1 Hz) and rather irregular. More than half the cells, however, had a more distinctive firing pattern which recurred every 1-3 min and consisted of a period of low or absent activity alternating with periods of quite marked discharge which occasionally exceeded 20 Hz. This periodicity was retained when the over-all activity was enhanced by an intracarotid injection of 0·2 ml. 5% (w/v) NaCl.
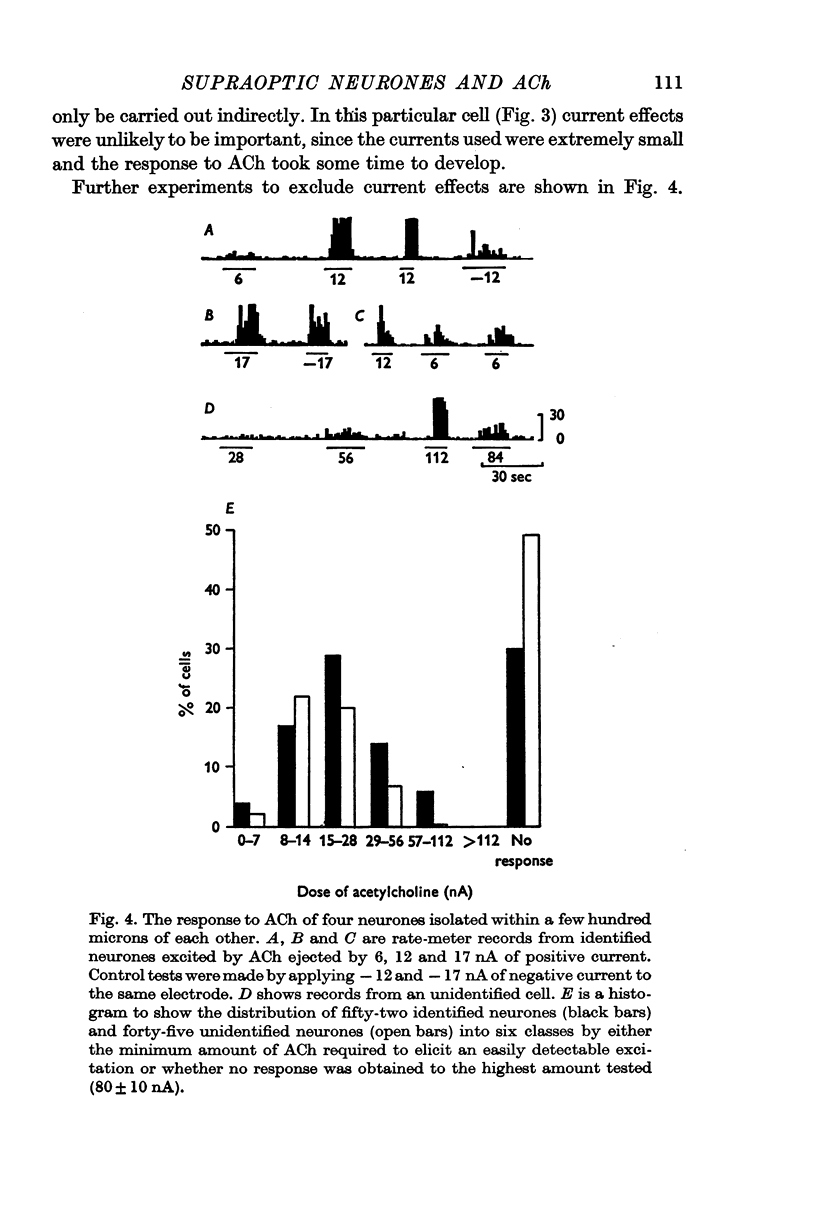
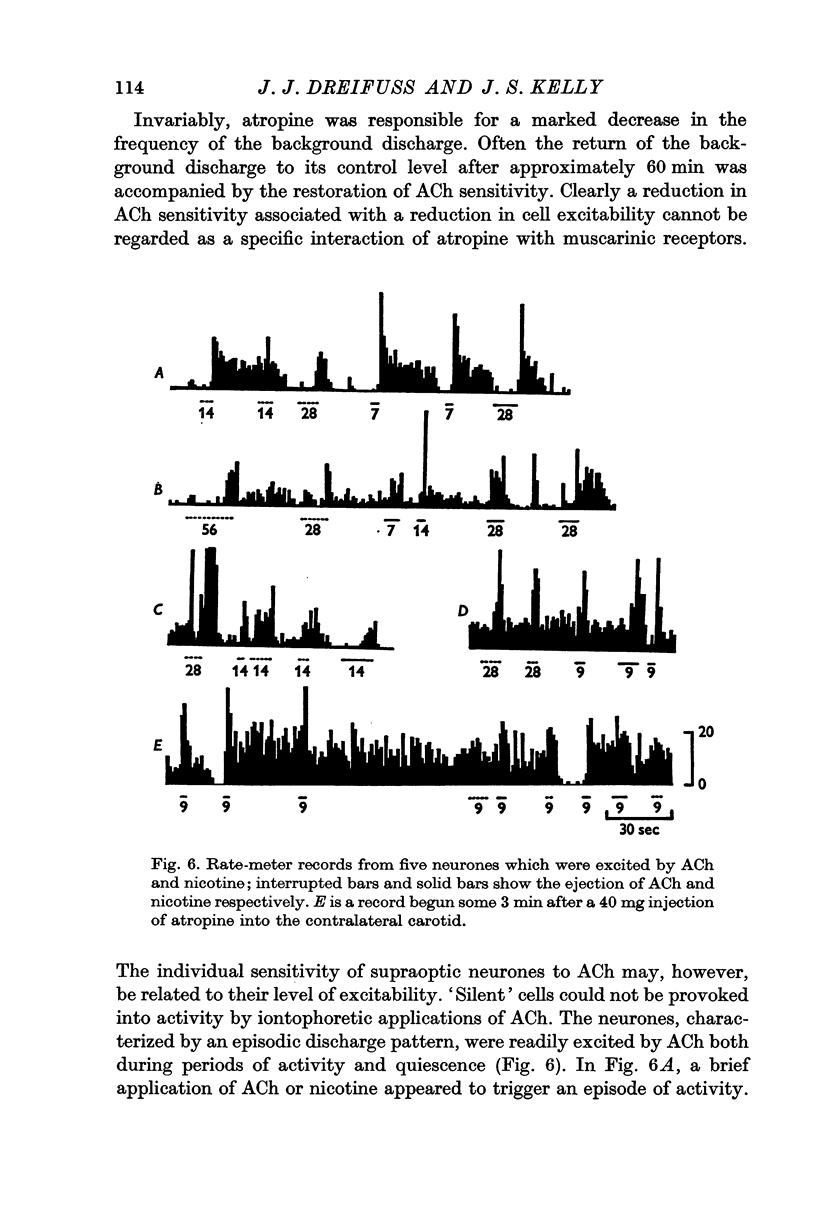
3. Of fifty-two cells tested, thirty-six were excited by acetylcholine applied by iontophoresis from a multibarrelled micropipette with average ejection currents of 35 ± 5 nA (S.D. of thirty-six observations). Ten of these cells were also excited by nicotine applied with similar currents.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAHAMS V. C., KOELLE G. B., SMART P. Histochemical demonstration of cholinesterases in the hypothalamus of the dog. J Physiol. 1957 Nov 14;139(1):137–144. doi: 10.1113/jphysiol.1957.sp005881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J. L., Crayton J. W., Nicoll R. A. Supraoptic neurosecretory cells: adrenergic and cholinergic sensitivity. Science. 1971 Jan 15;171(3967):208–210. doi: 10.1126/science.171.3967.208. [DOI] [PubMed] [Google Scholar]
- CROSS B. A., GREEN J. D. Activity of single neurones in the hypothalamus: effect of osmotic and other stimuli. J Physiol. 1959 Oct;148:554–569. doi: 10.1113/jphysiol.1959.sp006306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIS D. R., ECCLES R. M. The excitation of Renshaw cells by pharmacological agents applied electrophoretically. J Physiol. 1958 May 28;141(3):435–445. doi: 10.1113/jphysiol.1958.sp005987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford J. M., Curtis D. R. Pharmacological studies on feline Betz cells. J Physiol. 1966 Sep;186(1):121–138. doi: 10.1113/jphysiol.1966.sp008024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross B. A., Moss R. L., Urban I. Effect of iontophoretic application of acetylcholine and noradrenaline to antidromically identified paraventricular neurones. J Physiol. 1971;214 (Suppl):28P–30P. [PubMed] [Google Scholar]
- Curtis D. R., Ryall R. W. The acetylcholine receptors of Renshaw cells. Exp Brain Res. 1966;2(1):66–80. doi: 10.1007/BF00234361. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Ryall R. W. The excitation of Renshaw cells by cholinomimetics. Exp Brain Res. 1966;2(1):49–65. doi: 10.1007/BF00234360. [DOI] [PubMed] [Google Scholar]
- Dreifuss J. J., Kelly J. S. Excitation of identified supraoptic neurones by the iontophoretic application of acetylcholine. J Physiol. 1970 Sep;210(2):170P–172P. [PubMed] [Google Scholar]
- Dreifuss J. J., Kelly J. S. Recurrent inhibition of antidromically identified rat supraoptic neurones. J Physiol. 1972 Jan;220(1):87–103. doi: 10.1113/jphysiol.1972.sp009696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyball R. E., Koizumi K. Electrical activity in the supraoptic and paraventricular nuclei associated with neurohypophysial hormone release. J Physiol. 1969 May;201(3):711–722. doi: 10.1113/jphysiol.1969.sp008783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyball R. E. Oxytocin and ADH secretion in relation to electrical activity in antidromically identified supraoptic and paraventricular units. J Physiol. 1971 Apr;214(2):245–256. doi: 10.1113/jphysiol.1971.sp009430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES D. M., FATT P. Pharmacological investigations on a central synapse operated by acetylcholine. J Physiol. 1956 Jan 27;131(1):154–169. doi: 10.1113/jphysiol.1956.sp005452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., FATT P., KOKETSU K. Cholinergic and inhibitory synapses in a pathway from motor-axon collaterals to motoneurones. J Physiol. 1954 Dec 10;126(3):524–562. doi: 10.1113/jphysiol.1954.sp005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldberg W., Vogt M. Acetylcholine synthesis in different regions of the central nervous system. J Physiol. 1948 Jun 25;107(3):372–381. doi: 10.1113/jphysiol.1948.sp004282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay A. L., Hayward J. N. Spontaneous activity of single neurones in the hypothalamus of rabbits during sleep and waking. J Physiol. 1969 Mar;201(1):237–258. doi: 10.1113/jphysiol.1969.sp008753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEBB C. O., SILVER A. Choline acetylase in the central nervous system of man and some other mammals. J Physiol. 1956 Dec 28;134(3):718–728. doi: 10.1113/jphysiol.1956.sp005678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward J. N., Vincent J. D. Osmosensitive single neurones in the hypothalamus of unanaesthetized monkeys. J Physiol. 1970 Nov;210(4):947–972. doi: 10.1113/jphysiol.1970.sp009251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjević K., Phillis J. W. Pharmacological properties of acetylcholine-sensitive cells in the cerebral cortex. J Physiol. 1963 May;166(2):328–350. doi: 10.1113/jphysiol.1963.sp007107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjević K., Pumain R., Renaud L. The mechanism of excitation by acetylcholine in the cerebral cortex. J Physiol. 1971 May;215(1):247–268. doi: 10.1113/jphysiol.1971.sp009467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oomura Y., Ooyama H., Yamamoto T., Ono T., Kobayashi N. Behavior of hypothalamic unit activity during electrophoretic application of drugs. Ann N Y Acad Sci. 1969 May 15;157(2):642–665. doi: 10.1111/j.1749-6632.1969.tb12912.x. [DOI] [PubMed] [Google Scholar]
- PEPLER W. J., PEARSE A. G. The histochemistry of the esterases of rat brain, with special reference to those of the hypothalamic nuclei. J Neurochem. 1957;1(3):193–202. doi: 10.1111/j.1471-4159.1957.tb12072.x. [DOI] [PubMed] [Google Scholar]
- Pickford M. The inhibitory effect of acetylcholine on water diuresis in the dog, and its pituitary transmission. J Physiol. 1939 Feb 14;95(1):226–238. doi: 10.1113/jphysiol.1939.sp003721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakerly J. B., Lincoln D. W. Phasic discharge of antidromically identified units in the paraventricular nucleus of the hypothalamus. Brain Res. 1971 Jan 8;25(1):192–194. doi: 10.1016/0006-8993(71)90580-4. [DOI] [PubMed] [Google Scholar]


