Abstract
1. The properties of the surround response mechanism of on-centre cells and its interaction with the centre mechanism were studied by recording from single optic tract fibres. In many of the experiments the spatial distribution of the light within the retinal image of the stimuli was measured.
2. Pure surround responses of on-centre cells were isolated using a centrally located steady light which selectively desensitized (adapted) the centre mechanism. This permitted a peripheral flashing stimulus whose luminance varied over a range as great as 1·38 log units to elicit surround responses which, for any given cell, were of invariant shape. The rate of decay of the firing frequency of the spike burst at `off' varied from cell to cell. The general characteristics of such pure surround responses to squarewave stimuli were described. The plot of the magnitude of pure responses against stimulus luminance, at constant background conditions, was curvilinear.
3. The pure surround response of two off-centre cells was isolated; it was similar in shape to the pure centre response of on-centre cells.
4. Interaction of centre and surround mechanisms of on-centre cells was studied by eliciting a pure central and a pure surround response from the same cell. The electronically obtained algebraic sum of these two pure responses equalled the mixed response of the ganglion cell to simultaneous presentation of the stimuli which evoked the pure responses when presented singly. This is probably best explained by algebraic summation of centre and surround inputs.
5. The pure surround response from two cells to a fixed flashing stimulus was attenuated by a steady field adapting light, both when this was superimposed upon the stimulus and when not superimposed. In the latter case, (i) when the spatial separation between the flashing stimulus and the adapting light was at a minimum, less than 10% of the adapting flux fell inside the boundaries of the stimulating flux, and (ii) the response was attenuated also if the adapting light was in the geometric centre of the receptive field. These results indicate that the adaptation pool of the surround mechanism extends to the central portions of the receptive field.
6. Nearly half the cells tested did not yield pure surround responses. This was probably due to differences, within the ganglion cell population, (i) of the spatial distribution of the ratio of centre to surround signal sensitivity and (ii) of differences in the ratio of centre to surround adaptivity in the receptive field middle. It was not due to excess adaptive flux falling outside the region of maximal centre mechanism adaptivity, nor due to excess stimulus flux falling inside the region of maximal signal sensitivity.
Full text
PDF




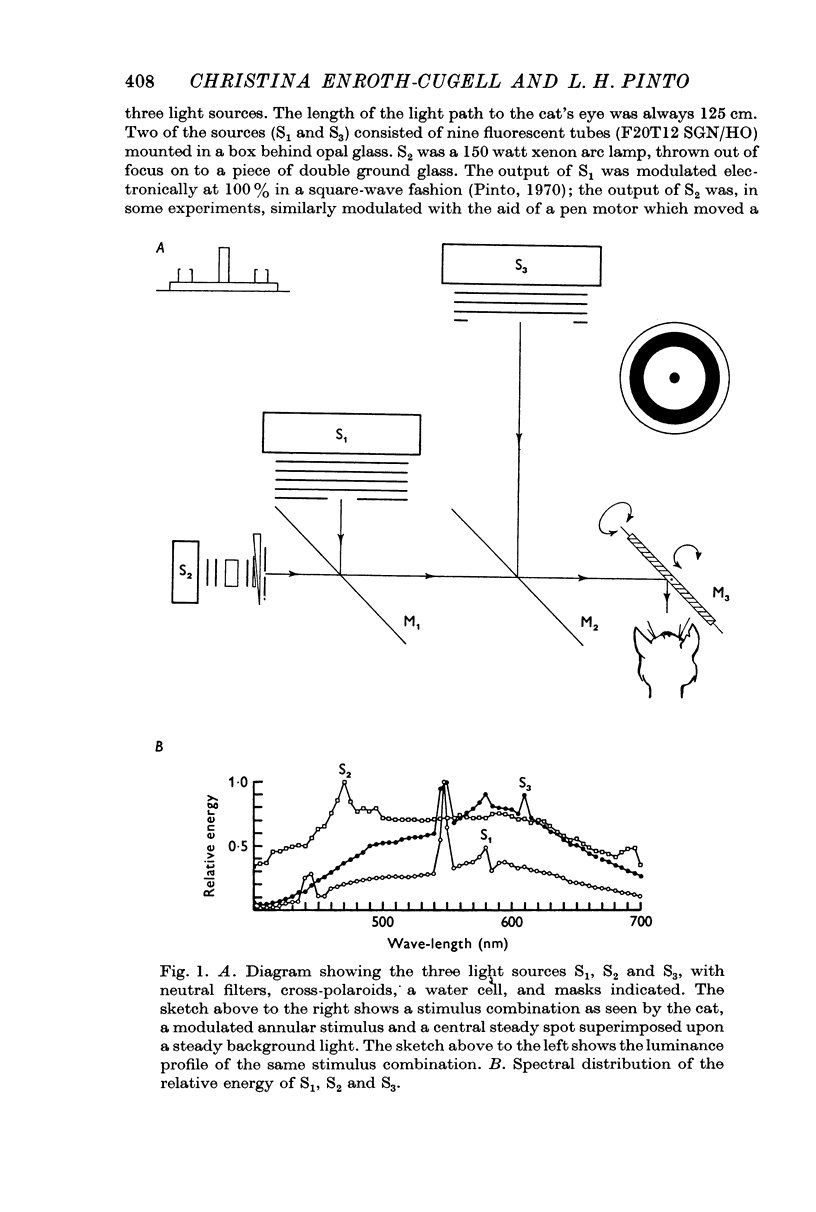




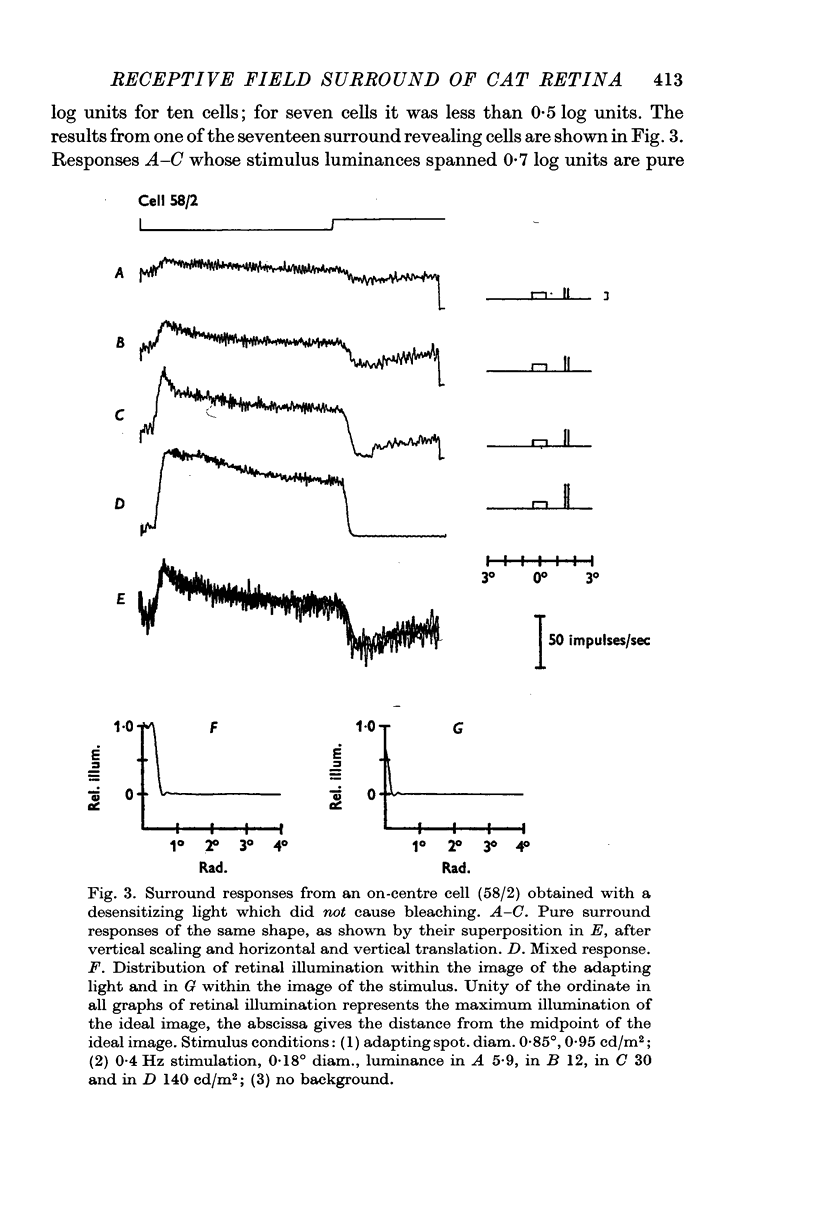
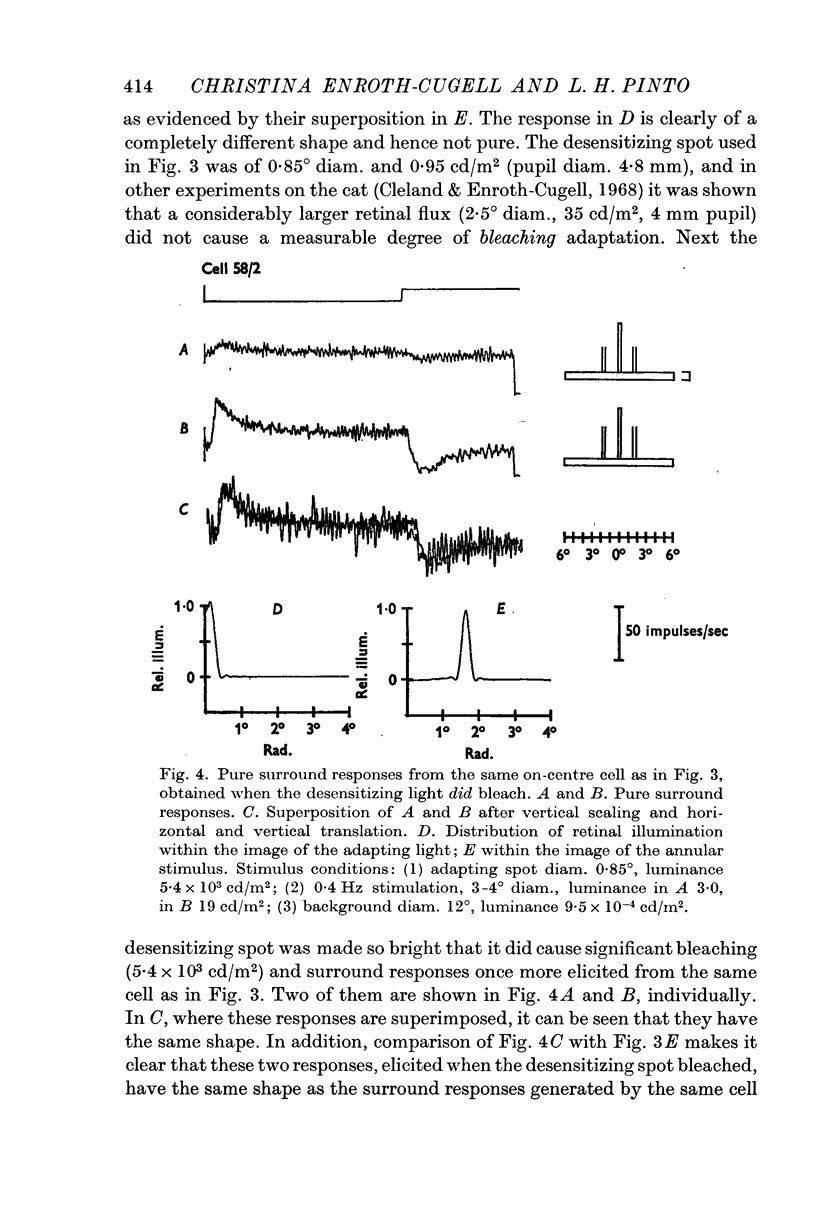

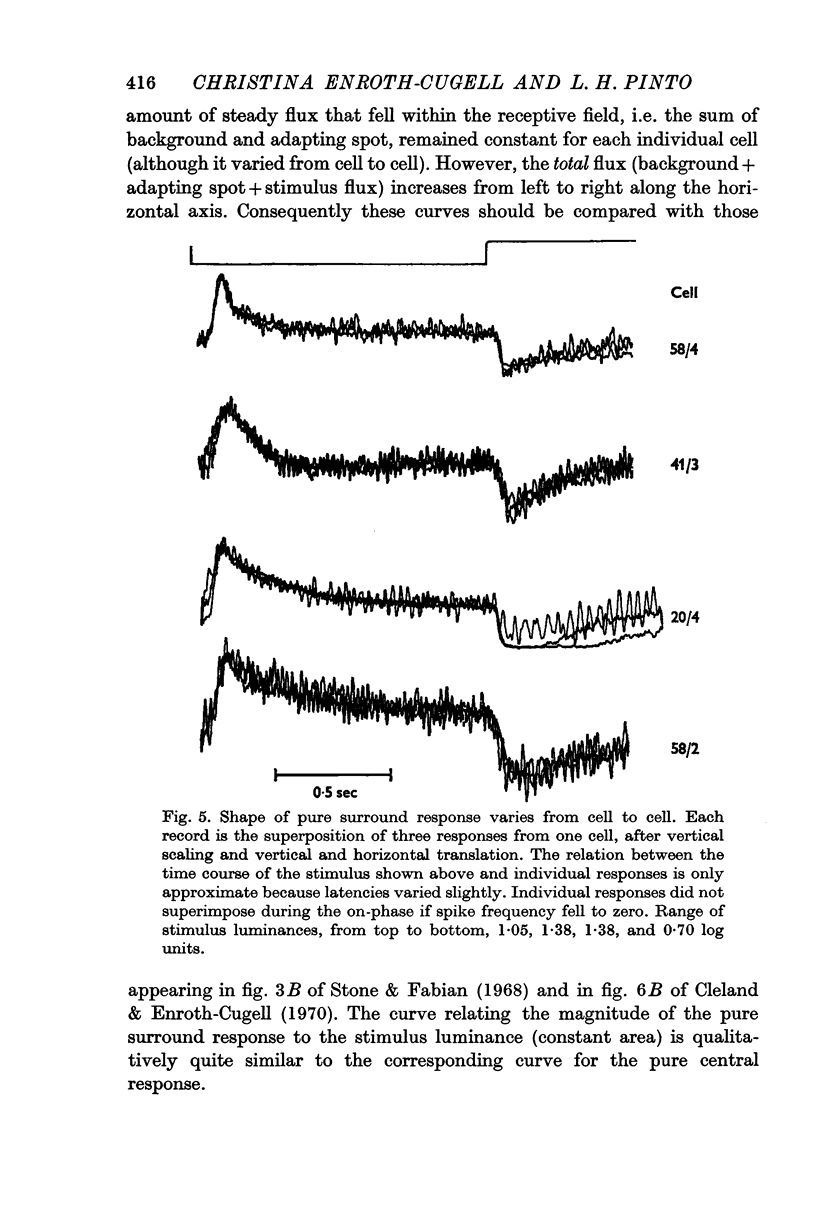























Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARLOW H. B., FITZHUGH R., KUFFLER S. W. Change of organization in the receptive fields of the cat's retina during dark adaptation. J Physiol. 1957 Aug 6;137(3):338–354. doi: 10.1113/jphysiol.1957.sp005817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R. Changes in the maintained discharge with adaptation level in the cat retina. J Physiol. 1969 Jun;202(3):699–718. doi: 10.1113/jphysiol.1969.sp008836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R. Three factors limiting the reliable detection of light by retinal ganglion cells of the cat. J Physiol. 1969 Jan;200(1):1–24. doi: 10.1113/jphysiol.1969.sp008679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonds A. B., Enroth-Cugell C., Pinto L. H. Image quality of the cat eye measured during retinal ganglion cell experiments. J Physiol. 1972 Jan;220(2):383–401. doi: 10.1113/jphysiol.1972.sp009713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Enroth-Cugell C. Quantitative aspects of gain and latency in the cat retina. J Physiol. 1970 Jan;206(1):73–91. doi: 10.1113/jphysiol.1970.sp008998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Enroth-cugell C. Quantitative aspects of sensitivity and summation in the cat retina. J Physiol. 1968 Sep;198(1):17–38. doi: 10.1113/jphysiol.1968.sp008591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Pinto L. H. Gallamine triethiodide (flaxedil) and cat retinal ganglion cell responses. J Physiol. 1970 Jul;208(3):677–689. doi: 10.1113/jphysiol.1970.sp009142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Pinto L. H. Pure central responses from off-centre cells and pure surround responses from on-centre cells. J Physiol. 1972 Jan;220(2):441–464. doi: 10.1113/jphysiol.1972.sp009715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITZHUGH R. The statistical detection of threshold signals in the retina. J Gen Physiol. 1957 Jul 20;40(6):925–948. doi: 10.1085/jgp.40.6.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D. H. Tungsten Microelectrode for Recording from Single Units. Science. 1957 Mar 22;125(3247):549–550. doi: 10.1126/science.125.3247.549. [DOI] [PubMed] [Google Scholar]
- KUFFLER S. W. Discharge patterns and functional organization of mammalian retina. J Neurophysiol. 1953 Jan;16(1):37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- KUFFLER S. W. Neurons in the retina; organization, inhibition and excitation problems. Cold Spring Harb Symp Quant Biol. 1952;17:281–292. doi: 10.1101/sqb.1952.017.01.026. [DOI] [PubMed] [Google Scholar]
- LIPETZ L. E. A mechanism of light adaptation. Science. 1961 Mar 3;133(3453):639–640. doi: 10.1126/science.133.3453.639. [DOI] [PubMed] [Google Scholar]
- Maffei L., Cervetto L. Dynamic interactions in retinal receptive fields. Vision Res. 1968 Oct;8(10):1299–1303. doi: 10.1016/0042-6989(68)90051-5. [DOI] [PubMed] [Google Scholar]
- Maffei L., Cervetto L., Fiorentini A. Transfer characteristics of excitation and inhibition in cat retinal ganglion cells. J Neurophysiol. 1970 Mar;33(2):276–284. doi: 10.1152/jn.1970.33.2.276. [DOI] [PubMed] [Google Scholar]
- RUSHTON W. A. VISUAL ADAPTATION. Proc R Soc Lond B Biol Sci. 1965 Mar 16;162:20–46. doi: 10.1098/rspb.1965.0024. [DOI] [PubMed] [Google Scholar]
- Rodieck R. W., Stone J. Response of cat retinal ganglion cells to moving visual patterns. J Neurophysiol. 1965 Sep;28(5):819–832. doi: 10.1152/jn.1965.28.5.819. [DOI] [PubMed] [Google Scholar]
- Spekreijse H. Rectification in the goldfish retina: analysis by sinusoidal and auxiliary stimulation. Vision Res. 1969 Dec;9(12):1461–1472. doi: 10.1016/0042-6989(69)90062-5. [DOI] [PubMed] [Google Scholar]
- Stone J., Fabian M. Summing properties of the cat's retinal ganglion cell. Vision Res. 1968 Aug;8(8):1023–1040. doi: 10.1016/0042-6989(68)90075-8. [DOI] [PubMed] [Google Scholar]
- WAGNER H. G., MACNICHOL E. F., Jr, WOLBARSHT M. L. Functional basis for "on"-center and "off"-center receptive fields in the retina. J Opt Soc Am. 1963 Jan;53:66–70. doi: 10.1364/josa.53.000066. [DOI] [PubMed] [Google Scholar]
- WIESEL T. N. Receptive fields of ganglion cells in the cat's retina. J Physiol. 1960 Oct;153:583–594. doi: 10.1113/jphysiol.1960.sp006557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters R. W., Walters J. W. Transient and steady state stimulus-response relations for cat retinal ganglion cells. Vision Res. 1970 Jun;10(6):461–477. doi: 10.1016/0042-6989(70)90003-9. [DOI] [PubMed] [Google Scholar]


