Abstract
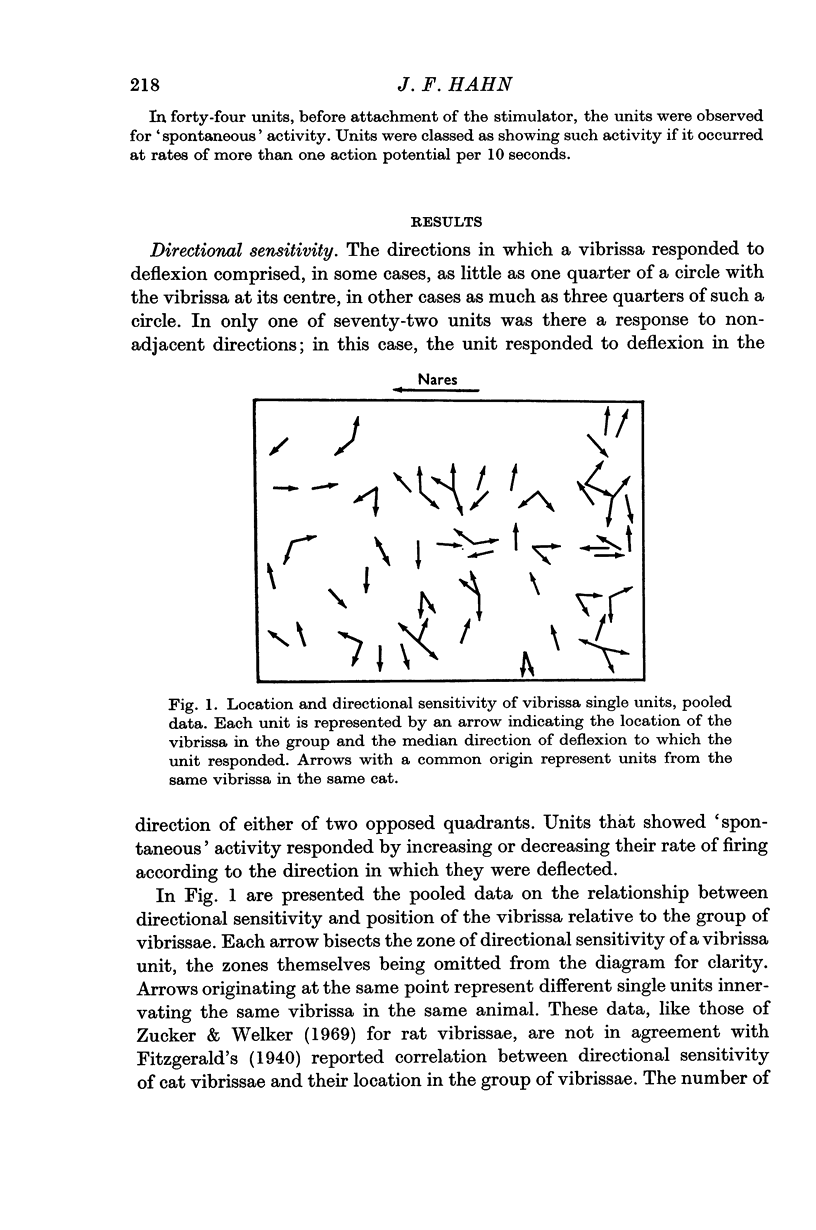
1. In the infraorbital nerve of anaesthetized cat, recordings were made from single units serving the vibrissae. The directional sensitivity of individual units was rather gross, but comparison of the outputs of several units from the same vibrissa could provide information that was more precise about the direction of vibrissa deflexion.
2. Many of the units showed `spontaneous' activity. The majority of rates were less than 1 impulse/sec, so that this activity is not likely to be an important carrier of information.
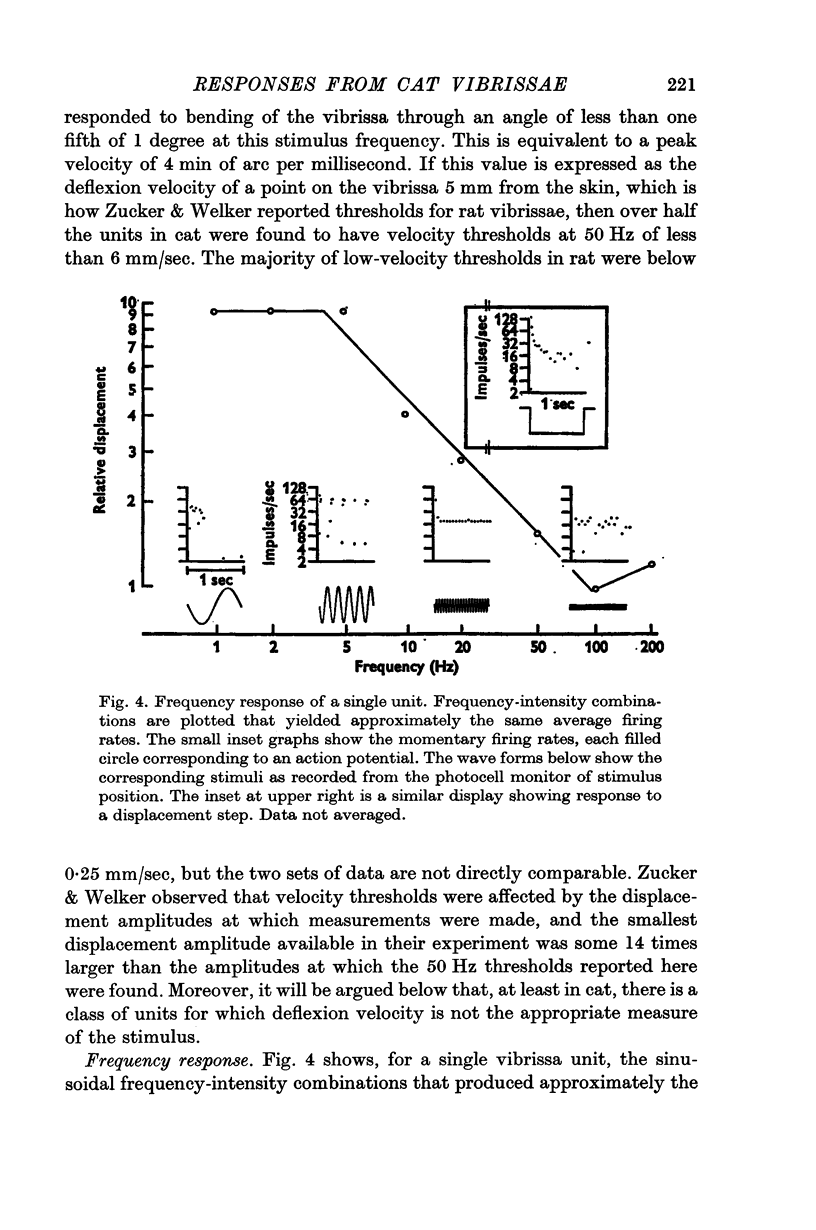
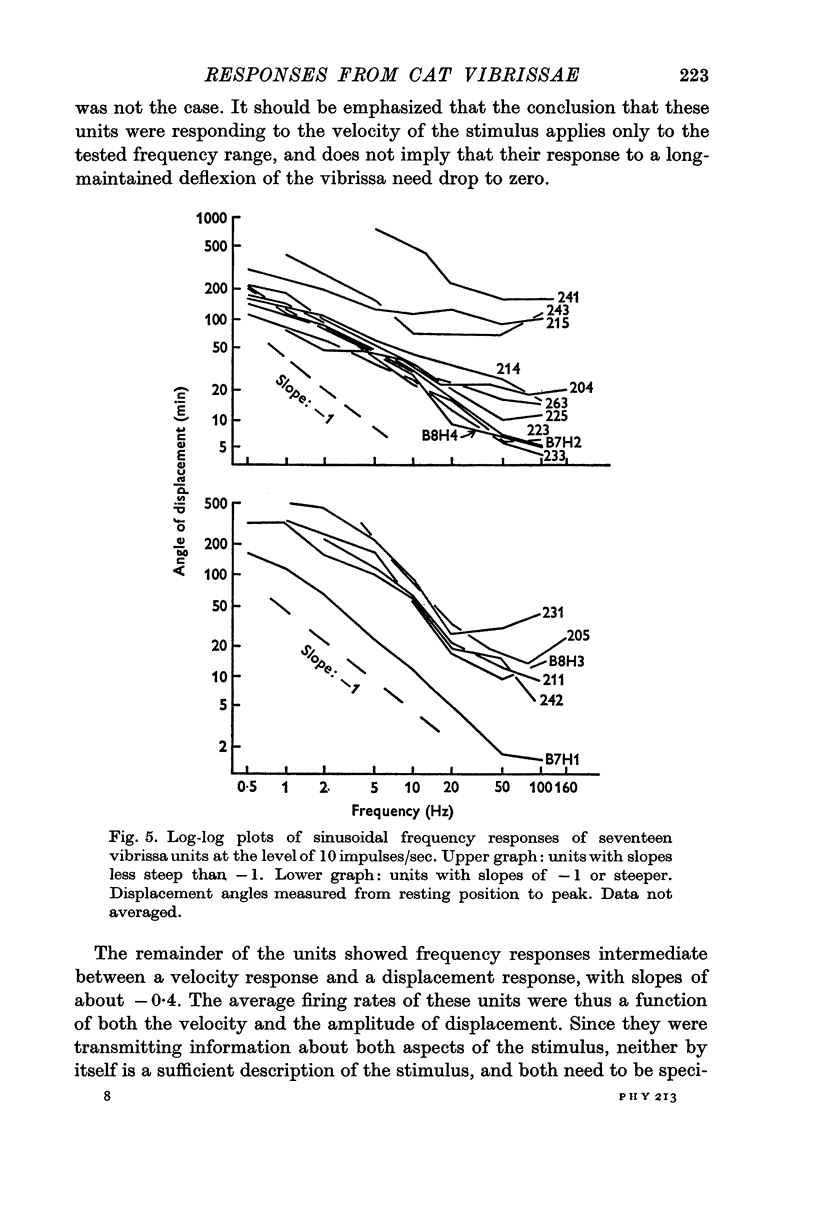
3. When sinusoidal deflexions in the frequency range of 0·5-100 Hz were applied, the frequency responses of about half of the units were those to be expected if they were responding to the velocity of the stimulus; most of the remainder responded to both the velocity and the magnitude of the deflexion. This form of information presentation has been shown to be well suited for compensatory tracking tasks, and it is suggested that the vibrissae are important for such tasks in the animal's life, rather than merely serving as obstacle detectors.
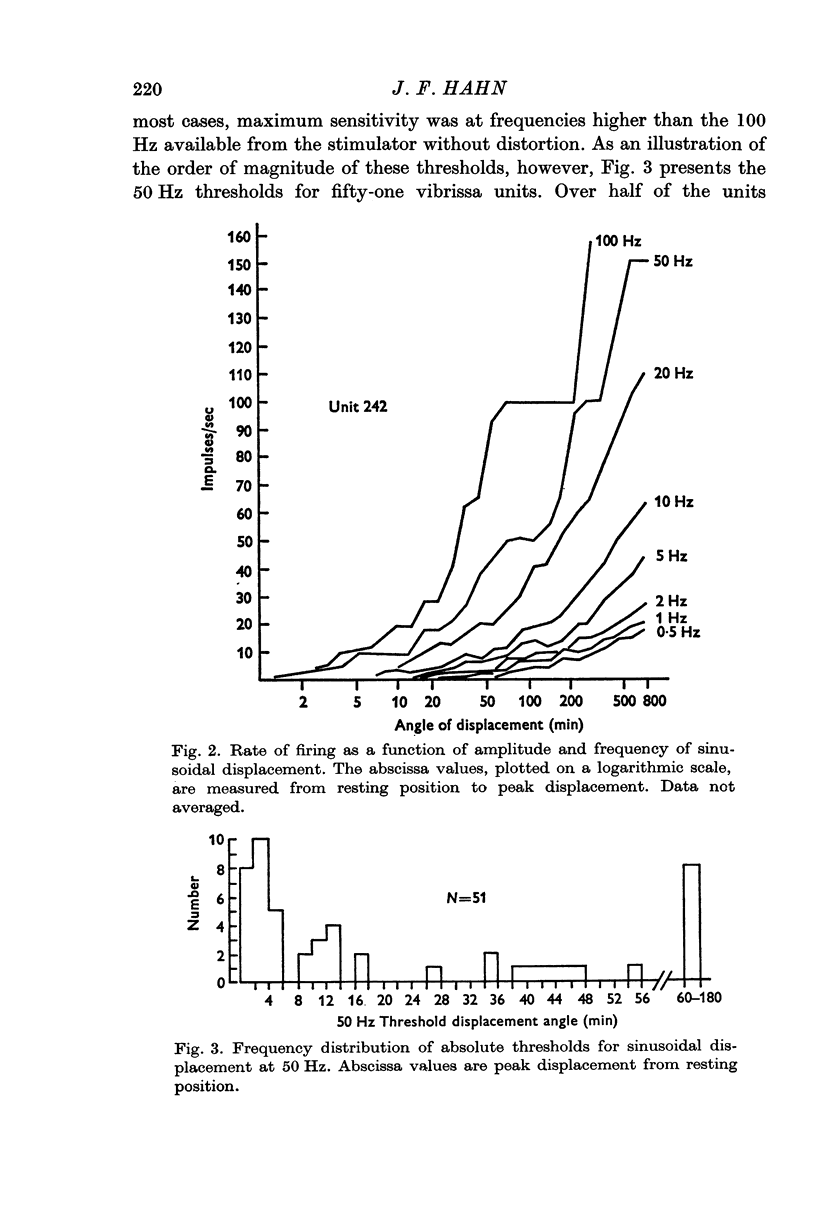
4. Greatest deflexion sensitivity typically was at higher frequencies than could be attained in this experiment, but would be greater than the median threshold deflexion angle of 12 min found at 50 Hz. It is suggested that these low thresholds are more appropriate for a tracking system than for an obstacle detector.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andres K. H. Uber die Feinstruktur der Rezeptoren an Sinushaaren. Z Zellforsch Mikrosk Anat. 1966;75(1):339–365. [PubMed] [Google Scholar]
- Fitzgerald O. Discharges from the sensory organs of the cat's vibrissae and the modification in their activity by ions. J Physiol. 1940 May 14;98(2):163–178. doi: 10.1113/jphysiol.1940.sp003841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindblom U., Tapper D. N. Terminal properties of a vibro-tactile sensor. Exp Neurol. 1967 Jan;17(1):1–15. doi: 10.1016/0014-4886(67)90117-3. [DOI] [PubMed] [Google Scholar]
- Patrizi G., Munger B. L. The ultrastructure and innervation of rat vibrissae. J Comp Neurol. 1966 Mar;126(3):423–435. doi: 10.1002/cne.901260305. [DOI] [PubMed] [Google Scholar]
- Talbot W. H., Darian-Smith I., Kornhuber H. H., Mountcastle V. B. The sense of flutter-vibration: comparison of the human capacity with response patterns of mechanoreceptive afferents from the monkey hand. J Neurophysiol. 1968 Mar;31(2):301–334. doi: 10.1152/jn.1968.31.2.301. [DOI] [PubMed] [Google Scholar]
- Zucker E., Welker W. I. Coding of somatic sensory input by vibrissae neurons in the rat's trigeminal ganglion. Brain Res. 1969 Jan;12(1):138–156. doi: 10.1016/0006-8993(69)90061-4. [DOI] [PubMed] [Google Scholar]


