Abstract
1. The% reduction in size of the externally recorded action potential produced by concentrations of tetrodotoxin (TTX) in the range 6-300 nM was determined for the small non-myelinated fibres of the rabbit cervical vagus nerve and of the walking leg nerves of crab and lobster. The concentration of TTX for 50% reduction was around 80 nM for rabbit vagus and 14 nM for crab nerve.
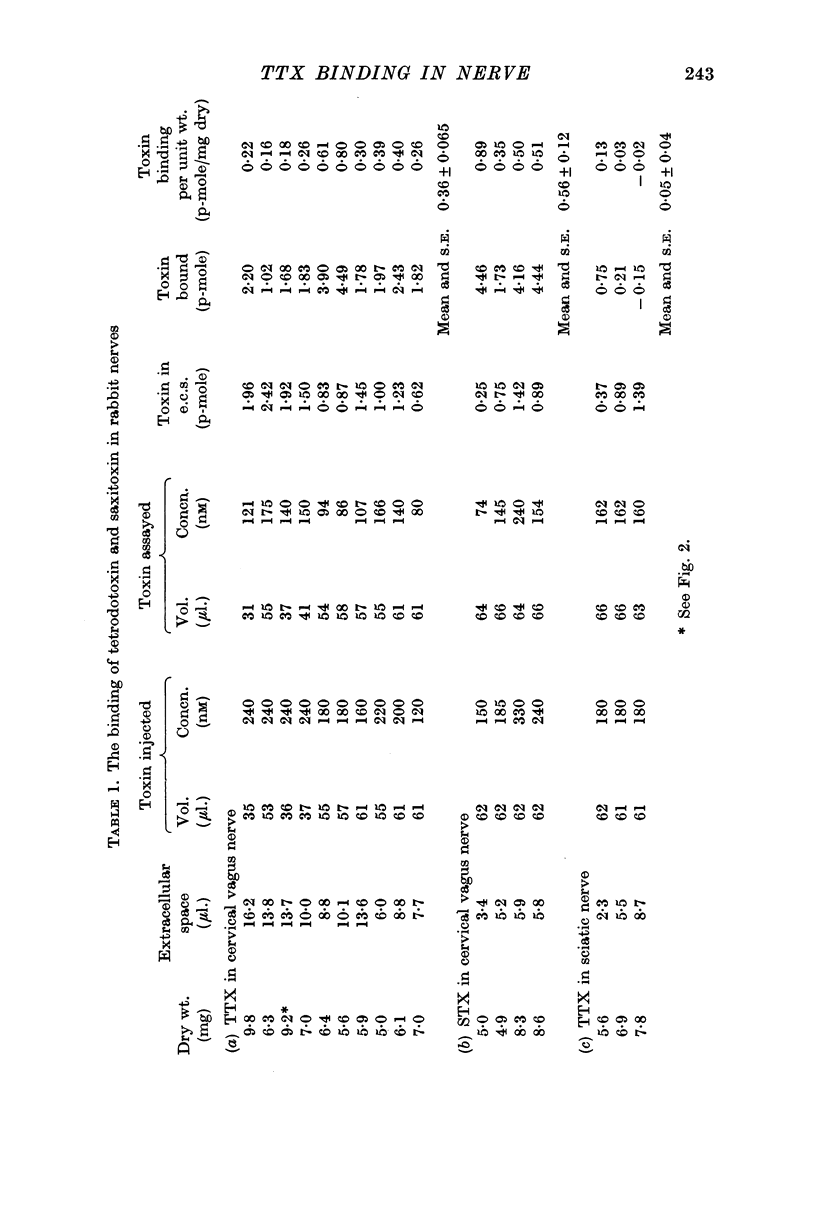
2. Bio-assay procedures were devised to measure the amount of TTX taken up by a nerve when it was exposed to a very small volume of a solution whose TTX content was just great enough to produce 100% block of conduction. The extracellular space of each nerve was determined with [14C]sugar so that an allowance could be made for extracellular dilution.
3. The TTX binding by rabbit, crab and lobster nerve was respectively 0·064, 0·053 and 0·036 p-mole/mg wet weight of nerve.
4. The binding of saxitoxin was measured in rabbit vagus nerve, and found to be much the same as that of TTX.
5. Control experiments on rabbit sciatic nerve, where the area of excitable membrane was much smaller, showed that there was relatively little unspecific binding of TTX.
6. In view of the evidence presented here and elsewhere that the blocking of sodium conductance by TTX involves the attachment of only one TTX molecule at each sodium site, and that unspecific binding of TTX does not cause serious errors, these results suggest that in 1 μm2 of nerve membrane the number of sodium sites is 75 for rabbit, 49 for crab, and 36 for lobster nerve.
Full text
PDF


















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABBOTT B. C., HILL A. V., HOWARTH J. V. The positive and negative heat production associated with a nerve impulse. Proc R Soc Lond B Biol Sci. 1958 Feb 18;148(931):149–187. doi: 10.1098/rspb.1958.0012. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Willis J. S. On the number of sodium pumping sites in cell membranes. Biochim Biophys Acta. 1969;183(3):646–649. doi: 10.1016/0005-2736(69)90180-1. [DOI] [PubMed] [Google Scholar]
- Cohen L. B., Hille B., Keynes R. D. Changes in axon birefringence during the action potential. J Physiol. 1970 Dec;211(2):495–515. doi: 10.1113/jphysiol.1970.sp009289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. B., Keynes R. D., Hille B. Light scattering and birefringence changes during nerve activity. Nature. 1968 May 4;218(5140):438–441. doi: 10.1038/218438a0. [DOI] [PubMed] [Google Scholar]
- Cuervo L. A., Adelman W. J., Jr Equilibrium and kinetic properties of the interaction between tetrodotoxin and the excitable membrane of the squid giant axon. J Gen Physiol. 1970 Mar;55(3):309–335. doi: 10.1085/jgp.55.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellory J. C., Keynes R. D. Binding of tritiated digoxin to human red cell ghosts. Nature. 1969 Feb 22;221(5182):776–776. doi: 10.1038/221776a0. [DOI] [PubMed] [Google Scholar]
- Furusawa K. The depolarization of crustacean nerve by stimulation or oxygen want. J Physiol. 1929 Jul 25;67(4):325–342. doi: 10.1113/jphysiol.1929.sp002573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth J. V., Keynes R. D., Ritchie J. M. The origin of the initial heat associated with a single impulse in mammalian non-myelinated nerve fibres. J Physiol. 1968 Feb;194(3):745–793. doi: 10.1113/jphysiol.1968.sp008434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao C. Y. Tetrodotoxin, saxitoxin and their significance in the study of excitation phenomena. Pharmacol Rev. 1966 Jun;18(2):997–1049. [PubMed] [Google Scholar]
- Keynes R. D., Ritchie J. M. The movements of labelled ions in mammalian non-myelinated nerve fibres. J Physiol. 1965 Jul;179(2):333–367. doi: 10.1113/jphysiol.1965.sp007666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landowne D., Ritchie J. M. The binding of tritiated ouabain to mammalian non-myelinated nerve fibres. J Physiol. 1970 Apr;207(2):529–537. doi: 10.1113/jphysiol.1970.sp009077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. W., Narahashi T., Shaw T. I. An upper limit to the number of sodium channels in nerve membrane? J Physiol. 1967 Jan;188(1):99–105. doi: 10.1113/jphysiol.1967.sp008126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NARAHASHI T., MOORE J. W., SCOTT W. R. TETRODOTOXIN BLOCKAGE OF SODIUM CONDUCTANCE INCREASE IN LOBSTER GIANT AXONS. J Gen Physiol. 1964 May;47:965–974. doi: 10.1085/jgp.47.5.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rang H. P., Ritchie J. M. The ionic content of mammalian non-myelinated nerve fibres and its alteration as a result of electrical activity. J Physiol. 1968 May;196(1):223–236. doi: 10.1113/jphysiol.1968.sp008503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAMPFLI R. A new method for measuring membrane potentials with external electrodes. Experientia. 1954 Dec 15;10(12):508–509. doi: 10.1007/BF02166189. [DOI] [PubMed] [Google Scholar]


