Abstract
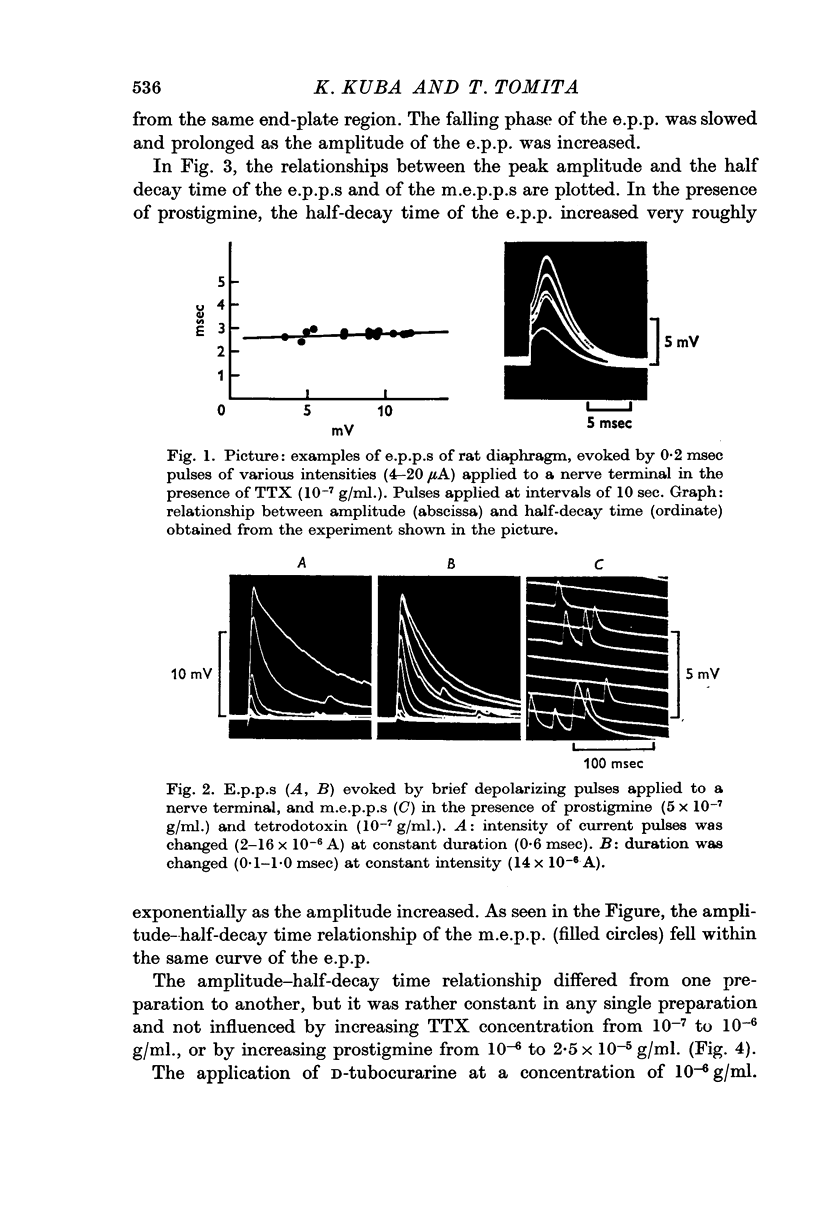
1. End-plate potentials (e.p.p.s) were evoked by applying brief depolarizing pulses to motor nerve endings in a phrenic nerve—diaphragm preparation paralysed by tetrodotoxin (TTX). Without prostigmine, the time to decay from the summit of the e.p.p. to half amplitude (the half-decay time) was roughly constant (2-4 msec) when the amplitude was increased by increasing stimulus intensity or duration.
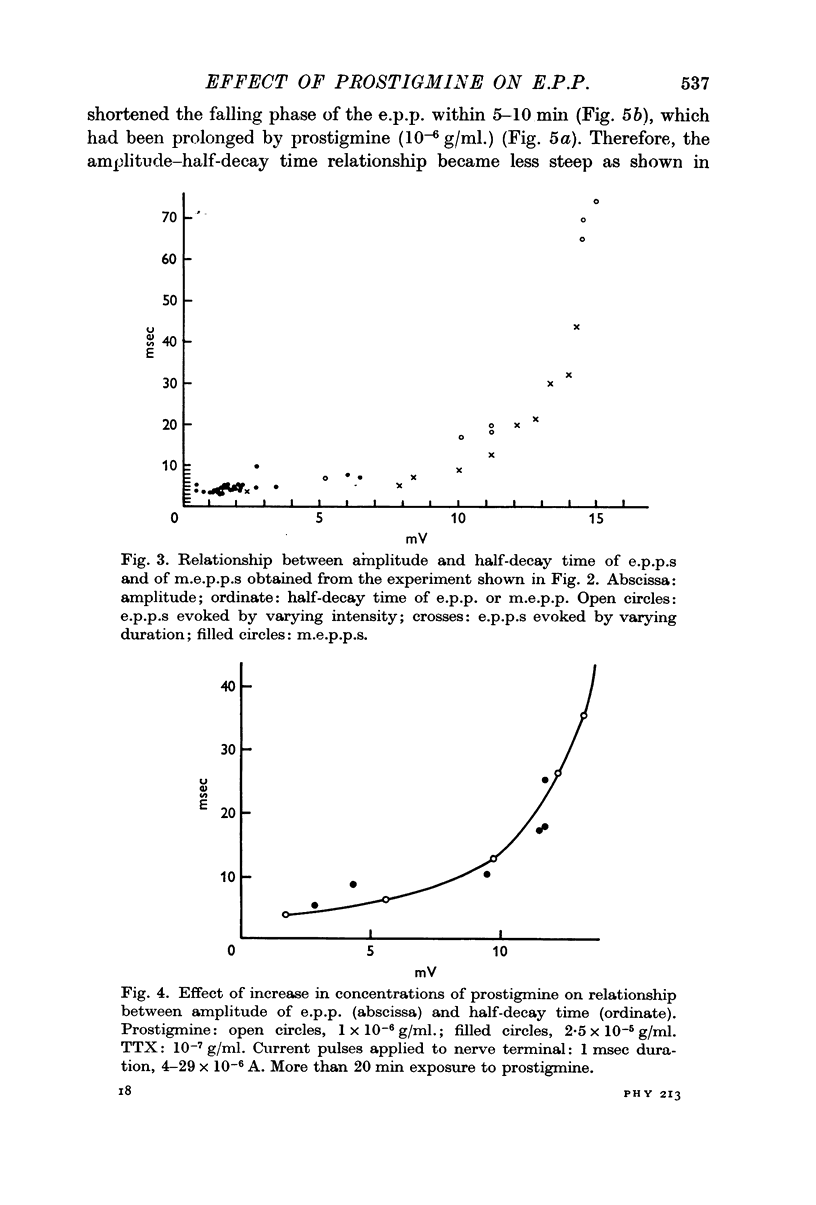
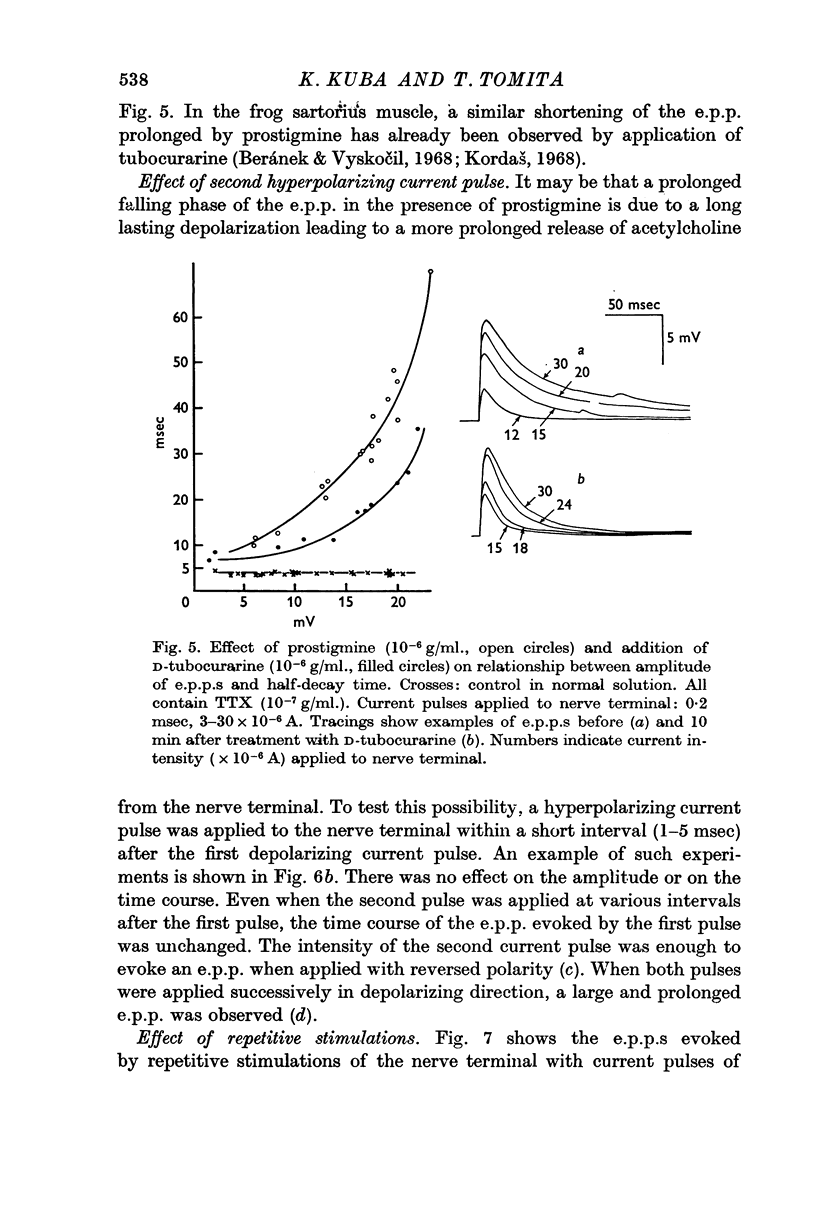
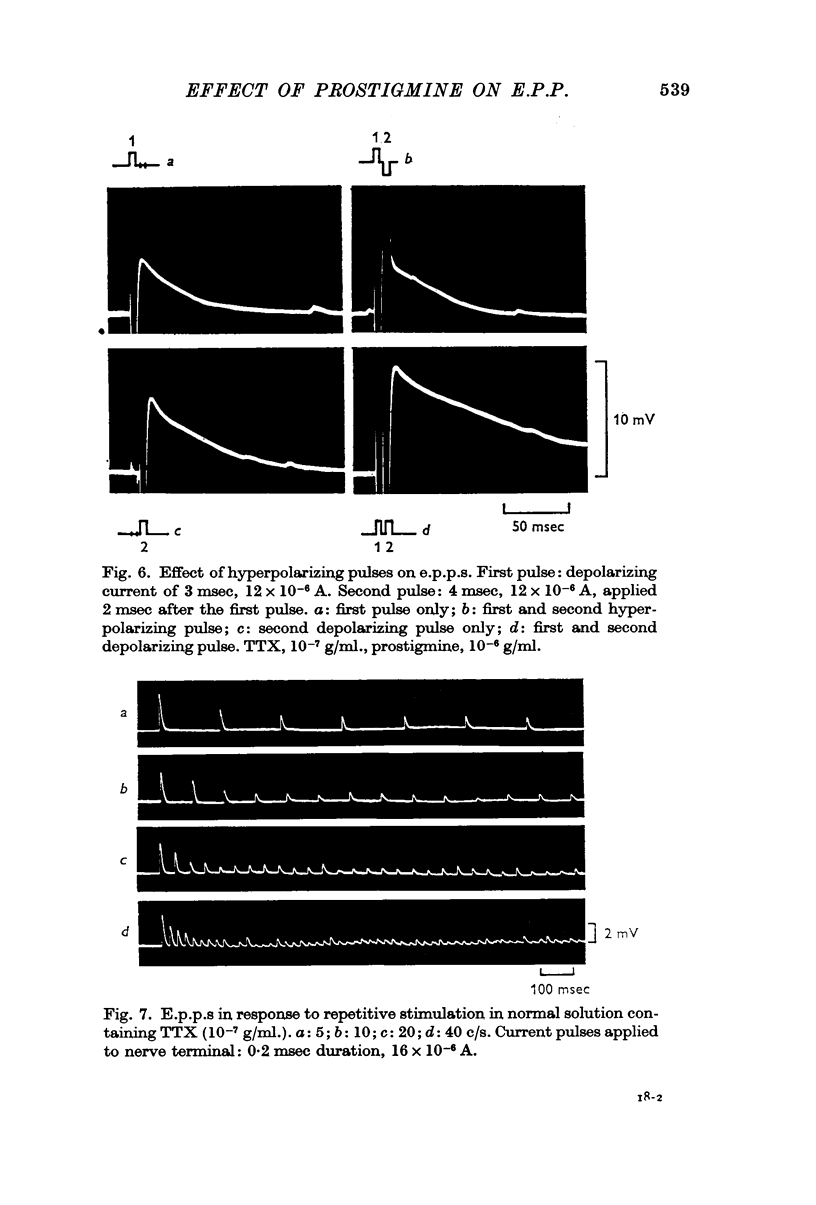
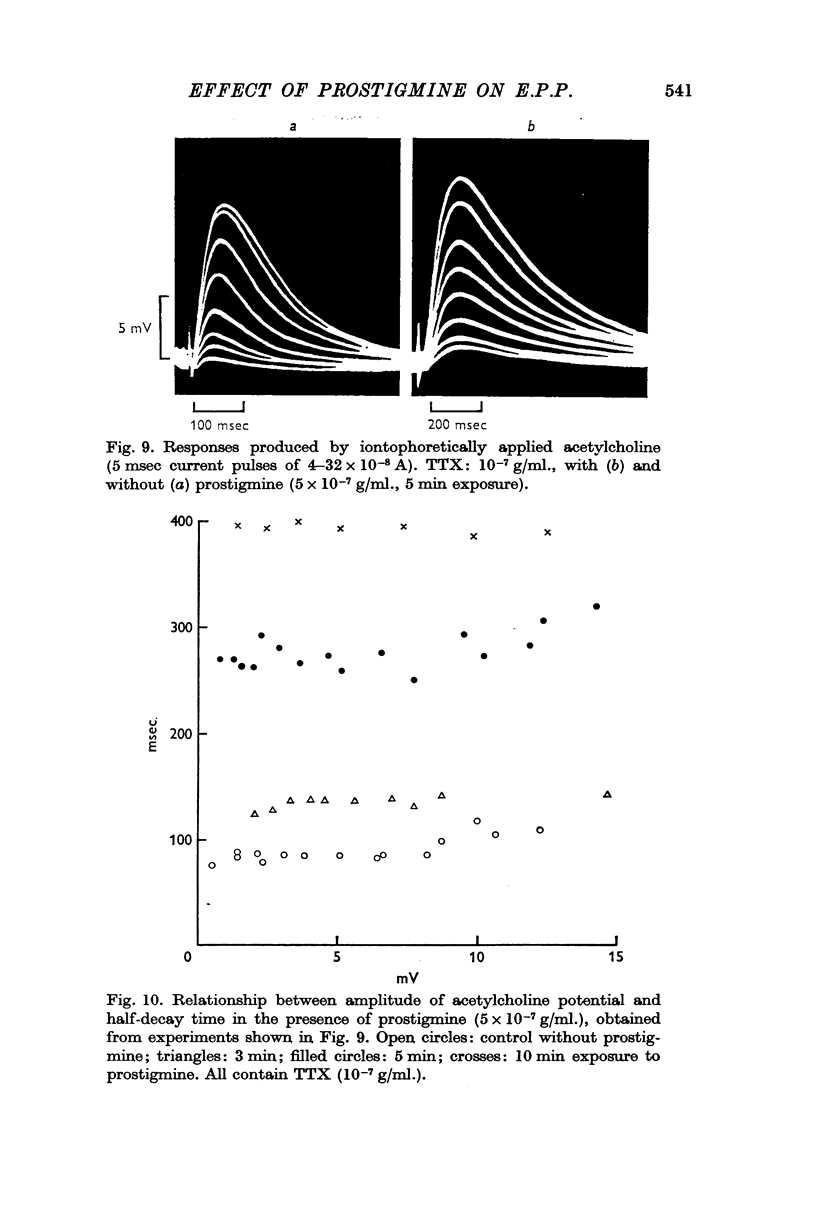
2. In the presence of prostigmine (5 × 10-7-2 × 10-5 g/ml.), the falling phase of the e.p.p. was different in time course depending on the amplitude. The half-decay time had, very roughly, an exponential dependence on amplitude. The relationship was not affected by increasing the TTX or the prostigmine concentration, but D-tubocurarine (10-6 g/ml.) made the relationship less steep.
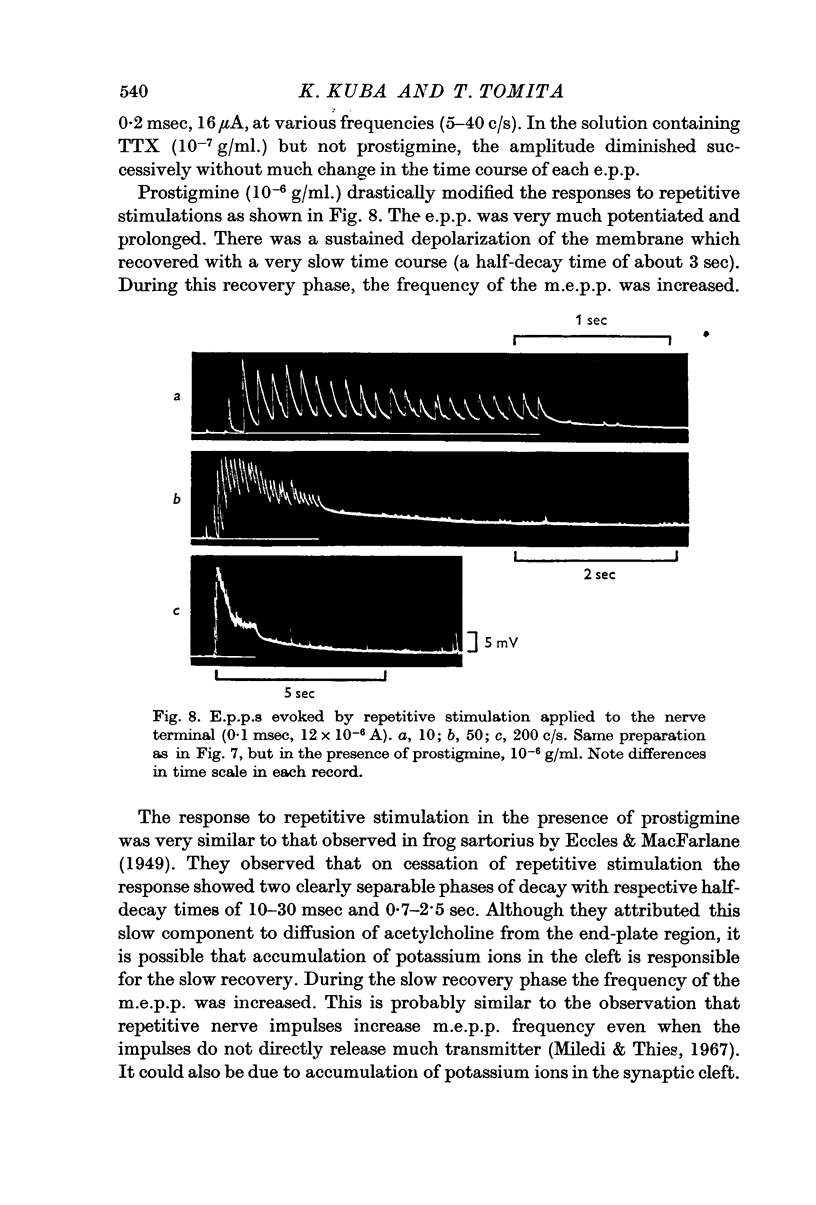
3. Hyperpolarizing current pulses, applied after the depolarizing current pulse which produced the e.p.p., had no effect on the time course of the e.p.p. No facilitating effect of repetitive stimulation was observed without prostigmine up to a frequency of 40 c/s, but there was a strong effect of repetitive stimulation in increasing the amplitude and duration of the e.p.p. in the presence of prostigmine. During stimulation, the endplate was continuously depolarized by 10-20 mV, and its recovery was very slow, the half-decay time being about 3 sec.
4. The half-decay time of the acetylcholine potentials produced by iontophoretically applied acetylcholine was almost independent of the amplitude, with or without prostigmine, although it increased the amplitude of the potential and prolonged the falling phase.
5. Possible mechanisms for the alteration of the falling phase of the e.p.p. were discussed. It is speculated that, in the presence of prostigmine, a process which is involved in a conductance increase of the post-synaptic membrane, after acetylcholine has combined with the receptor molecules, is the main factor determining the falling phase of the e.p.p.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beránek R., Vyskocil F. The effect of atropine on the frog sartorius neuromuscular junction. J Physiol. 1968 Mar;195(2):493–503. doi: 10.1113/jphysiol.1968.sp008470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., JAEGER J. C. The relationship between the mode of operation and the dimensions of the junctional regions at synapses and motor end-organs. Proc R Soc Lond B Biol Sci. 1958 Jan 1;148(930):38–56. doi: 10.1098/rspb.1958.0003. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., MacFARLANE W. V. Actions of anti-cholinesterases on endplate potential of frog muscle. J Neurophysiol. 1949 Jan;12(1):59–80. doi: 10.1152/jn.1949.12.1.59. [DOI] [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951 Nov 28;115(3):320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRNJEVIC K., MILEDI R. Acetylcholine in mammalian neuromuscular transmission. Nature. 1958 Sep 20;182(4638):805–806. doi: 10.1038/182805b0. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. Release of acetylcholine from a nerve terminal by electric pulses of variable strength and duration. Nature. 1965 Sep 4;207(5001):1097–1098. doi: 10.1038/2071097a0. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. Tetrodotoxin and neuromuscular transmission. Proc R Soc Lond B Biol Sci. 1967 Jan 31;167(1006):8–22. doi: 10.1098/rspb.1967.0010. [DOI] [PubMed] [Google Scholar]
- Kordas M. The effect of atropine and curarine on the time course of the end-palate potential in frog sartorius muscle. Int J Neuropharmacol. 1968 Nov;7(6):523–530. doi: 10.1016/0028-3908(68)90064-6. [DOI] [PubMed] [Google Scholar]
- Kuba K. Effects of catecholamines on the neuromuscular junction in the rat diaphragm. J Physiol. 1970 Dec;211(3):551–570. doi: 10.1113/jphysiol.1970.sp009293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILEDI R. Junctional and extra-junctional acetylcholine receptors in skeletal muscle fibres. J Physiol. 1960 Apr;151:24–30. [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Thies R. E. Post-tetanic increase in frequency of miniature end-plate potentials in calcium-free solutions. J Physiol. 1967 Sep;192(2):54P–55P. [PubMed] [Google Scholar]
- Riker W. F., Jr Actions of acetylcholine on mammalian motor nerve terminal. J Pharmacol Exp Ther. 1966 Jun;152(3):397–416. [PubMed] [Google Scholar]
- Steinbach A. B. A kinetic model for the action of xylocaine on receptors for acetylcholine. J Gen Physiol. 1968 Jul;52(1):162–180. doi: 10.1085/jgp.52.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach A. B. Alteration by xylocaine (lidocaine) and its derivatives of the time course of the end plate potential. J Gen Physiol. 1968 Jul;52(1):144–161. doi: 10.1085/jgp.52.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]


