Abstract
1. Micropuncture and micro-analytical techniques were used to study some of the electrochemical aspects of fluid and electrolyte transport in single seminiferous tubules, the epididymis and vas deferens.
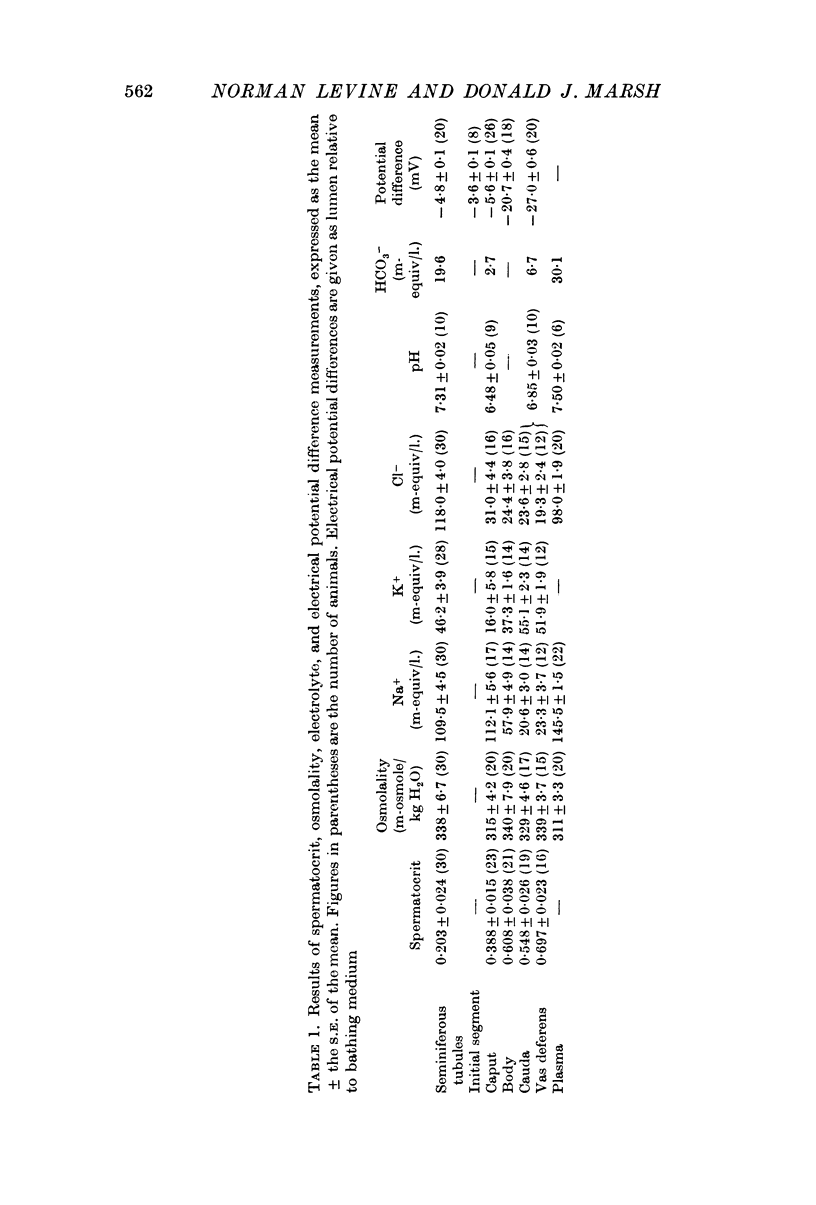
2. Seminiferous tubules contain a fluid that is slightly hypertonic to plasma, has a high potassium and chloride ion concentration, a lower sodium ion concentration and is slightly acidic relative to plasma.
3. The lumen of the seminiferous tubule is about 5 mV negative to a Ringers bathing solution.
4. Potassium and chloride ions enter the seminiferous tubule lumen against an electrochemical gradient, while the gradient for sodium ion favours its entry. This does not preclude possible active transport of sodium ion.
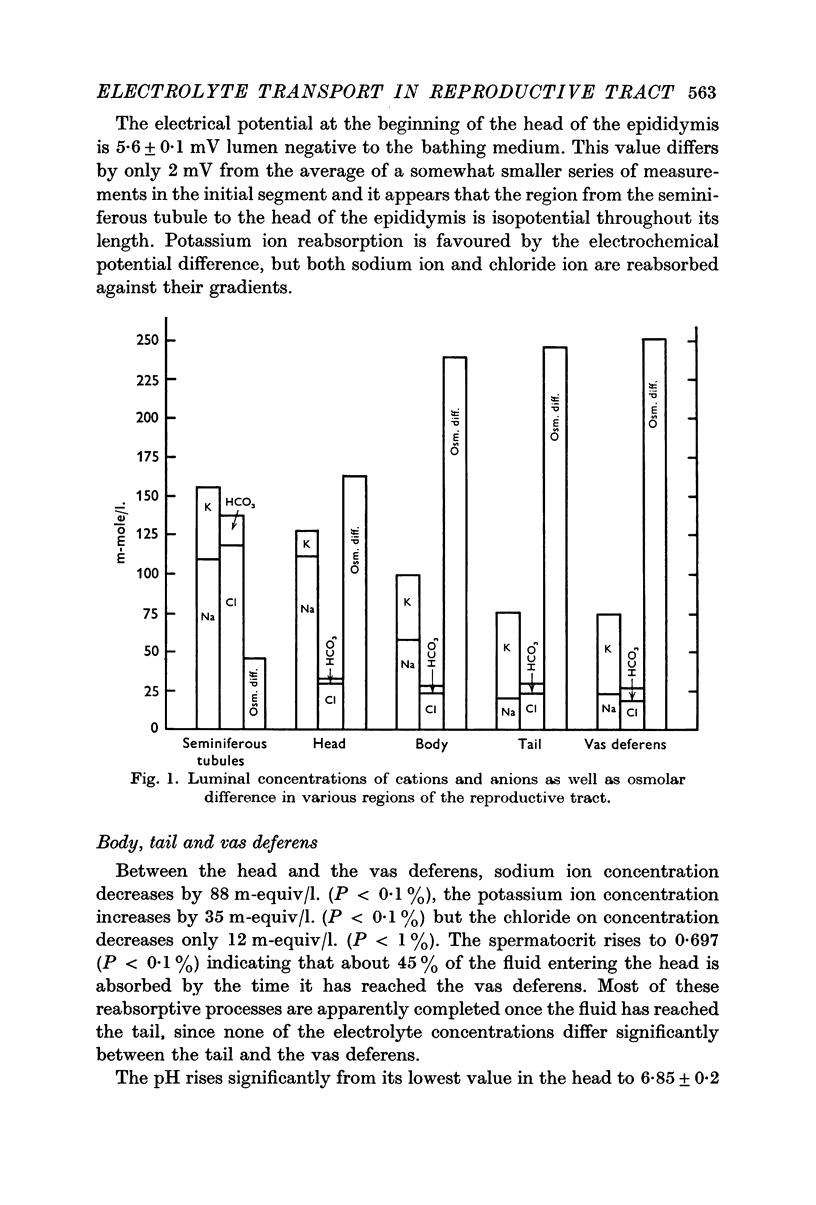
5. Between the seminiferous tubules and the beginning of the caput epididymis spermatocrit changes indicate that about 50% of the fluid leaving the testis is reabsorbed. Chloride ion and potassium ion are reabsorbed in concentrations greater than in lumen while sodium ion is reabsorbed in a concentration equal to that in the lumen. This region is also the site of intense hydrogen ion secretion.
6. The region between the seminiferous tubules and the caput is isopotential. Reabsorption of sodium and chloride ions are against electrochemical gradient. Potassium ion reabsorption is favoured by the electrochemical gradient.
7. Osmolar and electrical considerations indicate the probable secretion of organic acids between the seminiferous tubules and the caput epididymis.
8. Between the caput and the vas deferens 50% of the remaining fluid is reabsorbed. Sodium ion is reabsorbed in concentrations much greater than in lumen, potassium ion enters the lumen and the pH rises. Sodium reabsorption in this region is essentially independent of chloride reabsorption.
9. The corpus epididymis is 20 mV negative to a Ringers bathing medium while the beginning of the vas deferens is 27 mV negative. Reabsorption of sodium ion is against an electrochemical gradient as is potassium entry. Osmolality data and the concentration of sodium in the reabsorbate require further secretion of organic compounds in this region.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRICKER N. S., BIBER T., USSING H. H. Exposure of the isolated from skin to high potassium concentrations at the internal surface. I. Bioelectric phenomena and sodium transport. J Clin Invest. 1963 Jan;42:88–99. doi: 10.1172/JCI104699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRABO B., GUSTAFSSON B. DISTRIBUTION OF SODIUM AND POTASSIUM AND ITS RELATION TO SPERM CONCENTRATION IN THE EPIDIDYMAL PLASMA OF THE BULL. J Reprod Fertil. 1964 Jun;7:337–345. doi: 10.1530/jrf.0.0070337. [DOI] [PubMed] [Google Scholar]
- GERTZ K. H. [Transtubular sodium chloride transport and permeability for nonelectrolytes in the proximal and distal convolution of the rat kidney]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1963;276:336–356. [PubMed] [Google Scholar]
- Linzell J. L., Setchell B. P. Metabolism, sperm and fluid production of the isolated perfused testis of the sheep and goat. J Physiol. 1969 Mar;201(1):129–143. doi: 10.1113/jphysiol.1969.sp008747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PITTS R. F. RENAL PRODUCTION AND EXCRETION OF AMMONIA. Am J Med. 1964 May;36:720–742. doi: 10.1016/0002-9343(64)90182-2. [DOI] [PubMed] [Google Scholar]
- Rankin J., Meschia G., Makowski E. L., Battaglia F. C. Macroscopic distribution of blood flow in the sheep placenta. Am J Physiol. 1970 Jul;219(1):9–16. doi: 10.1152/ajplegacy.1970.219.1.9. [DOI] [PubMed] [Google Scholar]
- SCOTT T. W., WALES R. G., WALLACE J. C., WHITE I. G. COMPOSITION OF RAM EPIDIDYMAL AND TESTICULAR FLUID AND THE BIOSYNTHESIS OF GLYCERYLPHOSPHORYLCHOLINE BY THE RABBIT EPIDIDYMIS. J Reprod Fertil. 1963 Aug;6:49–59. doi: 10.1530/jrf.0.0060049. [DOI] [PubMed] [Google Scholar]
- Setchell B. P., Voglmayr J. K., Waites G. M. A blood-testis barrier restricting passage from blood into rete testis fluid but not into lymph. J Physiol. 1969 Jan;200(1):73–85. doi: 10.1113/jphysiol.1969.sp008682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon S., Alpert H. A method for determining titratable acidity in nanoliter samples of biological fluids. Anal Biochem. 1969 Nov;32(2):291–296. doi: 10.1016/0003-2697(69)90088-8. [DOI] [PubMed] [Google Scholar]
- Tuck R. R., Waites G. M., Young J. A., Setchell B. P. Compoition of fluid secreted by the seminiferous tubules of the rat collected by catheterization and micropuncture technics. J Reprod Fertil. 1970 Mar;21(2):367–368. doi: 10.1530/jrf.0.0210367. [DOI] [PubMed] [Google Scholar]
- Vieira F. L., Malnic G. Hydrogen ion secretion by rat renal cortical tubules as studied by an antimony microelectrode. Am J Physiol. 1968 Apr;214(4):710–718. doi: 10.1152/ajplegacy.1968.214.4.710. [DOI] [PubMed] [Google Scholar]
- Voglmayr J. K., Waites G. M., Setchell B. P. Studies on spermatozoa and fluid collected directly from the testis of the conscious ram. Nature. 1966 May 21;210(5038):861–863. doi: 10.1038/210861b0. [DOI] [PubMed] [Google Scholar]
- Wales R. G., Wallace J. C., White I. G. Composition of bull epididymal and testicular fluid. J Reprod Fertil. 1966 Aug;12(1):139–144. doi: 10.1530/jrf.0.0120139. [DOI] [PubMed] [Google Scholar]


