Abstract
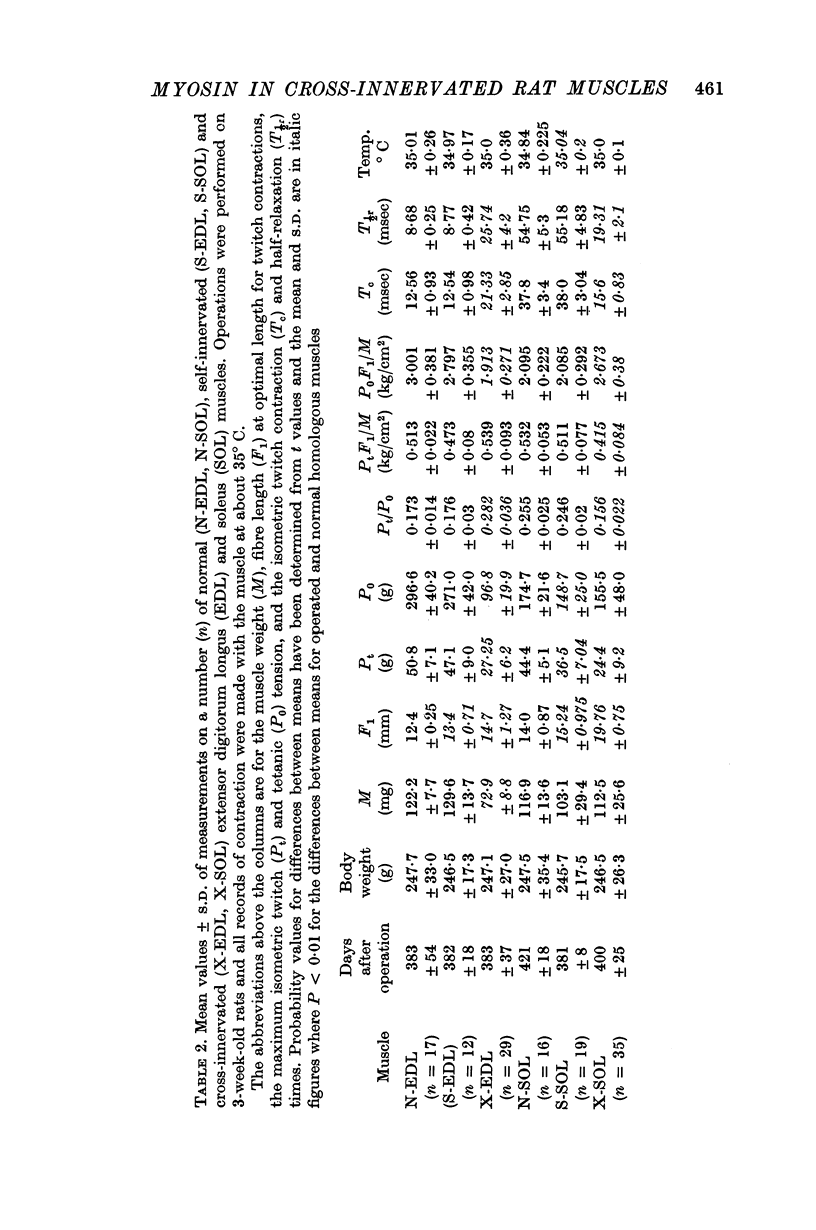
1. The characteristics of isometric twitch and tetanic contractions have been determined for normal (N-EDL, N-SOL), self-innervated (S-EDL, S-SOL) and cross-innervated (X-EDL, X-SOL) extensor digitorum longus (EDL) and soleus (SOL) muscles of the rat at 35° C. The muscles were then used for biochemical analyses of properties of myosin and actomyosin.
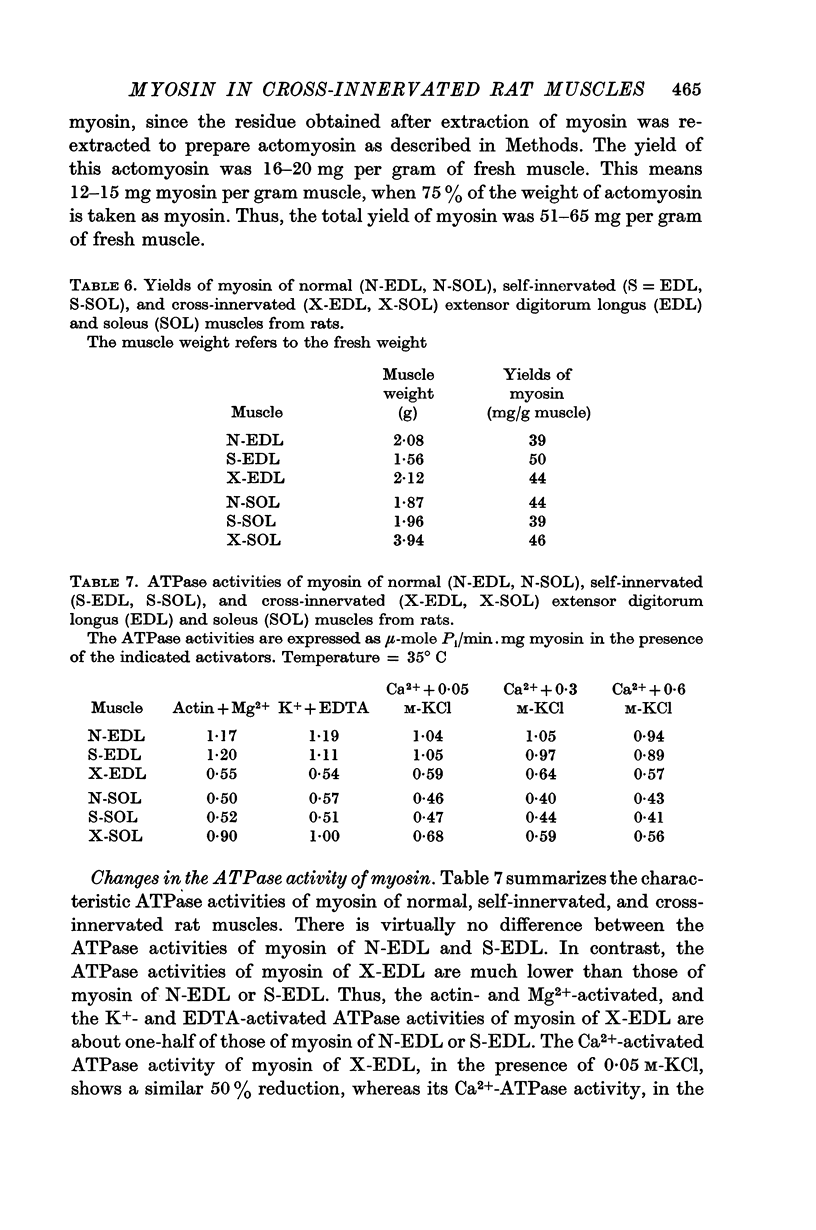
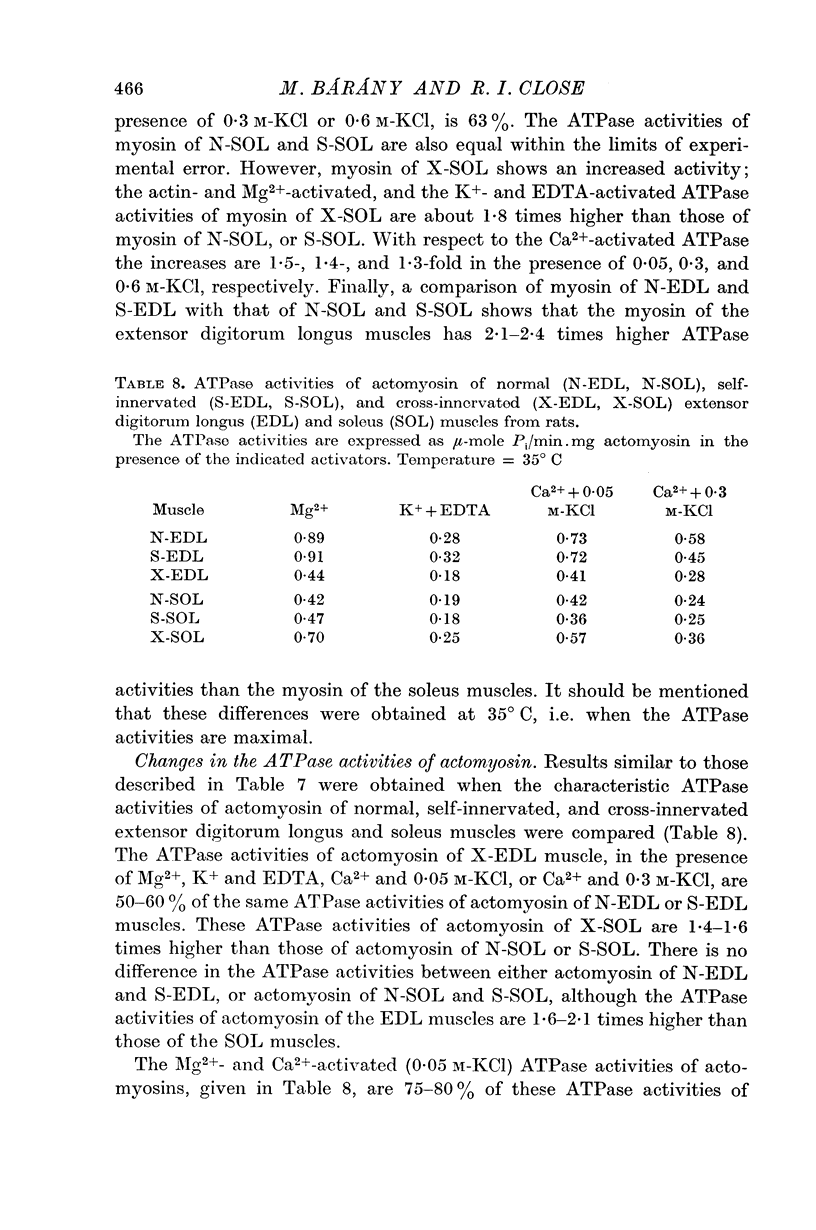
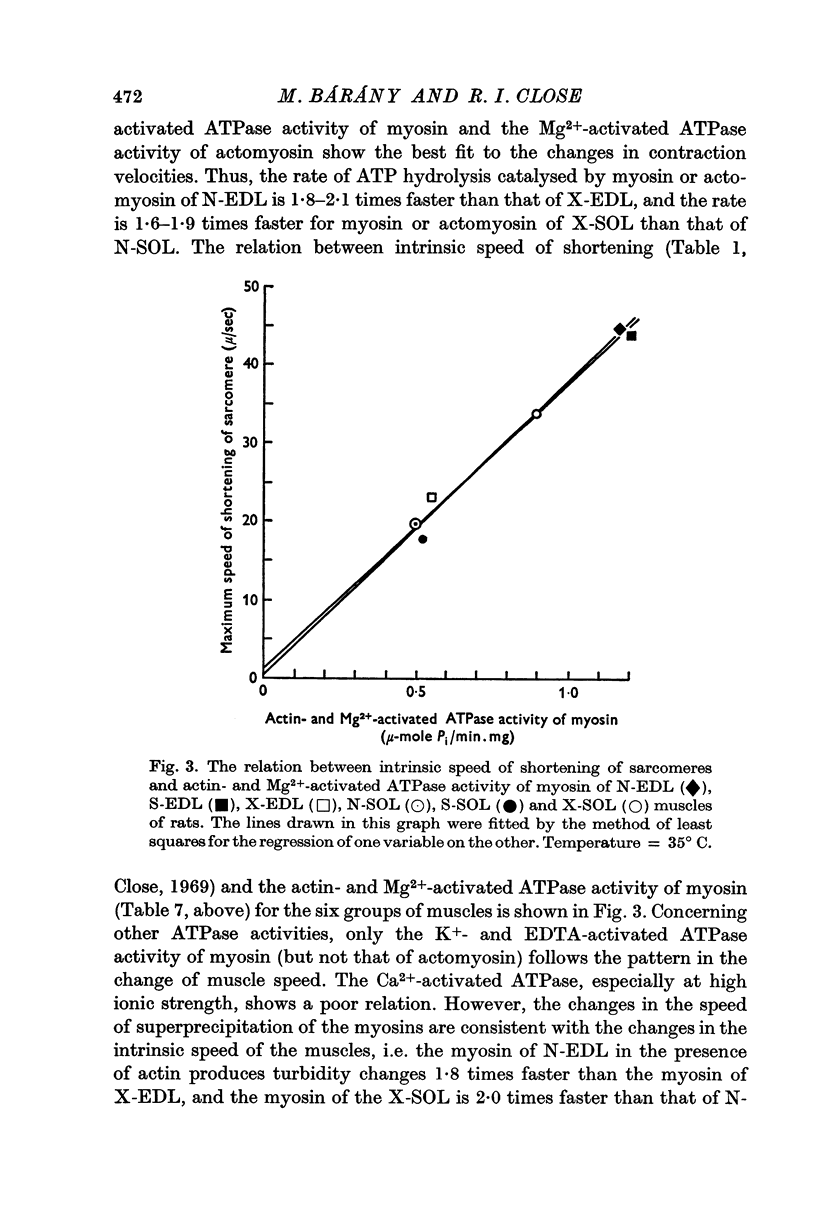
2. The ATPase activities of myosin and actomyosin of X-EDL decreased to the level of those of N-SOL or S-SOL, and the ATPase activities of X-SOL approached those of N-EDL or S-EDL. Of the various ATPase activities, the actin- and Mg2+-activated ATPase activity of myosin and the Mg2+-activated ATPase activity of actomyosin showed the highest degree of correlation with the intrinsic speed of shortening of the muscles.
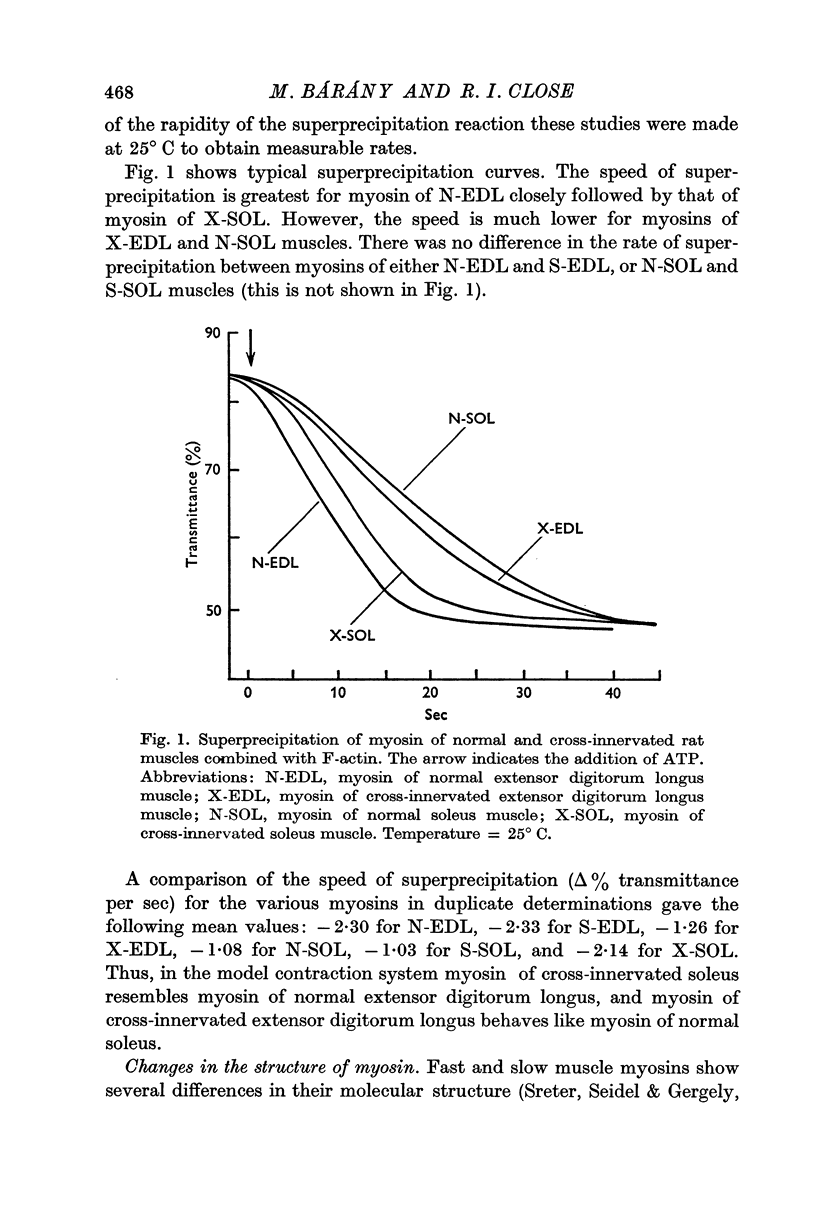
3. Myosin of normal, self-innervated, and cross-innervated muscles combined with F-actin superprecipitated at rates which were proportional to the speed of muscle contraction.
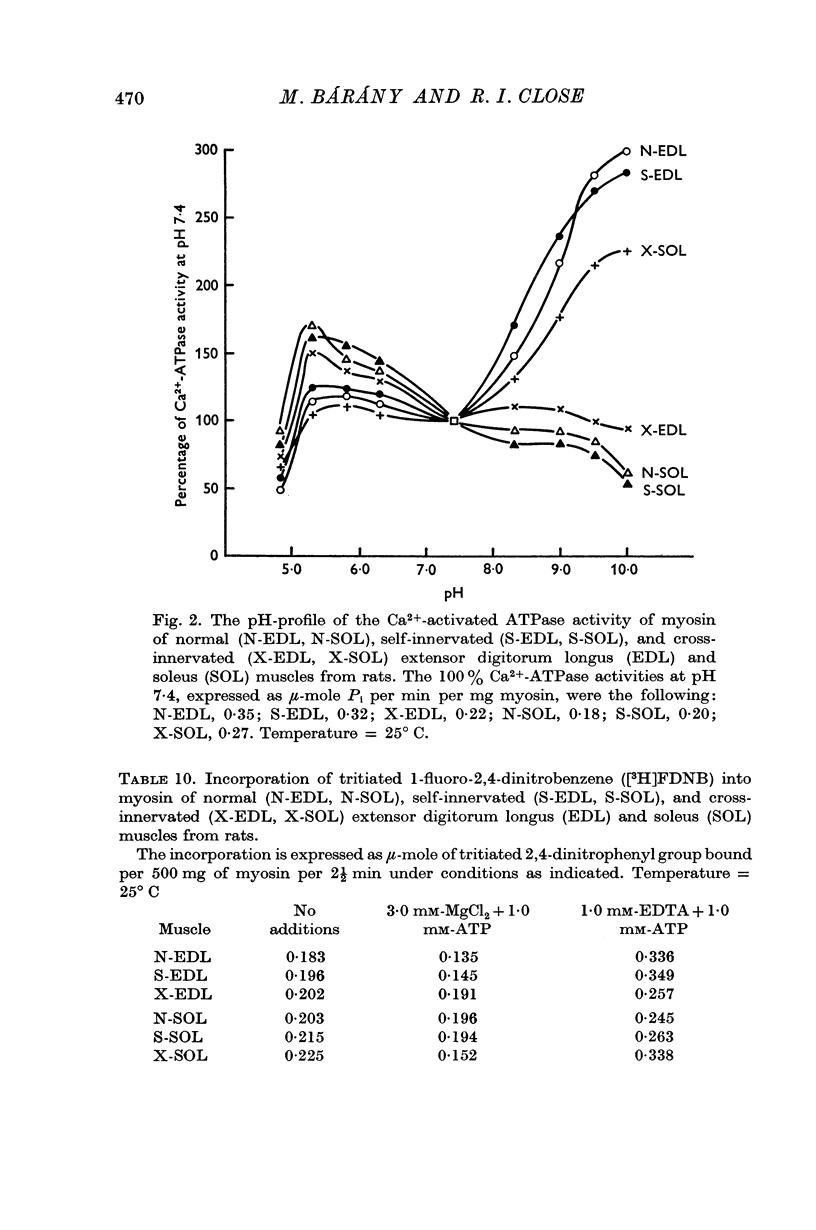
4. The pH profile curve and the ATP-induced dinitrophenylation reaction revealed that the structure of myosin of X-EDL was altered to that of N-SOL or S-SOL, and the structure of myosin of X-SOL was modified to that of N-EDL or S-EDL.
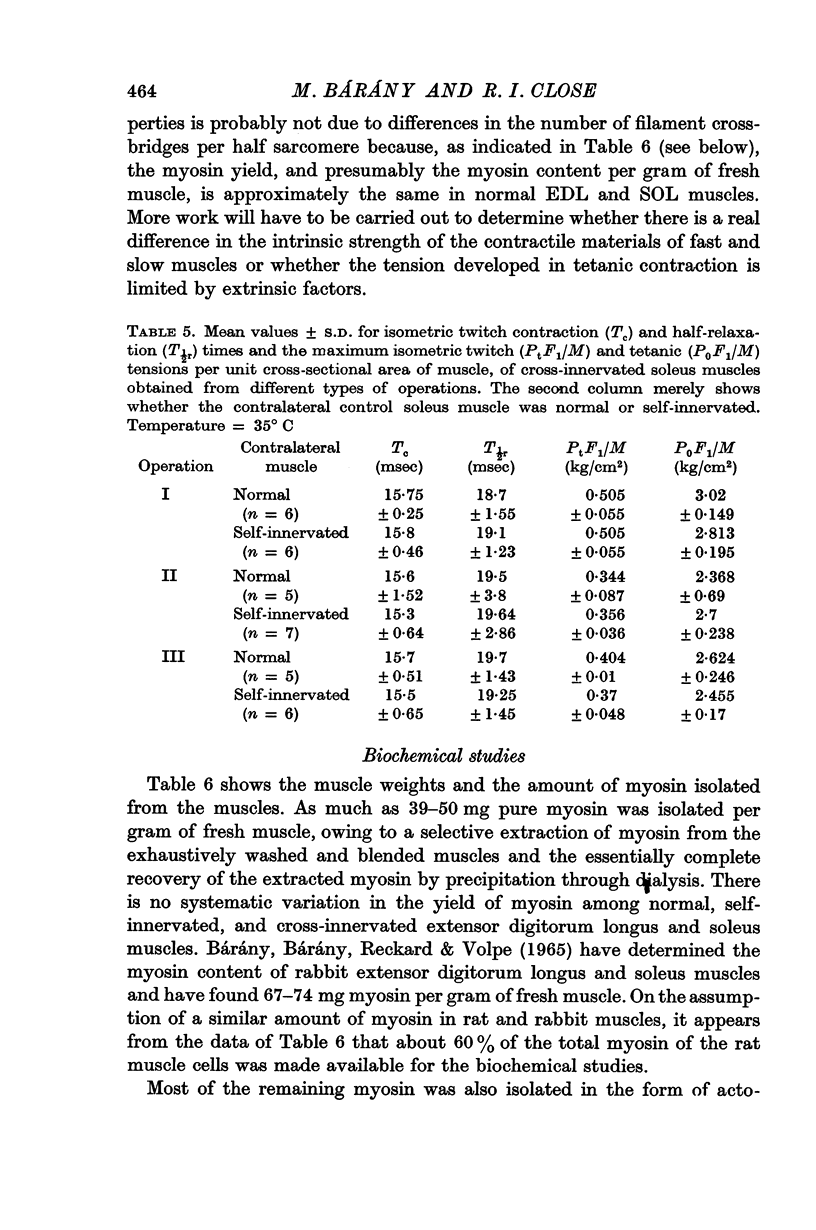
5. No differences were found in the yield of myosin of normal, self-innervated, and cross-innervated extensor digitorum longus and soleus muscles.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARANY M., BARANY K., RECKARD T., VOLPE A. MYOSIN OF FAST AND SLOW MUSCLES OF THE RABBIT. Arch Biochem Biophys. 1965 Jan;109:185–191. doi: 10.1016/0003-9861(65)90304-8. [DOI] [PubMed] [Google Scholar]
- BARANY M., BARANY K. Studies on "active centers" of L-myosin. Biochim Biophys Acta. 1959 Oct;35:293–309. doi: 10.1016/0006-3002(59)90378-6. [DOI] [PubMed] [Google Scholar]
- Buller A. J., Mommaerts W. F., Seraydarian K. Enzymic properties of myosin in fast and slow twitch muscles of the cat following cross-innervation. J Physiol. 1969 Dec;205(3):581–597. doi: 10.1113/jphysiol.1969.sp008984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bárány M. ATPase activity of myosin correlated with speed of muscle shortening. J Gen Physiol. 1967 Jul;50(6 Suppl):197–218. doi: 10.1085/jgp.50.6.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bárány M., Bailin G., Bárány K. Reaction of myosin with 1-fluoro-2, 4-dinitrobenzene at low ionic strength. J Biol Chem. 1969 Feb 25;244(4):648–657. [PubMed] [Google Scholar]
- Bárány M., Bárány K. Adenosine triphosphate-dependent reaction of 1-fluoro-2,4-dinitrobenzene with various myosins. J Biol Chem. 1969 Oct 10;244(19):5206–5212. [PubMed] [Google Scholar]
- Bárány M., Conover T. E., Schliselfeld L. H., Gaetjens E., Goffart M. Relation of properties of isolated myosin to those of intact muscles of the cat and sloth. Eur J Biochem. 1967 Sep;2(2):156–164. doi: 10.1111/j.1432-1033.1967.tb00120.x. [DOI] [PubMed] [Google Scholar]
- Bárány M., Tucci A. F., Conover T. E. The removal of the bound ADP of F-actin. J Mol Biol. 1966 Aug;19(2):483–502. doi: 10.1016/s0022-2836(66)80018-9. [DOI] [PubMed] [Google Scholar]
- CLOSE R. DYNAMIC PROPERTIES OF FAST AND SLOW SKELETAL MUSCLES OF THE RAT DURING DEVELOPMENT. J Physiol. 1964 Sep;173:74–95. doi: 10.1113/jphysiol.1964.sp007444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close R. Dynamic properties of fast and slow skeletal muscles of the rat after nerve cross-union. J Physiol. 1969 Oct;204(2):331–346. doi: 10.1113/jphysiol.1969.sp008916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close R. Effects of cross-union of motor nerves to fast and slow skeletal muscles. Nature. 1965 May 22;206(4986):831–832. doi: 10.1038/206831a0. [DOI] [PubMed] [Google Scholar]
- Close R. The relation between intrinsic speed of shortening and duration of the active state of muscle. J Physiol. 1965 Oct;180(3):542–559. doi: 10.1113/jphysiol.1965.sp007716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORNALL A. G., BARDAWILL C. J., DAVID M. M. Determination of serum proteins by means of the biuret reaction. J Biol Chem. 1949 Feb;177(2):751–766. [PubMed] [Google Scholar]
- Samaha F. J., Guth L., Albers R. W. Differences between slow and fast muscle myosin. Adenosine triphosphatase activity and release of associated proteins by p-chloromercuriphenylsulfonate. J Biol Chem. 1970 Jan 25;245(2):219–224. [PubMed] [Google Scholar]
- Samaha F. J., Guth L., Albers R. W. The neural regulation of gene expression in the muscle cell. Exp Neurol. 1970 May;27(2):276–282. doi: 10.1016/0014-4886(70)90220-7. [DOI] [PubMed] [Google Scholar]
- Sreter F. A., Seidel J. C., Gergely J. Studies on myosin from red and white skeletal muscles of the rabbit. I. Adenosine triphosphatase activity. J Biol Chem. 1966 Dec 25;241(24):5772–5776. [PubMed] [Google Scholar]
- WEBER H. H., PORTZEHL H. Muscle contraction and fibrous muscle proteins. Adv Protein Chem. 1952;7:161–252. doi: 10.1016/s0065-3233(08)60019-4. [DOI] [PubMed] [Google Scholar]


