Abstract
1. Colour sensitivity and spatial organization were determined for the dominant-eye receptive fields of thirty-eight simple or complex cells in cat primary visual vortex. Receptive fields were all from the cortical area associated with central vision. Each cell was investigated with threshold or suprathreshold monochromatic stimuli, under scotopic, low and high mesopic adaptation.
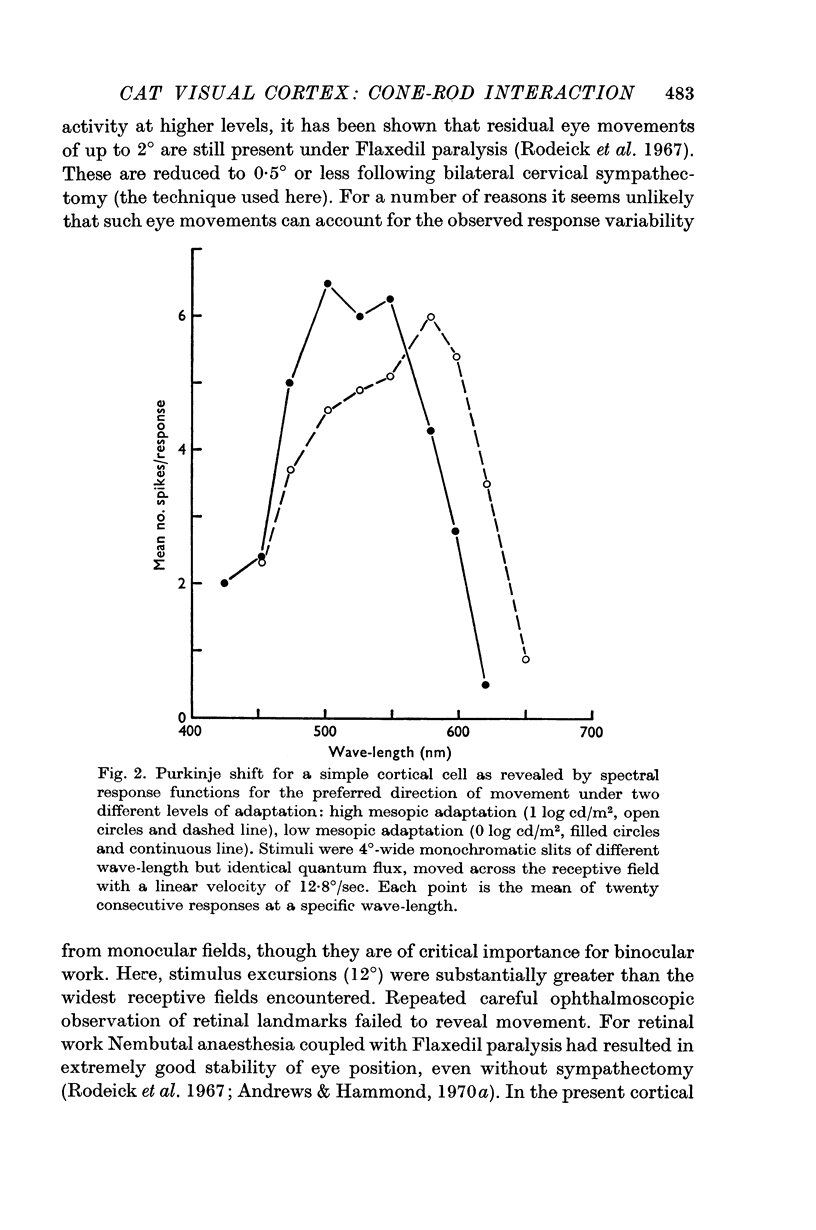
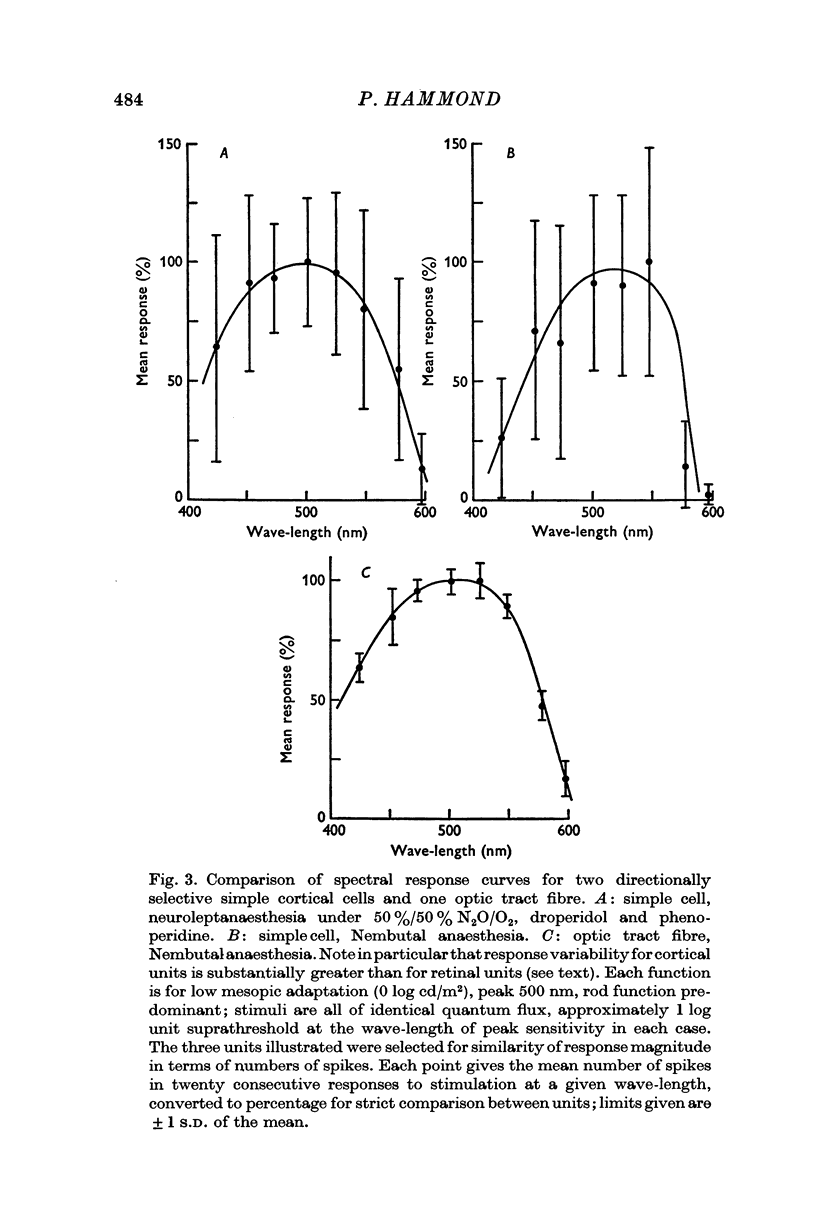
2. The Purkinje shift, well defined for all units, was consistent with dual input from each of only two receptor mechanisms, viz. 556 nm cones and 500 nm rods. With change of adaptation level there was a systematic change in the peak sensitivity of spectral response curves to suprathreshold monochromatic stimuli, equated for quantum flux but of different wave-length. Equally with change of adaptation, the relative shift in threshold between wave-lengths selective for cone or rod activation was in close agreement with the change predicted from the Dartnall nomogram curves for visual pigments 556 and 507 respectively.
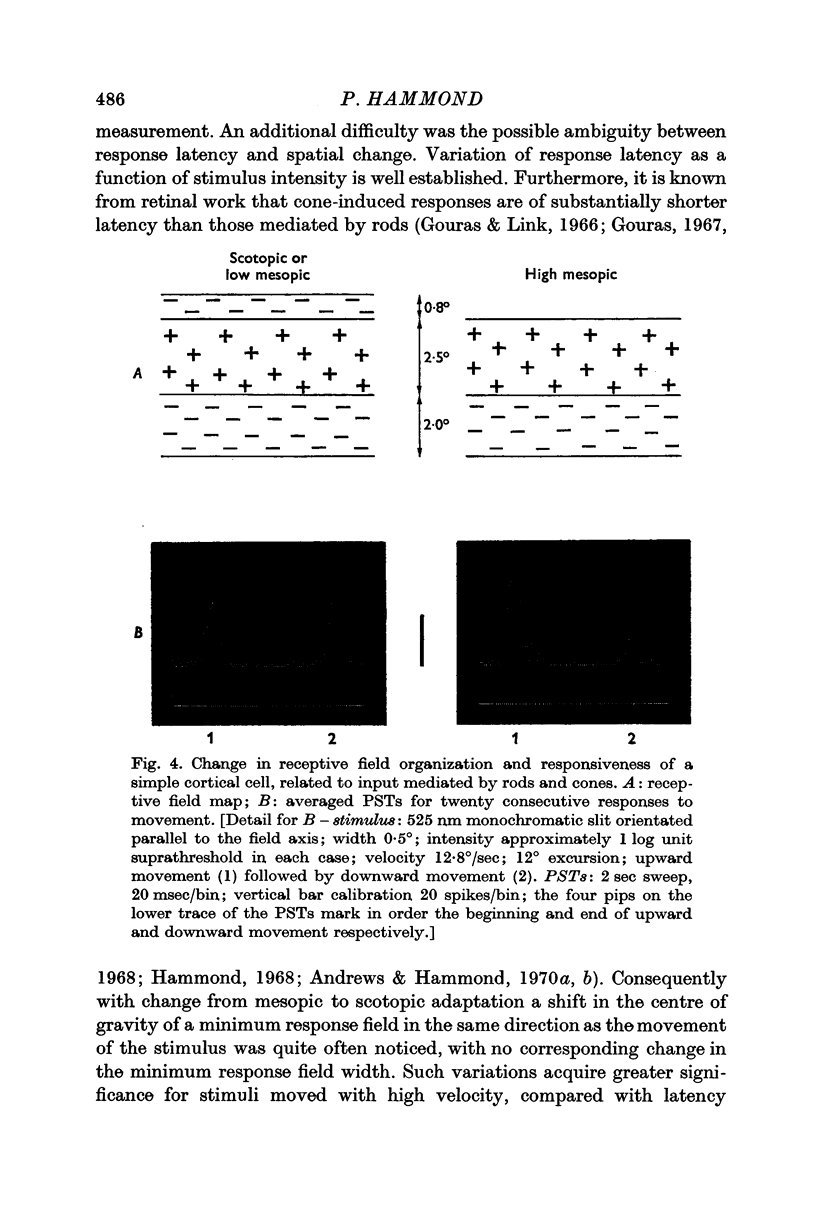
3. For ganglion cells with concentric fields rod input derives from a spatially larger area than cone input. Rod field centre and rod field surround are substantially larger than the corresponding centre and surround for cones (Andrews & Hammond, 1970b). For cortical cells a conclusive comparable change could only be demonstrated for one simple unit. Its receptive field consisted of a horizontal excitatory stripe with asymmetric inhibitory flanks. When light-adapted the weaker, upper flank was functionally undetectable, indicative of purely rod input to this sideband, and the preference for upward movement was enhanced.
4. No difference in receptive field configuration, or in spatial extent of input mediated by cones or by rods, was detected for any other unit. The discrepancy between retinal and cortical findings is discussed. It is inferred that cortical fields are compounded essentially by convergent input from geniculate cell field-centres.
Full text
PDF





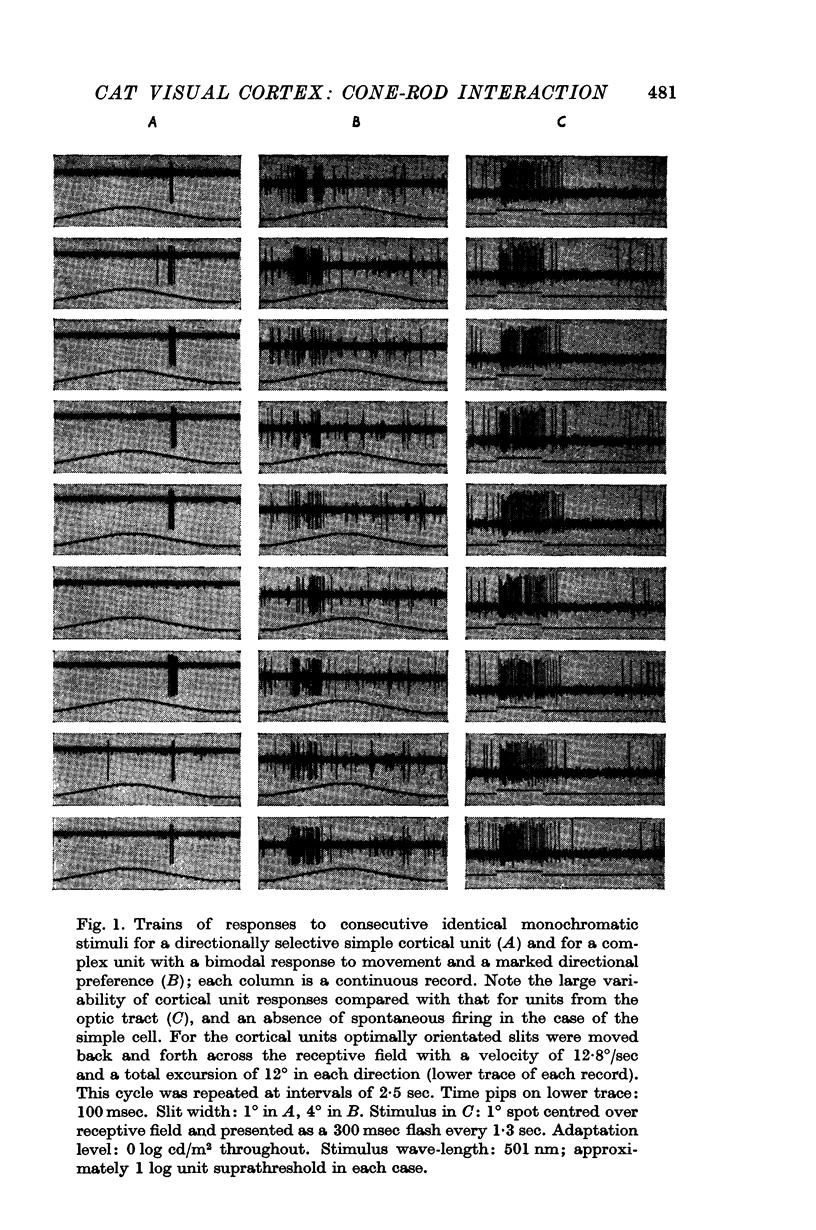





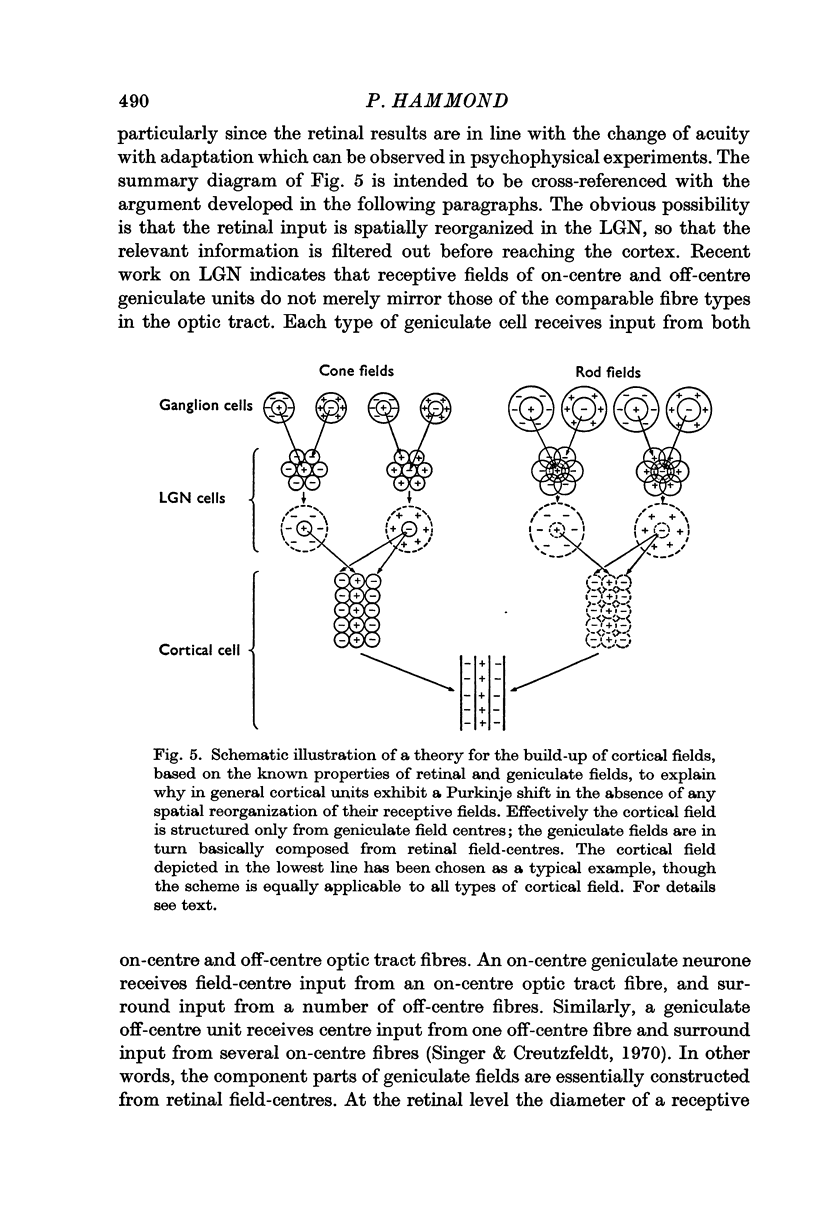




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews D. P., Hammond P. Mesopic increment threshold spectral sensitivity of single optic tract fibres in the cat: cone-rod interaction. J Physiol. 1970 Jul;209(1):65–81. doi: 10.1113/jphysiol.1970.sp009156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews D. P., Hammond P. Suprathreshold spectral properties of single optic tract fibres in cat, under mesopic adaptation: cone-rod interaction. J Physiol. 1970 Jul;209(1):83–103. doi: 10.1113/jphysiol.1970.sp009157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW H. B., FITZHUGH R., KUFFLER S. W. Change of organization in the receptive fields of the cat's retina during dark adaptation. J Physiol. 1957 Aug 6;137(3):338–354. doi: 10.1113/jphysiol.1957.sp005817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW H. B., FITZHUGH R., KUFFLER S. W. Dark adaptation, absolute threshold and Purkinje shift in single units of the cat's retina. J Physiol. 1957 Aug 6;137(3):327–337. doi: 10.1113/jphysiol.1957.sp005816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Blakemore C., Pettigrew J. D. The neural mechanism of binocular depth discrimination. J Physiol. 1967 Nov;193(2):327–342. doi: 10.1113/jphysiol.1967.sp008360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C. The representation of three-dimensional visual space in the cat's striate cortex. J Physiol. 1970 Jul;209(1):155–178. doi: 10.1113/jphysiol.1970.sp009160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell F. W., Cleland B. G., Cooper G. F., Enroth-Cugell C. The angular selectivity of visual cortical cells to moving gratings. J Physiol. 1968 Sep;198(1):237–250. doi: 10.1113/jphysiol.1968.sp008604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DARTNALL H. J. A. The interpretation of spectral sensitivity curves. Br Med Bull. 1953;9(1):24–30. doi: 10.1093/oxfordjournals.bmb.a074302. [DOI] [PubMed] [Google Scholar]
- DE VALOIS R. L., SMITH C. J., KITAI S. T., KAROLY A. J. Response of single cells in monkey lateral geniculate nucleus to monochromatic light. Science. 1958 Jan 31;127(3292):238–239. doi: 10.1126/science.127.3292.238. [DOI] [PubMed] [Google Scholar]
- Daw N. W., Pearlman A. L. Cat colour vision: one cone process or several? J Physiol. 1969 May;201(3):745–764. doi: 10.1113/jphysiol.1969.sp008785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Valois R. L., Abramov I., Jacobs G. H. Analysis of response patterns of LGN cells. J Opt Soc Am. 1966 Jul;56(7):966–977. doi: 10.1364/josa.56.000966. [DOI] [PubMed] [Google Scholar]
- De Valois R. L., Abramov I., Mead W. R. Single cell analysis of wavelength discrimination at the lateral geniculate nucleus in the macaque. J Neurophysiol. 1967 May;30(3):415–433. doi: 10.1152/jn.1967.30.3.415. [DOI] [PubMed] [Google Scholar]
- De Valois R. L. Analysis and coding of color vision in the primate visual system. Cold Spring Harb Symp Quant Biol. 1965;30:567–579. doi: 10.1101/sqb.1965.030.01.055. [DOI] [PubMed] [Google Scholar]
- GUNTER R. The spectral sensitivity of dark-adapted cats. J Physiol. 1952 Nov;118(3):395–404. doi: 10.1113/jphysiol.1952.sp004803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUNTER R. The spectral sensitivity of light-adapted cats. J Physiol. 1954 Feb 26;123(2):409–415. doi: 10.1113/jphysiol.1954.sp005061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouras P. Identification of cone mechanisms in monkey ganglion cells. J Physiol. 1968 Dec;199(3):533–547. doi: 10.1113/jphysiol.1968.sp008667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouras P., Link K. Rod and cone interaction in dark-adapted monkey ganglion cells. J Physiol. 1966 May;184(2):499–510. doi: 10.1113/jphysiol.1966.sp007928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouras P. The effects of light-adaptation on rod and cone receptive field organization of monkey ganglion cells. J Physiol. 1967 Oct;192(3):747–760. doi: 10.1113/jphysiol.1967.sp008328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Integrative action in the cat's lateral geniculate body. J Physiol. 1961 Feb;155:385–398. doi: 10.1113/jphysiol.1961.sp006635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields of optic nerve fibres in the spider monkey. J Physiol. 1960 Dec;154:572–580. doi: 10.1113/jphysiol.1960.sp006596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond P. Spectral properties of dark-adapted retinal ganglion cells in the plaice (Pleuronectes platessa, L.). J Physiol. 1968 Apr;195(3):535–556. doi: 10.1113/jphysiol.1968.sp008473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Receptive fields and functional architecture of monkey striate cortex. J Physiol. 1968 Mar;195(1):215–243. doi: 10.1113/jphysiol.1968.sp008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUFFLER S. W. Discharge patterns and functional organization of mammalian retina. J Neurophysiol. 1953 Jan;16(1):37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- MELLO N. K., PETERSON N. J. BEHAVIORAL EVIDENCE FOR COLOR DISCRIMINATION IN CAT. J Neurophysiol. 1964 May;27:323–333. doi: 10.1152/jn.1964.27.3.323. [DOI] [PubMed] [Google Scholar]
- Narth C. S. An inexpensive field effect transistor preamplifier, for use with entracellular micro-electrodes. J Physiol. 1969 Feb;200(2):102P–103P. [PubMed] [Google Scholar]
- Nikara T., Bishop P. O., Pettigrew J. D. Analysis of retinal correspondence by studying receptive fields of binocular single units in cat striate cortex. Exp Brain Res. 1968;6(4):353–372. doi: 10.1007/BF00233184. [DOI] [PubMed] [Google Scholar]
- Pearlman A. L., Daw N. W. Opponent color cells in the cat lateral geniculate nucleus. Science. 1970 Jan 2;167(3914):84–86. doi: 10.1126/science.167.3914.84. [DOI] [PubMed] [Google Scholar]
- Pettigrew J. D., Nikara T., Bishop P. O. Binocular interaction on single units in cat striate cortex: simultaneous stimulation by single moving slit with receptive fields in correspondence. Exp Brain Res. 1968;6(4):391–410. doi: 10.1007/BF00233186. [DOI] [PubMed] [Google Scholar]
- Pettigrew J. D., Nikara T., Bishop P. O. Responses to moving slits by single units in cat striate cortex. Exp Brain Res. 1968;6(4):373–390. doi: 10.1007/BF00233185. [DOI] [PubMed] [Google Scholar]
- Rodieck R. W., Pettigrew J. D., Bishop P. O., Nikara T. Residual eye movements in receptive-field studies of paralyzed cats. Vision Res. 1967 Jan;7(1):107–110. doi: 10.1016/0042-6989(67)90031-4. [DOI] [PubMed] [Google Scholar]
- SECHZER J. A., BROWN J. L. COLOR DISCRIMINATION IN THE CAT. Science. 1964 Apr 24;144(3617):427–429. doi: 10.1126/science.144.3617.427. [DOI] [PubMed] [Google Scholar]
- Singer W., Creutzfeldt O. D. Reciprocal lateral inhibition of on- and off-center neurones in the lateral geniculate body of the cat. Exp Brain Res. 1970;10(3):311–330. doi: 10.1007/BF00235054. [DOI] [PubMed] [Google Scholar]
- WEALE R. A. Photochemical reactions in the living cat's retina. J Physiol. 1953 Nov 28;122(2):322–331. doi: 10.1113/jphysiol.1953.sp005002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel T. N., Hubel D. H. Spatial and chromatic interactions in the lateral geniculate body of the rhesus monkey. J Neurophysiol. 1966 Nov;29(6):1115–1156. doi: 10.1152/jn.1966.29.6.1115. [DOI] [PubMed] [Google Scholar]



