Abstract
1. The precentral and postcentral banks of the Rolandic fissure of the arm area of the baboon's cortex have been probed to their depths with extracellular micro-electrodes under nitrous oxide and oxygen anaesthesia, supplemented by minimal intravenous pentobarbitone or chloralose.
2. Afferent volleys were sent in from the deep (motor) radial nerve and the deep palmar (motor) branch of the ulnar nerve. Their entry into the central nervous system was timed at the dorsal root entry zone. The nerves were stimulated in continuity and the effects of stimuli below threshold for the motor axons were investigated.
3. Area 3a, in the depths of the postcentral bank, which is cytoarchitectonically transitional between areas 3 and 4, is the receiving area for afferent impulses from muscle.
4. Evoked potential waves and unitary discharges began 4 msec, and the majority of units discharged between 5 and 10 msec, after the afferent volley reached the dorsal root entry zone.
5. Similar responses were elicited by a brief pull (70 μ in 1 msec) or brief vibration (50 μ at 250-400 Hz) applied to the tendons of m. extensor digitorum communis.
6. No potential waves were evoked in area 4, even in the depths adjacent to area 3a, by muscle afferent volleys.
Full text
PDF












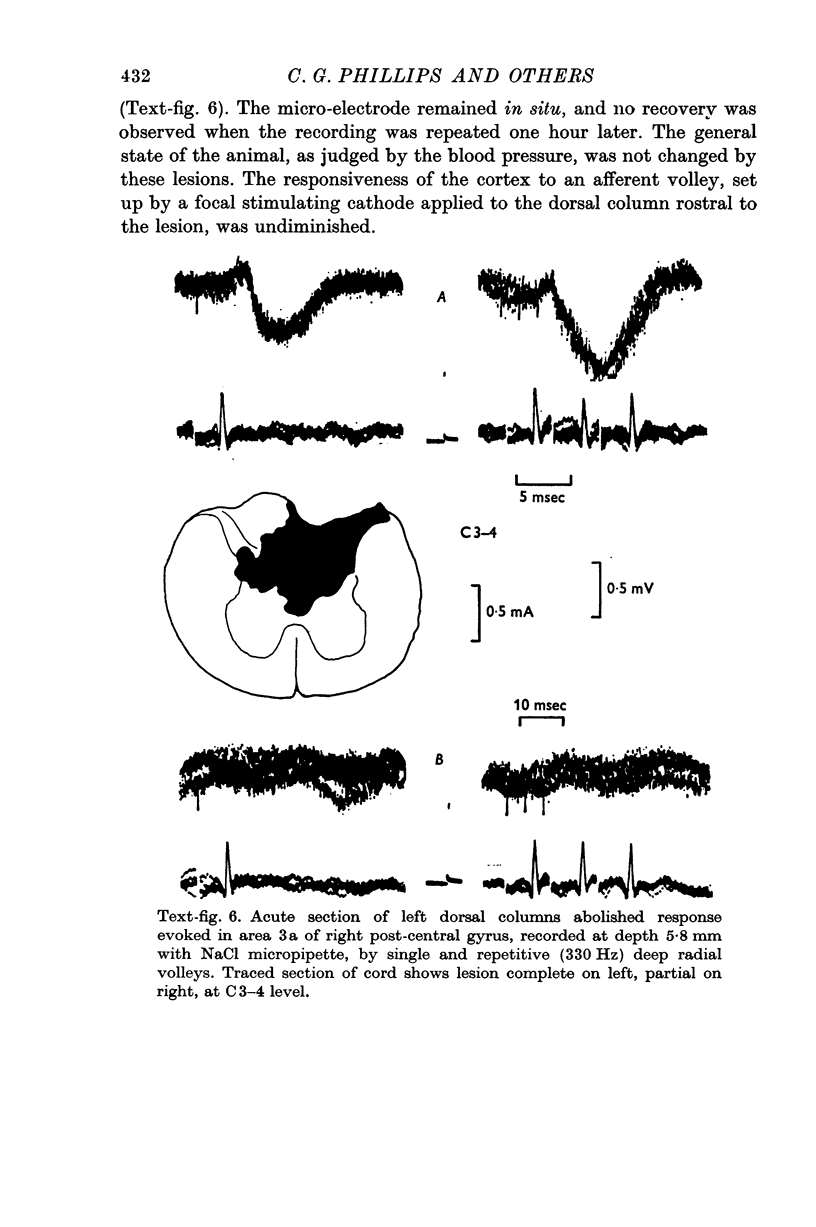





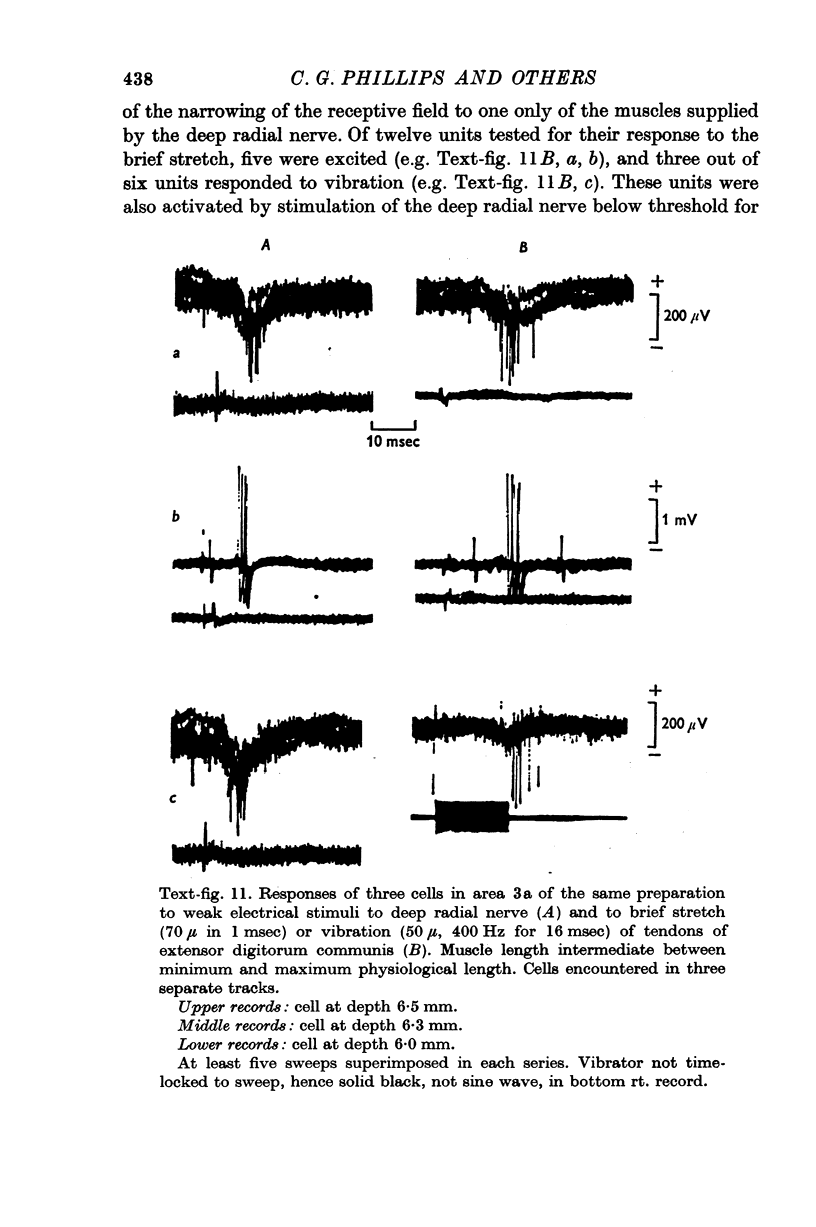












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albe-Fessard D., Liebeskind J., Lamarre Y. Projection au niveau du cortex somato-moteur du singe d'afférences provenant des récepteurs musculaires. C R Acad Sci Hebd Seances Acad Sci D. 1965 Nov 8;261(19):3891–3894. [PubMed] [Google Scholar]
- Albe-Fessard D., Liebeskind J. Origine des messages somato-sensitifs activant les cellules du cortex moteur chez le singe. Exp Brain Res. 1966;1(2):127–146. doi: 10.1007/BF00236866. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Engberg I., Matthews P. B. The relative sensitivity to vibration of muscle receptors of the cat. J Physiol. 1967 Oct;192(3):773–800. doi: 10.1113/jphysiol.1967.sp008330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORDA M., EKLUND G., VON EULER EXTERNAL INTERCOSTAL AND PHRENIC ALPHA-MOTOR RESPONSES TO CHANGES IN RESPIRATORY LOAD. Acta Physiol Scand. 1965 Mar;63:391–400. doi: 10.1111/j.1748-1716.1965.tb04079.x. [DOI] [PubMed] [Google Scholar]
- Clough J. F., Kernell D., Phillips C. G. The distribution of monosynaptic excitation from the pyramidal tract and from primary spindle afferents to motoneurones of the baboon's hand and forearm. J Physiol. 1968 Sep;198(1):145–166. doi: 10.1113/jphysiol.1968.sp008598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles R. M., Phillips C. G., Chien-Ping W. Motor innervation, motor unit organization and afferent innervation of m. extensor digitorum communis of the baboon's forearm. J Physiol. 1968 Sep;198(1):179–192. doi: 10.1113/jphysiol.1968.sp008600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon G., Miller R. Identification of cortical cells projecting to the dorsal column nuclei of the cat. Q J Exp Physiol Cogn Med Sci. 1969 Jan;54(1):85–98. doi: 10.1113/expphysiol.1969.sp002009. [DOI] [PubMed] [Google Scholar]
- HASSLER R., MUHS-CLEMENT K. ARCHITEKTONISCHER AUFBAU DES SENSOMOTORISCHEN UND PARIETALEN CORTEX DE KATZE. J Hirnforsch. 1964;7:377–420. [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. RECEPTIVE FIELDS AND FUNCTIONAL ARCHITECTURE IN TWO NONSTRIATE VISUAL AREAS (18 AND 19) OF THE CAT. J Neurophysiol. 1965 Mar;28:229–289. doi: 10.1152/jn.1965.28.2.229. [DOI] [PubMed] [Google Scholar]
- Jones E. G., Powell T. P. Connexions of the somatic sensory cortex of the rhesus monkey. 3. Thalamic connexions. Brain. 1970;93(1):37–56. doi: 10.1093/brain/93.1.37. [DOI] [PubMed] [Google Scholar]
- Jones E. G., Powell T. P. Connexions of the somatic sensory cortex of the rhesus monkey. I. Ipsilateral cortical connexions. Brain. 1969;92(3):477–502. doi: 10.1093/brain/92.3.477. [DOI] [PubMed] [Google Scholar]
- Jones E. G., Powell T. P. The ipsilateral cortical connexions of the somatic sensory areas in the cat. Brain Res. 1968 Jun;9(1):71–94. doi: 10.1016/0006-8993(68)90258-8. [DOI] [PubMed] [Google Scholar]
- LUNDBERG A., WINSBURY G. Selective adequate activation of large afferents from muscle spindles and Golgi tendon organs. Acta Physiol Scand. 1960 Jul 15;49:155–164. doi: 10.1111/j.1748-1716.1960.tb01939.x. [DOI] [PubMed] [Google Scholar]
- Landgren S., Silfvenius H. Projection to cerebral cortex of group I muscle afferents from the cat's hind limb. J Physiol. 1969 Feb;200(2):353–372. doi: 10.1113/jphysiol.1969.sp008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATTHEWS P. B. MUSCLE SPINDLES AND THEIR MOTOR CONTROL. Physiol Rev. 1964 Apr;44:219–288. doi: 10.1152/physrev.1964.44.2.219. [DOI] [PubMed] [Google Scholar]
- Matthews P. B. The reflex excitation of the soleus muscle of the decerebrate cat caused by vibbration applied to its tendon. J Physiol. 1966 May;184(2):450–472. doi: 10.1113/jphysiol.1966.sp007926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSCARSSON O., ROSEN I. PROJECTION TO CEREBRAL CORTEX OF LARGE MUSCLE-SPINDLE AFFERENTS IN FORELIMB NERVES OF THE CAT. J Physiol. 1963 Dec;169:924–945. doi: 10.1113/jphysiol.1963.sp007305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscarsson O., Rosén I. Short-latency projections to the cat's cerebral cortex from skin and muscle afferents in the contralateral forelimb. J Physiol. 1966 Jan;182(1):164–184. doi: 10.1113/jphysiol.1966.sp007816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHILLIPS C. G., POWELL T. P., SHEPHERD G. M. RESPONSES OF MITRAL CELLS TO STIMULATION OF THE LATERAL OLFACTORY TRACT IN THE RABBIT. J Physiol. 1963 Aug;168:65–88. doi: 10.1113/jphysiol.1963.sp007178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POWELL T. P., MOUNTCASTLE V. B. Some aspects of the functional organization of the cortex of the postcentral gyrus of the monkey: a correlation of findings obtained in a single unit analysis with cytoarchitecture. Bull Johns Hopkins Hosp. 1959 Sep;105:133–162. [PubMed] [Google Scholar]
- POWELL T. P., MOUNTCASTLE V. B. The cytoarchitecture of the postcentral gyrus of the monkey Macaca mulatta. Bull Johns Hopkins Hosp. 1959 Sep;105:108–131. [PubMed] [Google Scholar]
- Phillips C. G., Powell T. P., Wiesendanger M. Projection from low-threshold muscle afferents of hand and forearm to area 3a of baboon's cortex. J Physiol. 1970 Sep;210(1):59P–60P. [PubMed] [Google Scholar]
- Phillips C. G. The Ferrier lecture, 1968. Motor apparatus of the baboon's hand. Proc R Soc Lond B Biol Sci. 1969 May 20;173(1031):141–174. doi: 10.1098/rspb.1969.0044. [DOI] [PubMed] [Google Scholar]
- ROBERTS T. S., AKERT K. Insular and opercular cortex and its thalamic projection in Macaca mulatta. Schweiz Arch Neurol Neurochir Psychiatr. 1963;92:1–43. [PubMed] [Google Scholar]
- ROSE J. E., WOOLSEY C. N. The relations of thalamic connections, cellular structure and evocable electrical activity in the auditory region of the cat. J Comp Neurol. 1949 Dec;91(3):441–466. doi: 10.1002/cne.900910306. [DOI] [PubMed] [Google Scholar]
- Thomas R. C., Wilson V. J. Precise localization of Renshaw cells with a new marking technique. Nature. 1965 Apr 10;206(980):211–213. doi: 10.1038/206211b0. [DOI] [PubMed] [Google Scholar]







