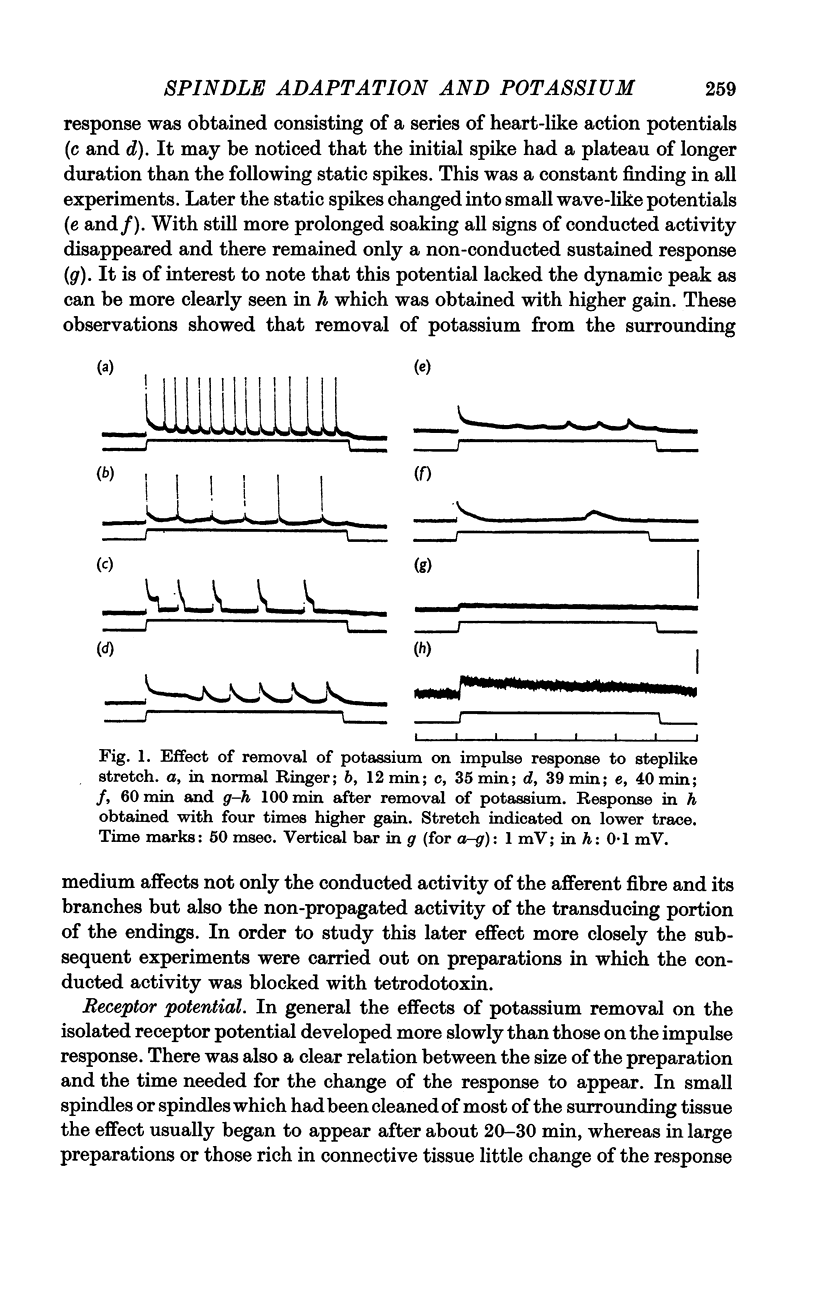
Abstract
1. Effects of changes in ionic environment on the receptor potential were studied in isolated frog spindle. Particular attention was focused on the action of potassium removal on the early adaptive decline of the response.
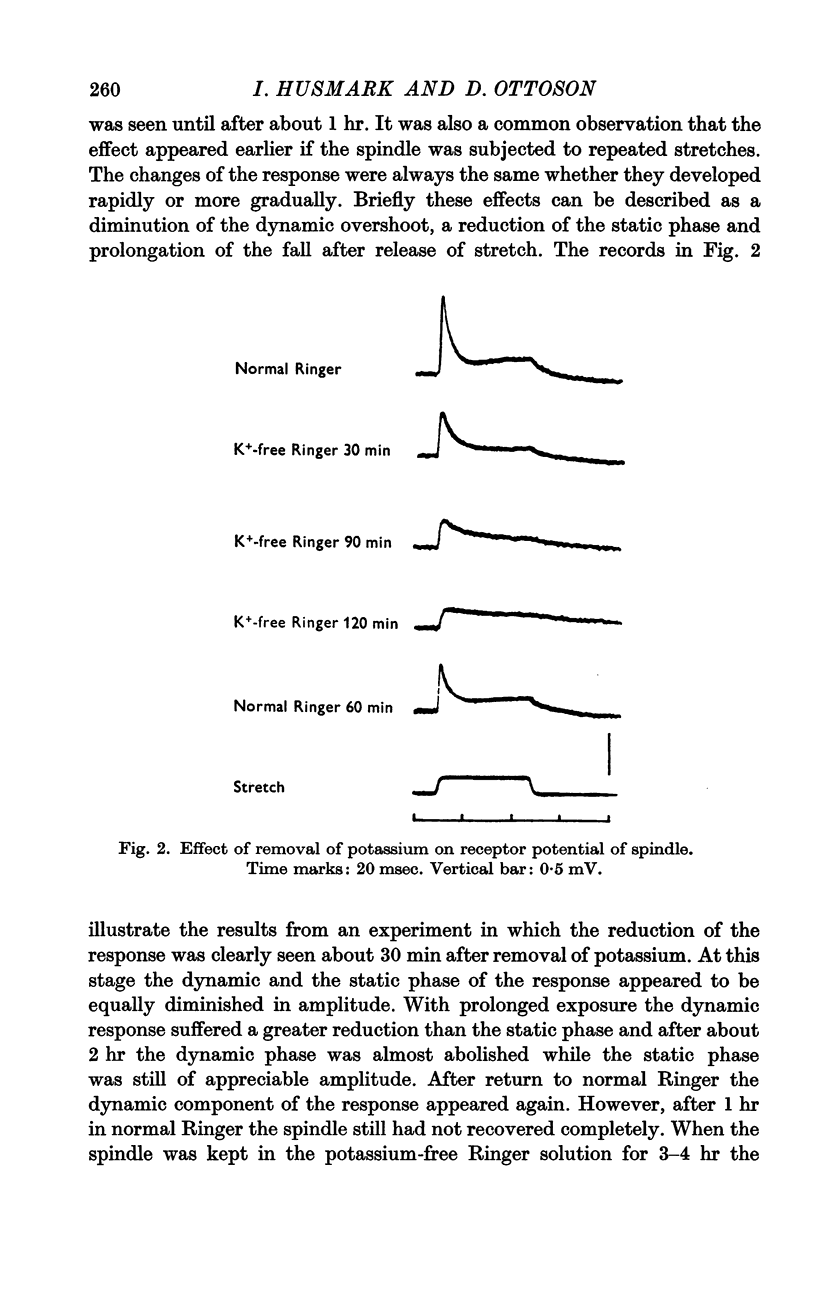
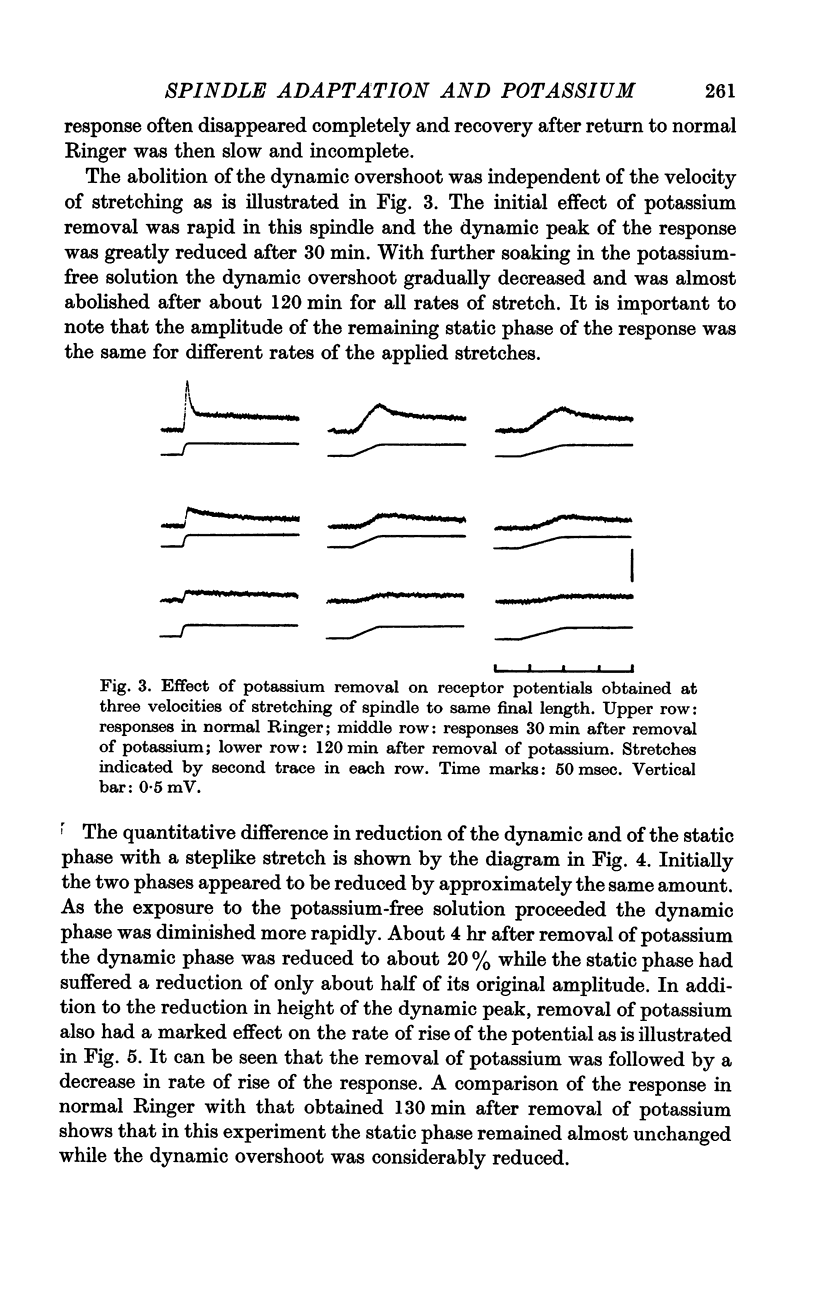
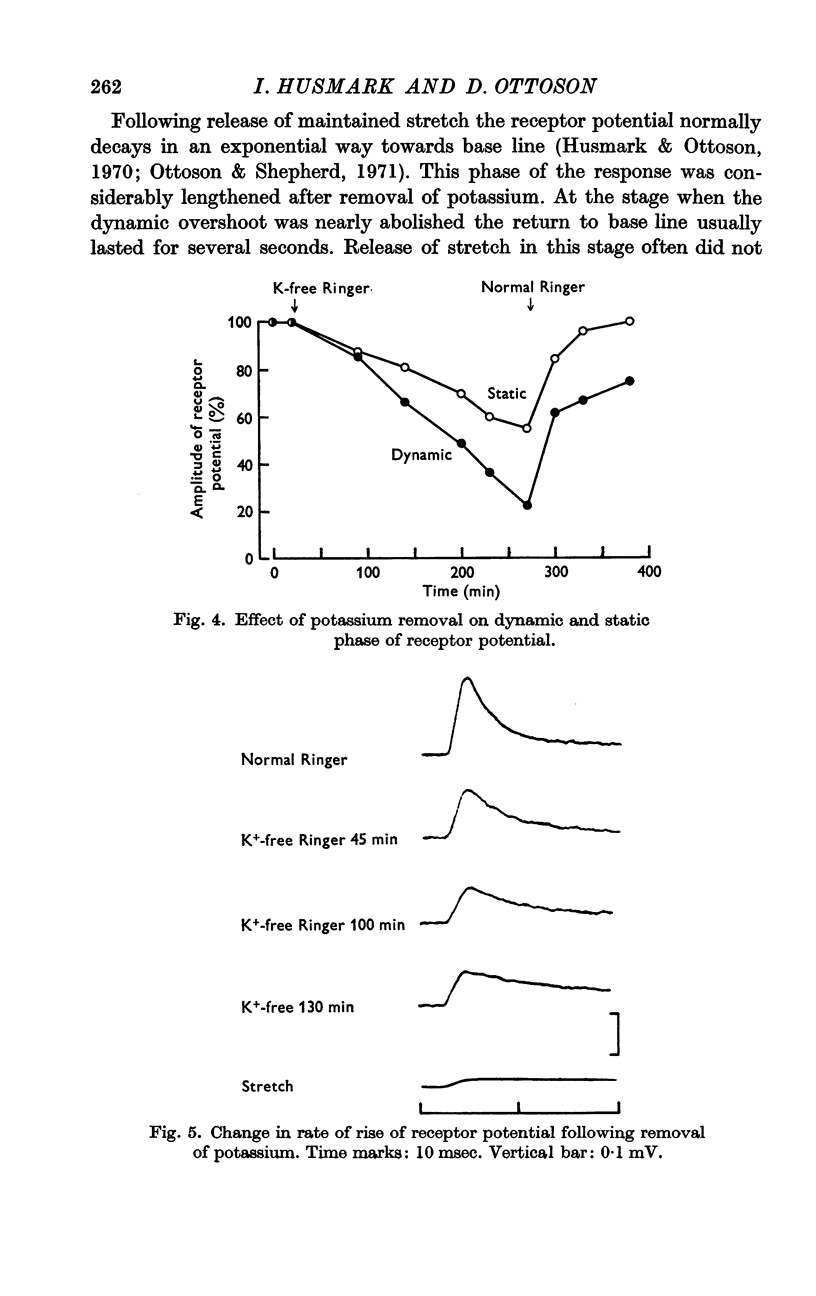
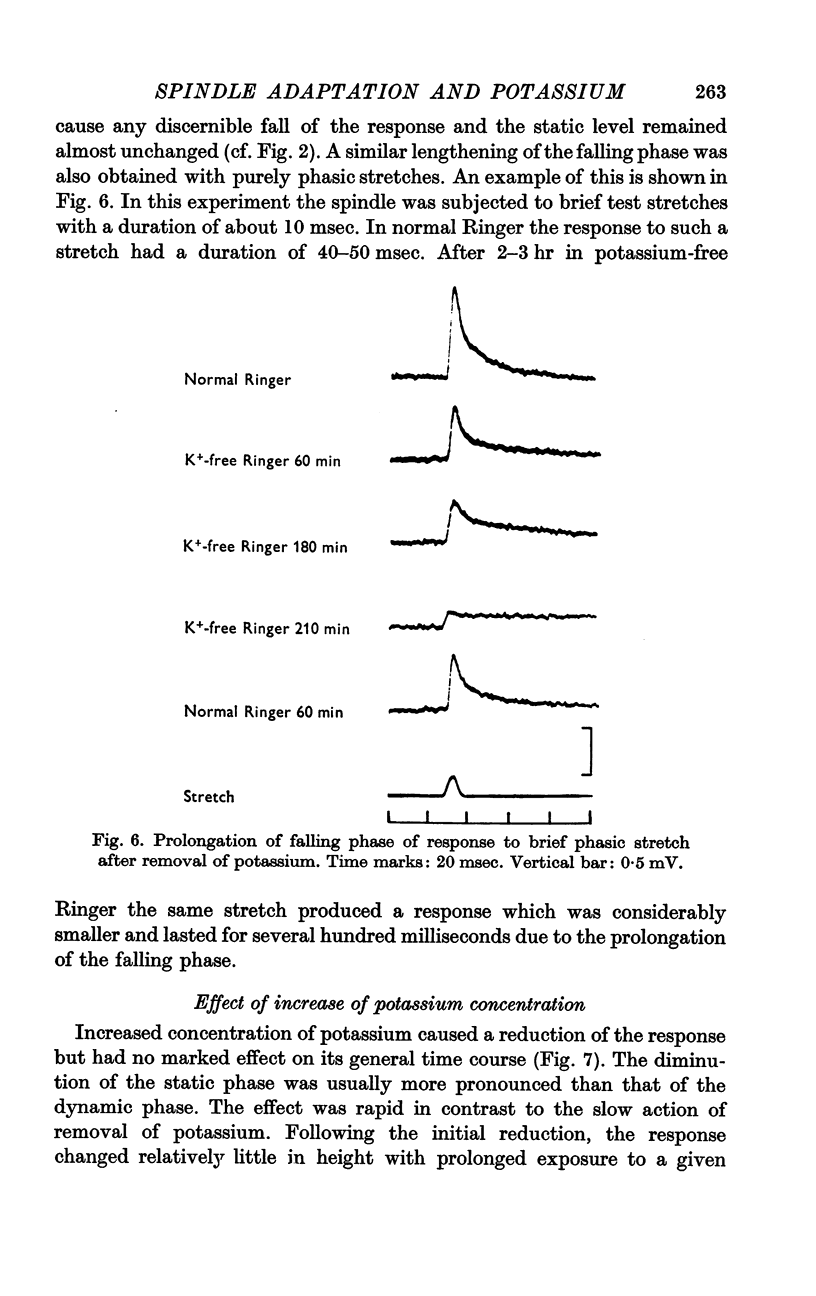
2. Removal of potassium caused a reduction and final disappearance of the dynamic overshoot of the receptor potential. The static phase of the response was also reduced although to less extent. The repolarization phase of the response following release of phasic or maintained stretch was greatly prolonged.
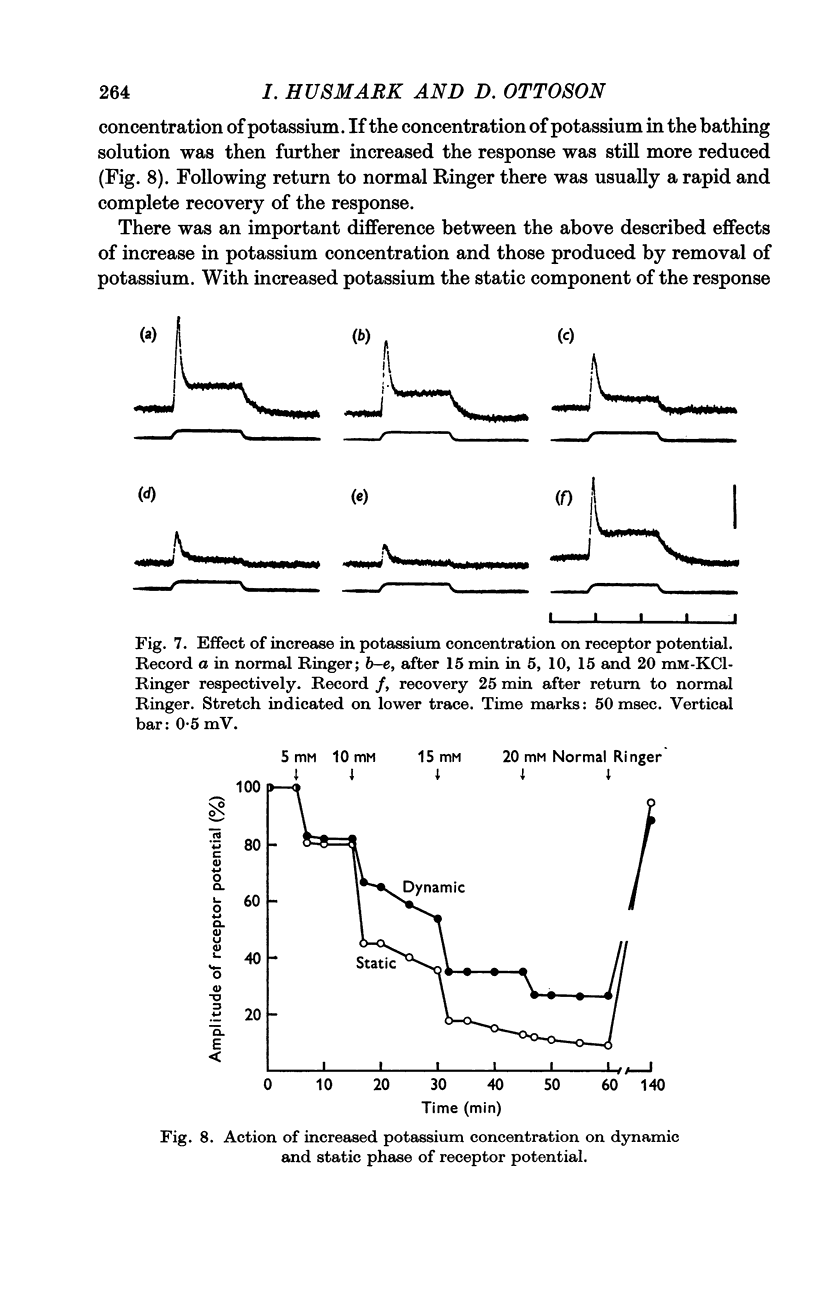
3. Increased potassium concentration caused a reduction of the response, but did not change its general time course. The amount of reduction was related to the potassium concentration.
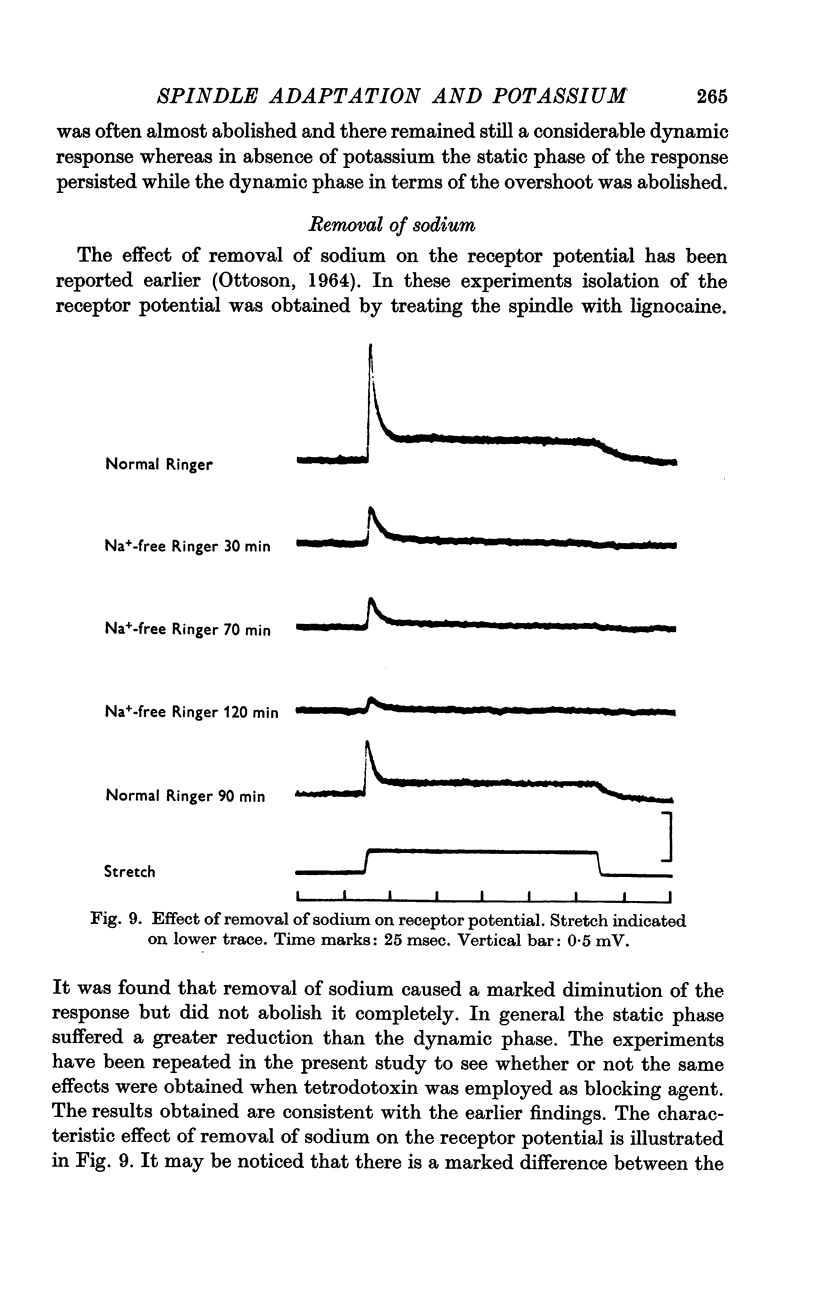
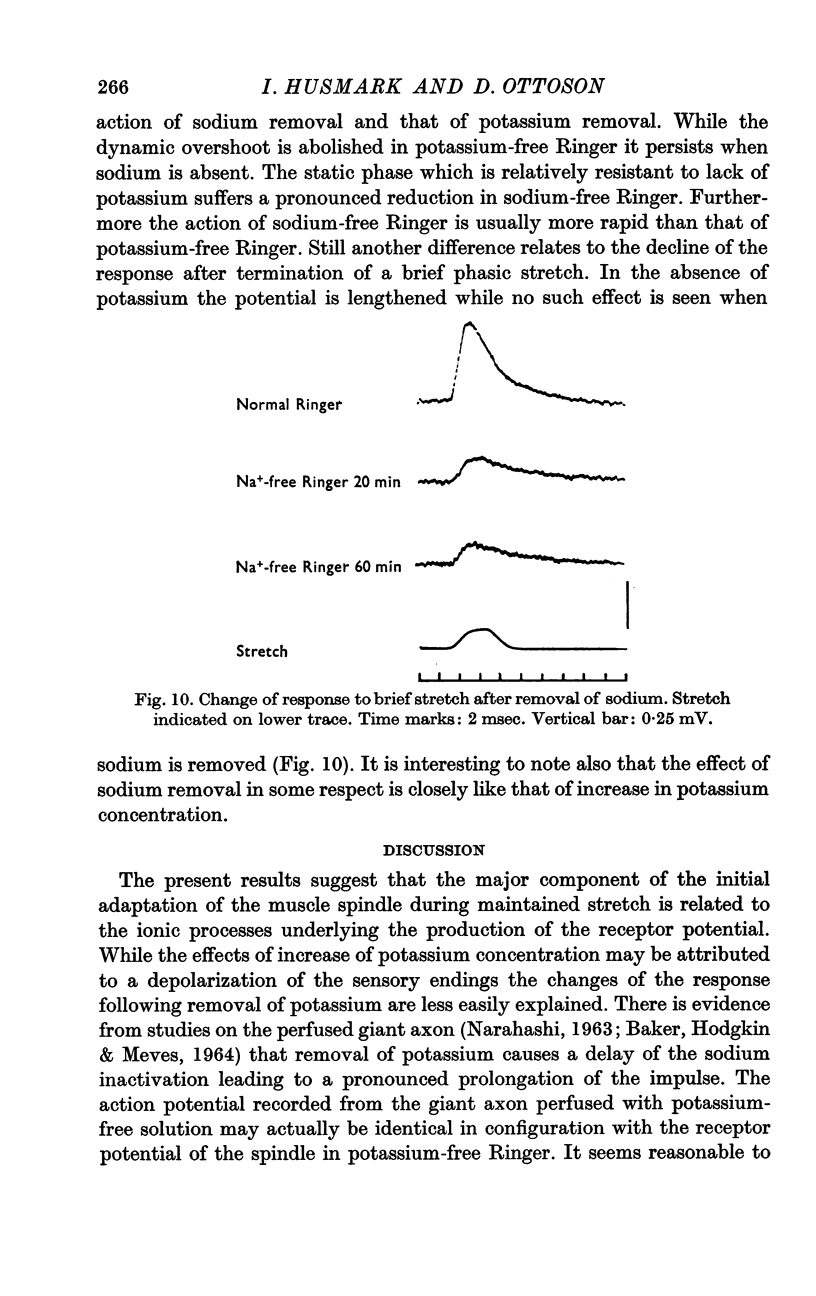
4. Removal of sodium caused a marked diminution of the response, the static phase being in general more affected than the dynamic phase.
5. It is suggested that the effects of potassium removal are caused by a delay in sodium inactivation and a partial depolarization of the endings. It is concluded that the greater part of the early adaptation of the spindle proper may be attributed to ionic mechanisms in the transducer membrane.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAKER P. F., HODGKIN A. L., MEVES H. THE EFFECT OF DILUTING THE INTERNAL SOLUTION ON THE ELECTRICAL PROPERTIES OF A PERFUSED GIANT AXON. J Physiol. 1964 Apr;170:541–560. doi: 10.1113/jphysiol.1964.sp007348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeckh J., Priesner E., Schneider D., Jacobson M. Olfactory Receptor Response to the Cockroach Sexual Attractant. Science. 1963 Aug 23;141(3582):716–717. doi: 10.1126/science.141.3582.716. [DOI] [PubMed] [Google Scholar]
- FRANKENHAEUSER B. Delayed currents in myelinated nerve fibres of Xenopus laevis investigated with voltage clamp technique. J Physiol. 1962 Jan;160:40–45. doi: 10.1113/jphysiol.1962.sp006832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B. Instantaneous potassium currents in myelinated nerve fibres of Xenopus laevis. J Physiol. 1962 Jan;160:46–53. doi: 10.1113/jphysiol.1962.sp006833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B. Potassium permeability in myelinated nerve fibres of Xenopus laevis. J Physiol. 1962 Jan;160:54–61. doi: 10.1113/jphysiol.1962.sp006834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulpius B., Baumann F. Effects of sodium, potassium, and calcium ions on slow and spike potentials in single photoreceptor cells. J Gen Physiol. 1969 May;53(5):541–561. doi: 10.1085/jgp.53.5.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husmark I., Ottoson D. Is the adaptation of the muscle spindle of ionic origin? Acta Physiol Scand. 1971 Jan;81(1):138–140. doi: 10.1111/j.1748-1716.1971.tb04883.x. [DOI] [PubMed] [Google Scholar]
- Husmark I., Ottoson D. The contribution of mechanical factors to the early adaptation of the spindle response. J Physiol. 1971 Feb;212(3):577–592. doi: 10.1113/jphysiol.1971.sp009343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B. Depolarization of sensory terminals and the initiation of impulses in the muscle spindle. J Physiol. 1950 Oct 16;111(3-4):261–282. doi: 10.1113/jphysiol.1950.sp004479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millecchia R., Mauro A. The ventral photoreceptor cells of Limulus. 3. A voltage-clamp study. J Gen Physiol. 1969 Sep;54(3):331–351. doi: 10.1085/jgp.54.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NARAHASHI T. DEPENDENCE OF RESTING AND ACTION POTENTIALS ON INTERNAL POTASSIUM IN PERFUSED SQUID GIANT AXONS. J Physiol. 1963 Nov;169:91–115. doi: 10.1113/jphysiol.1963.sp007243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OTTOSON D. THE ACTION OF CALCIUM ON THE FROG'S ISOLATED MUSCLE SPINDLE. J Physiol. 1965 May;178:68–79. doi: 10.1113/jphysiol.1965.sp007614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottoson D., McReynolds J. S., Shepherd G. M. Sensitivity of isolated frog muscle spondle during and after stretching. J Neurophysiol. 1969 Jan;32(1):24–34. doi: 10.1152/jn.1969.32.1.24. [DOI] [PubMed] [Google Scholar]
- Ottoson D., Shepherd G. M. Changes of length within the frog muscle spindle during stretch as shown by stroboscopic photomicroscopy. Nature. 1968 Nov 30;220(5170):912–914. doi: 10.1038/220912a0. [DOI] [PubMed] [Google Scholar]
- Ottoson D., Shepherd G. M. Length changes within isolated frog muscle spindle during and after stretching. J Physiol. 1970 May;207(3):747–759. doi: 10.1113/jphysiol.1970.sp009092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd G. M., Ottoson D. Response of the isolated muscle spindle to different rates of stretching. Cold Spring Harb Symp Quant Biol. 1965;30:95–103. doi: 10.1101/sqb.1965.030.01.013. [DOI] [PubMed] [Google Scholar]
- TASAKII, SHIMAMURA M. Further observations on resting and action potential of intracellularly perfused squid axon. Proc Natl Acad Sci U S A. 1962 Sep 15;48:1571–1577. doi: 10.1073/pnas.48.9.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]


