Abstract
1. A study has been made to see whether active and passive movements of sodium and potassium in human red blood cells are influenced by changing the chloride gradient and hence the potential difference across the cell membrane.
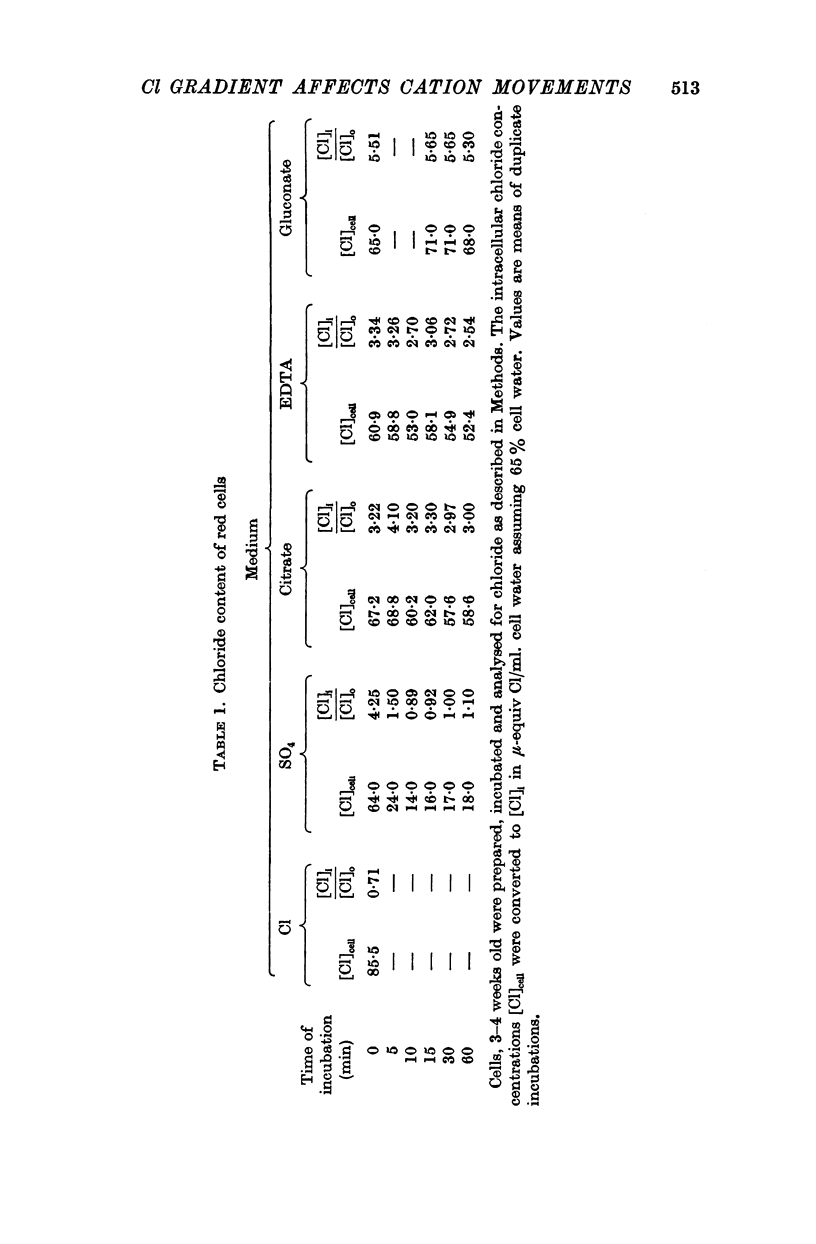
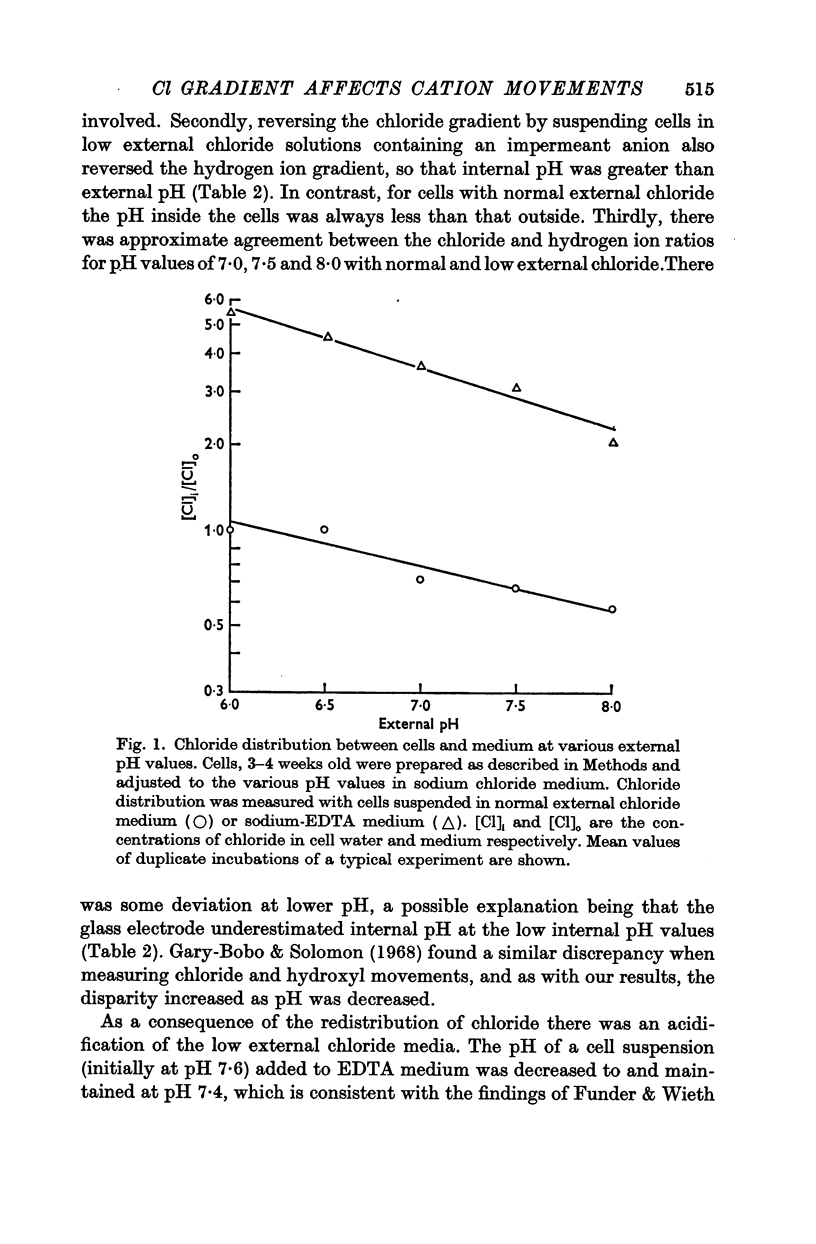
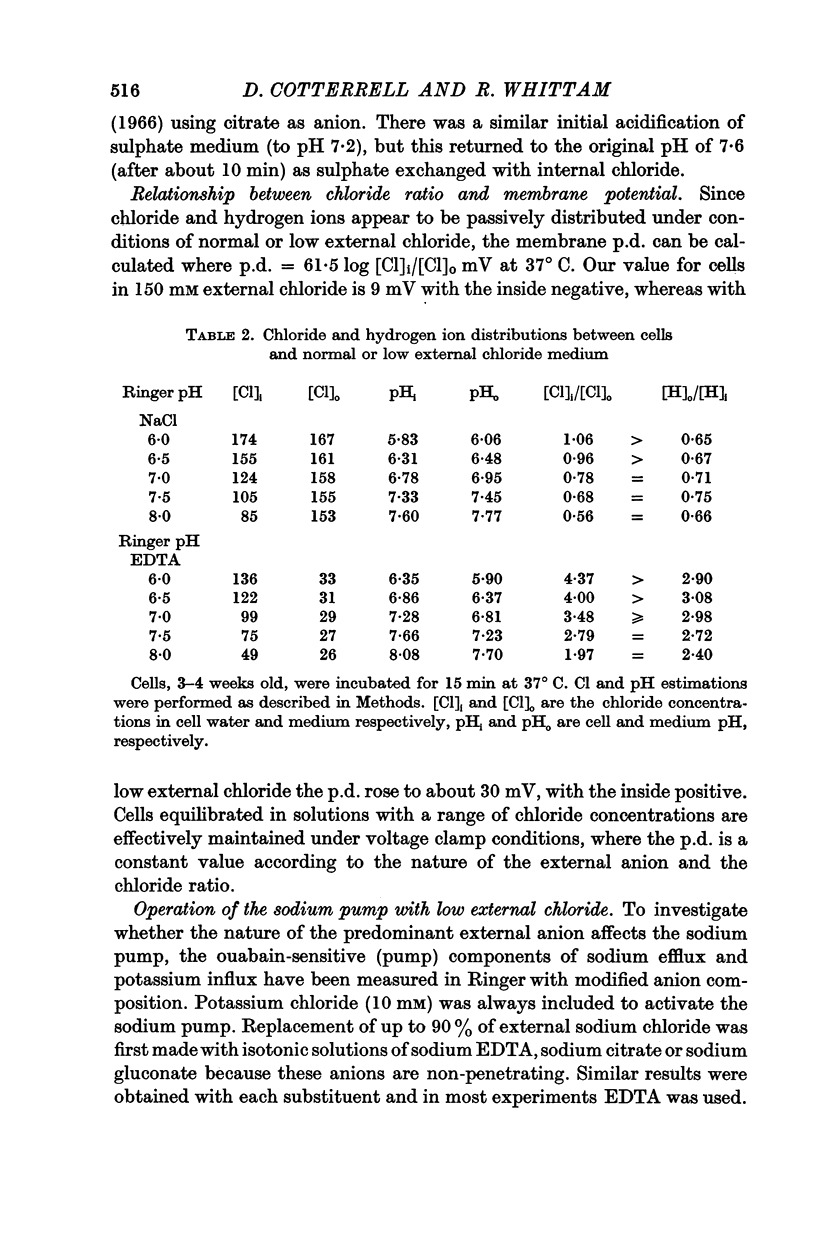
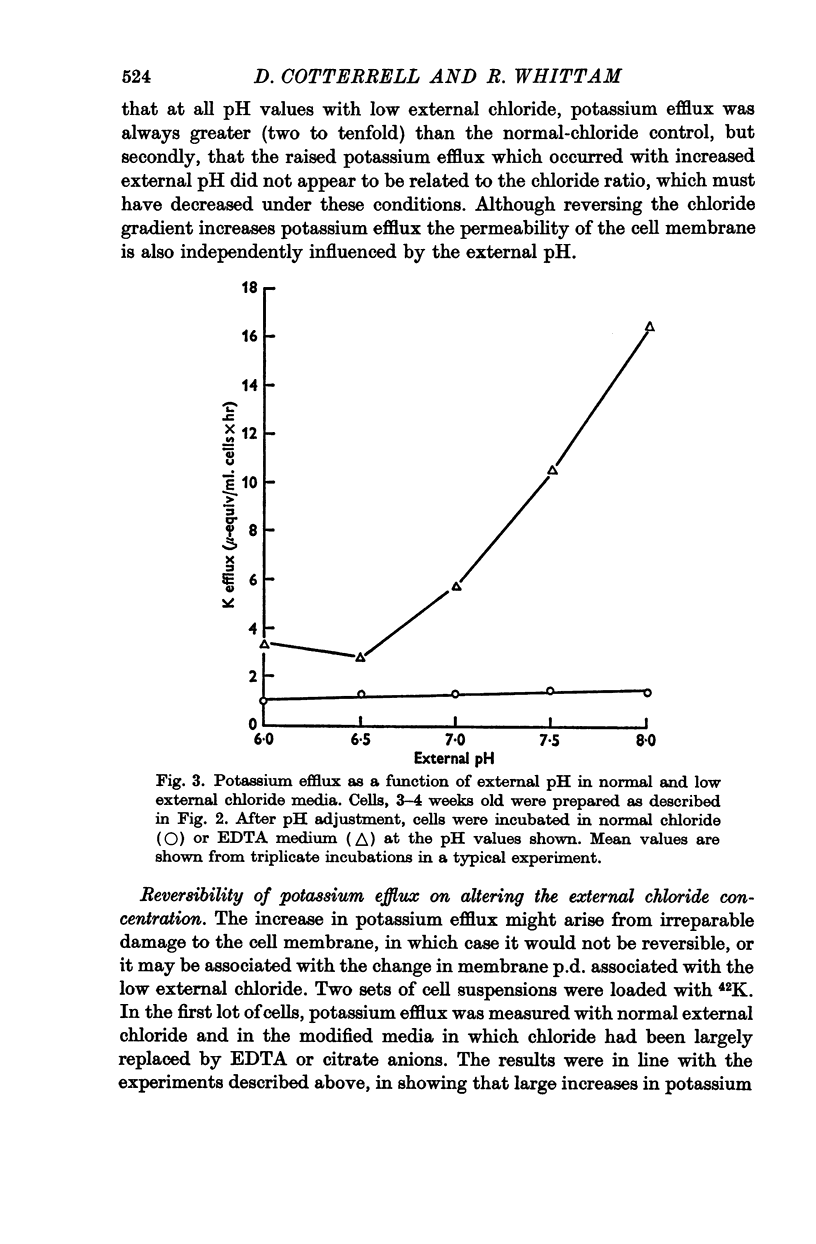
2. Chloride distribution was measured between red cells and isotonic solutions with a range of concentrations of chloride and non-penetrating anions (EDTA, citrate, gluconate). The cell chloride concentration was greater than that outside with low external chloride, suggesting that the sign of the membrane potential was reversed. The chloride ratio (internal/external) was approximately equal to the inverse of the hydrogen ion ratio at normal and low external chloride, and inversely proportional to external pH. These results show that chloride is passively distributed, making it valid to calculate the membrane potential from the chloride ratio.
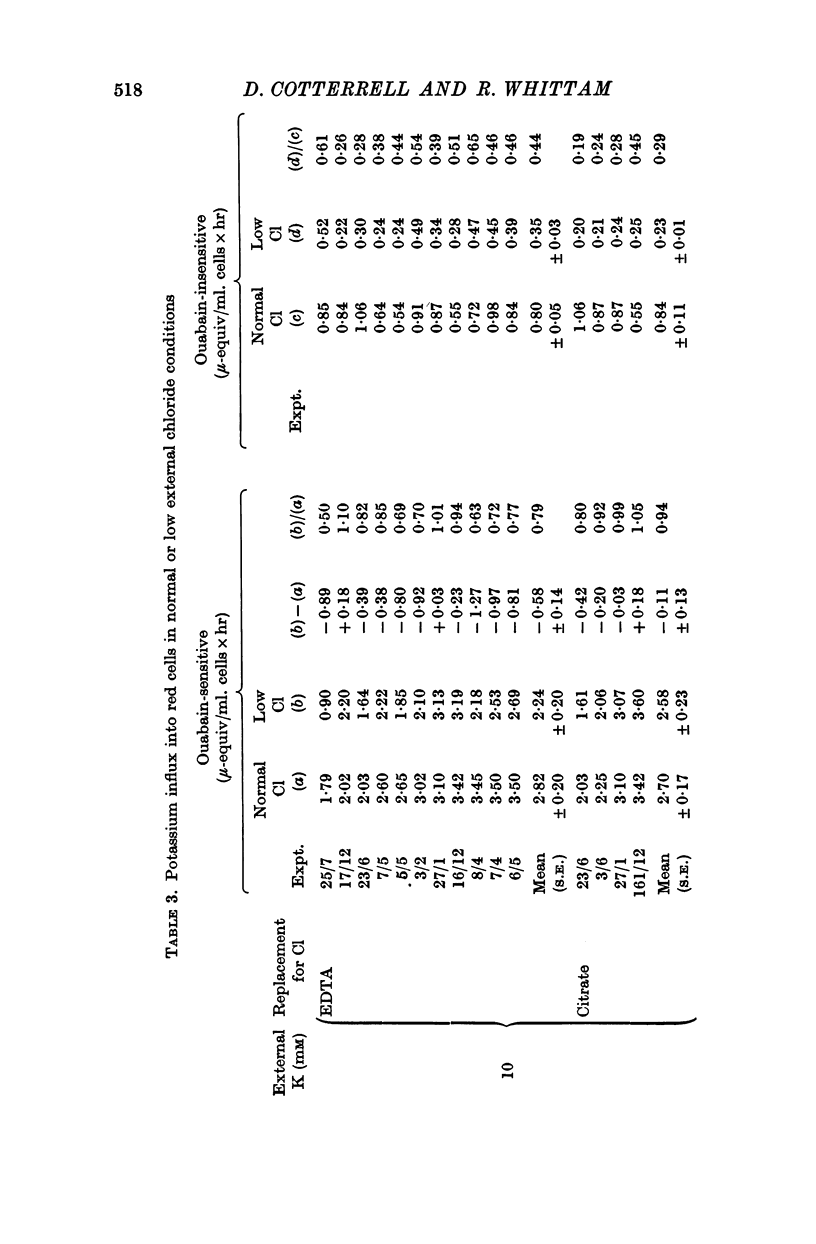
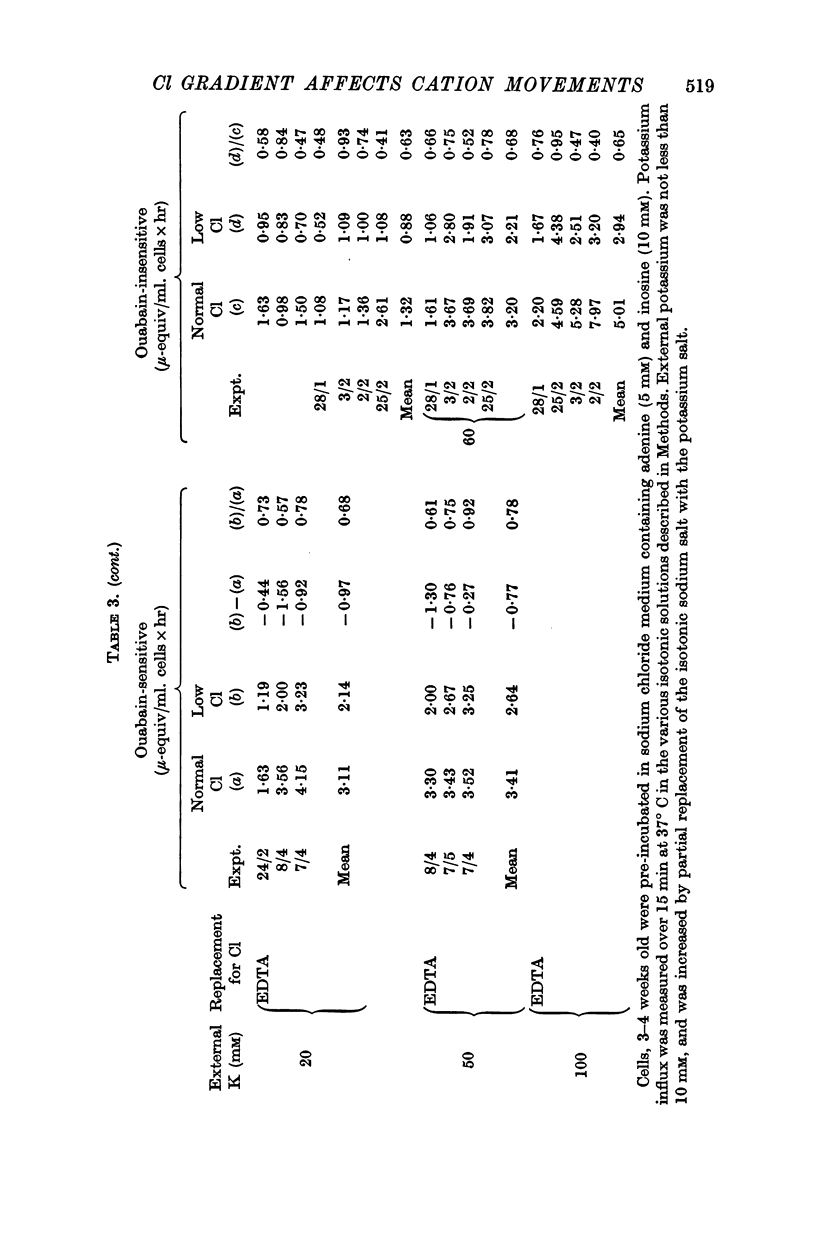
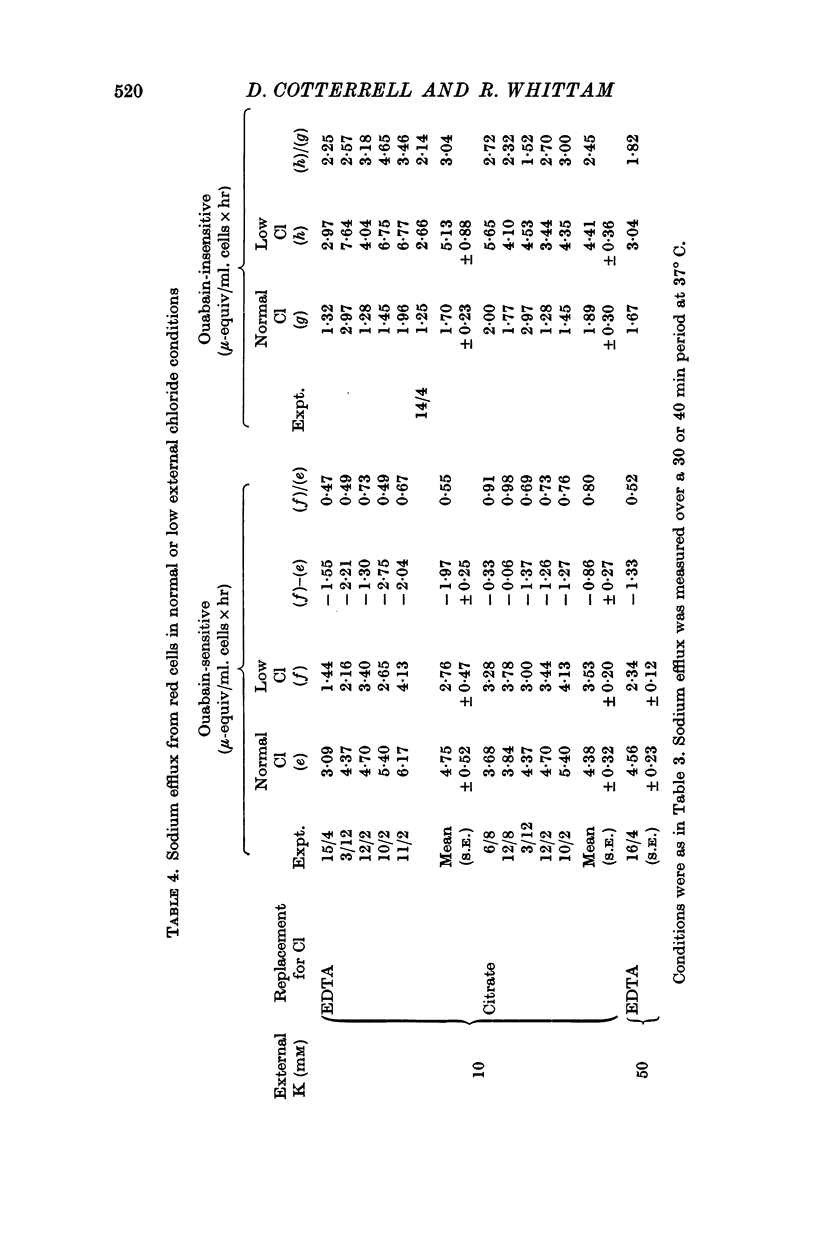
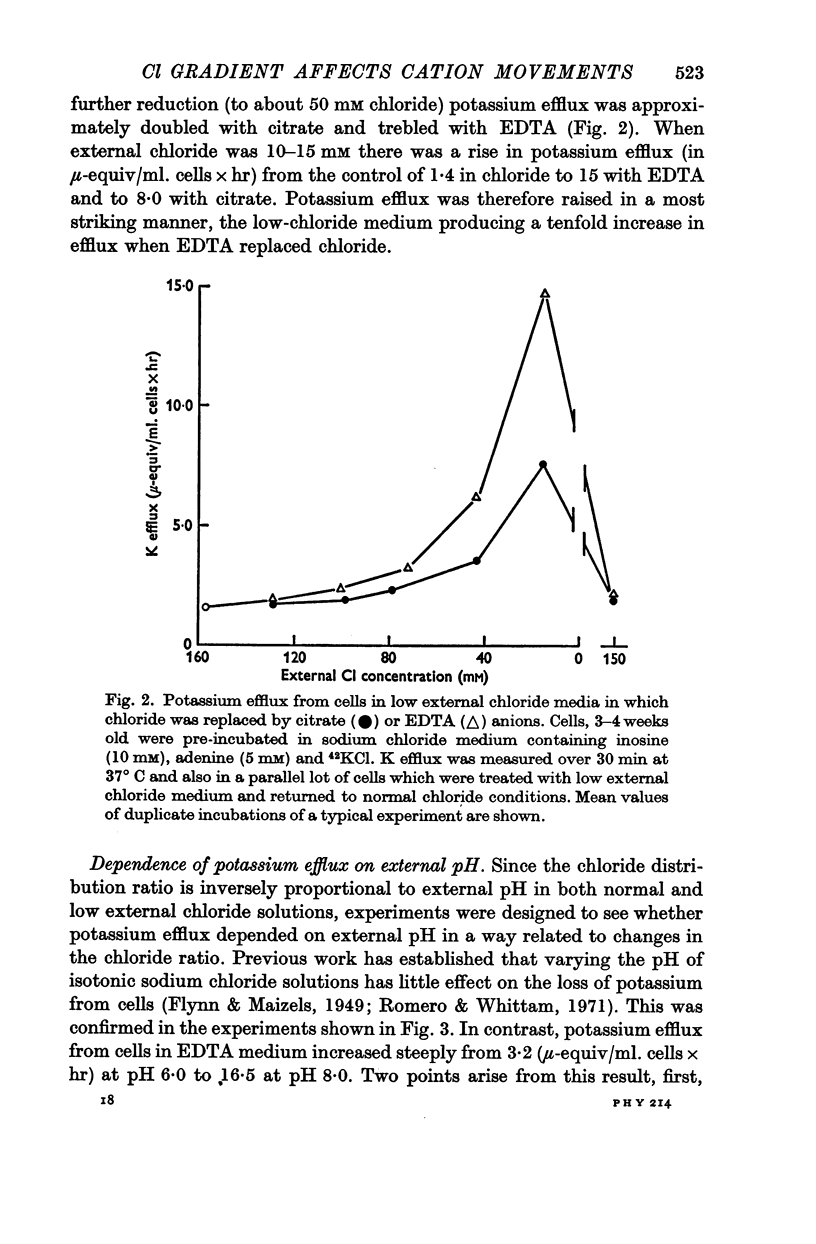
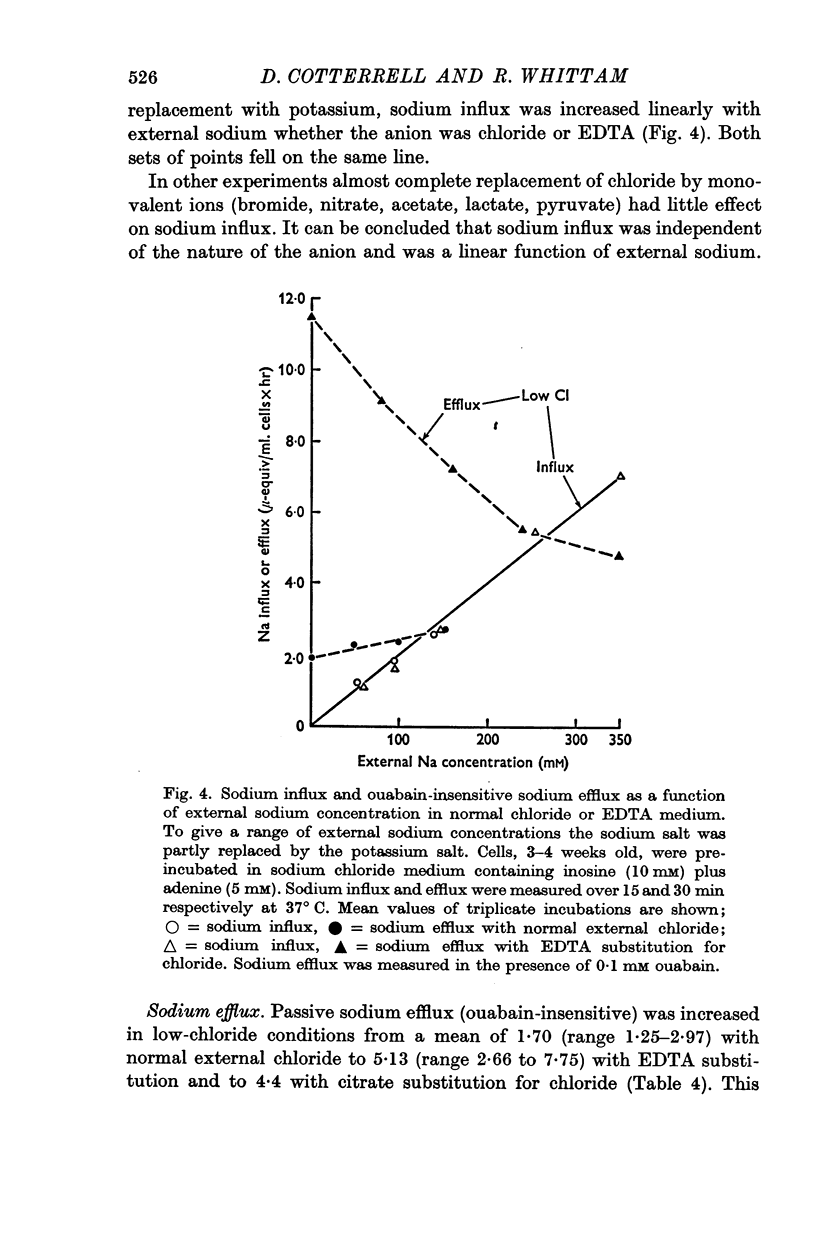
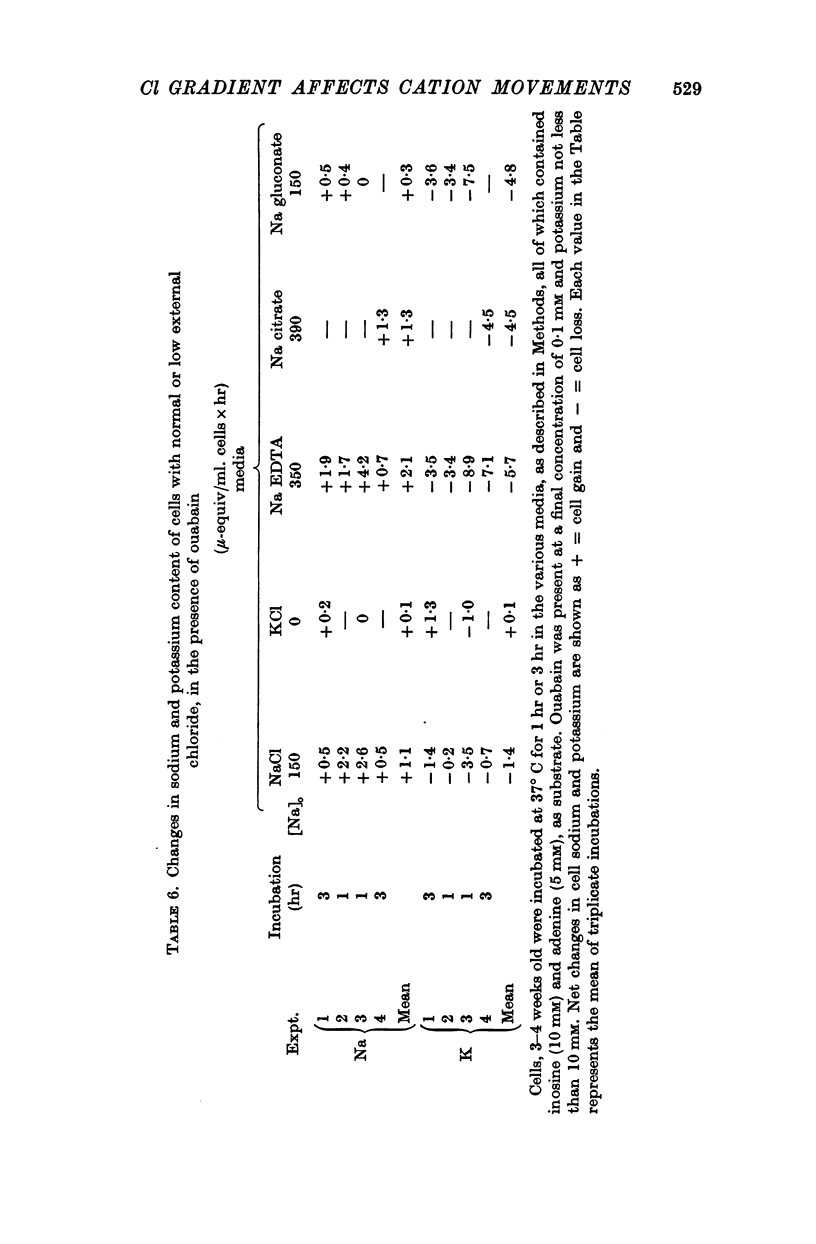
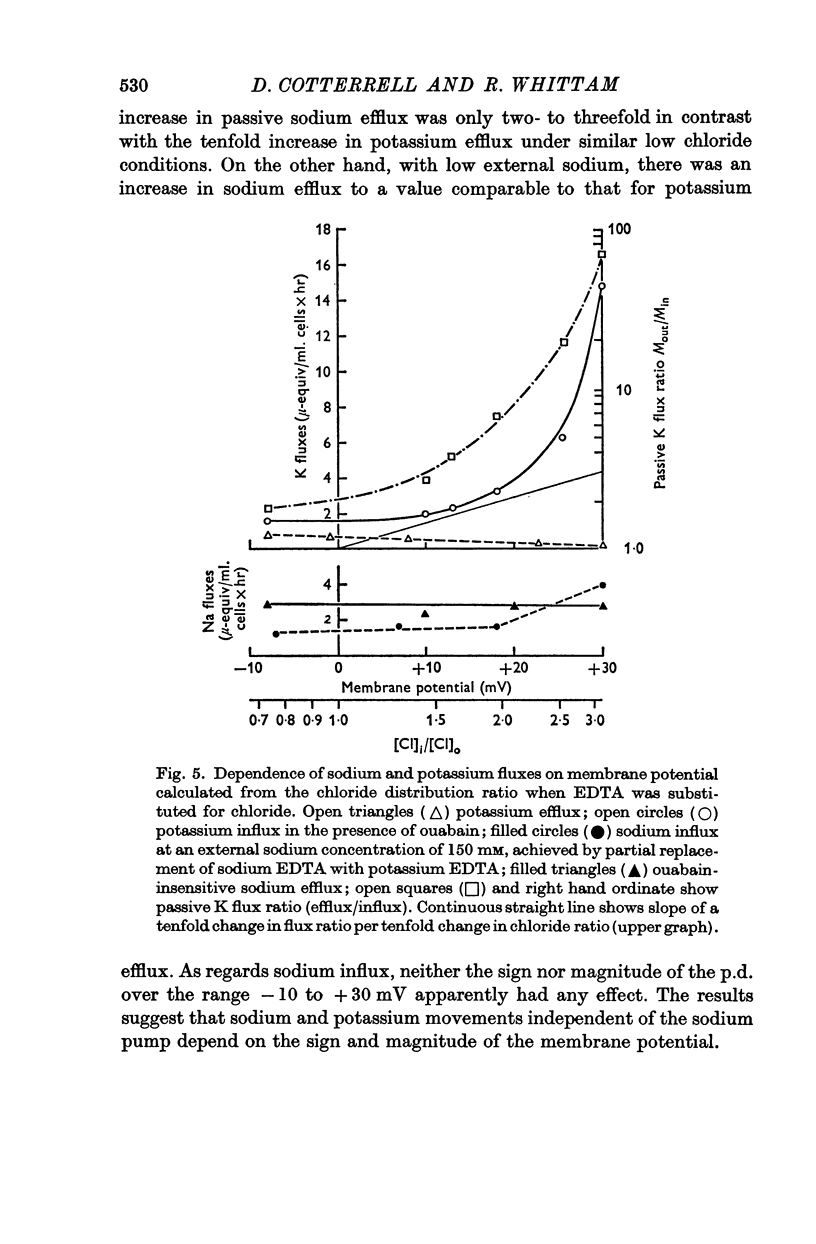
3. Ouabain-sensitive (pump) potassium influx and sodium efflux were decreased by not more than 20 and 40% respectively on reversing the chloride gradient, corresponding to a change in membrane potential from -9 to +30 mV. In contrast, passive (ouabain-insensitive) movements were reversibly altered — potassium influx was decreased about 60% and potassium efflux was increased some tenfold. Sodium influx was unaffected by the nature of the anion and depended only on the external sodium concentration, whereas ouabain-insensitive sodium efflux was increased about threefold. When external sodium was replaced by potassium there was a decrease in ouabain-insensitive sodium efflux with normal chloride, but an increase in low-chloride medium.
4. Net movements of sodium and potassium were roughly in accord with the unidirectional fluxes.
5. The results suggest that reversing the chloride gradient and, therefore, the sign of the membrane potential, had little effect on the sodium pump, but caused a marked increase in passive outward movements of both sodium and potassium ions.
Full text
PDF
















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRAUNSBERG H., GUYVER A. AUTOMATIC LIQUID SCINTILLATION COUNTING OF HIGH-ENERGY BETA-EMITTERS IN TISSUE SLICES AND AQUEOUS SOLUTIONS IN THE ABSENCE OF ORGANIC SCINTILLATOR. Anal Biochem. 1965 Jan;10:86–95. doi: 10.1016/0003-2697(65)90241-1. [DOI] [PubMed] [Google Scholar]
- Cotterrell D., Whittam R. An increase in potassium efflux in human red cells associated with reversing the sign of the membrane potential. J Physiol. 1970 Sep;210(2):136P–137P. [PubMed] [Google Scholar]
- Davson H. Studies on the permeability of erythrocytes: The effect of reducing the salt content of the medium surrounding the cell. Biochem J. 1939 Mar;33(3):389–401. doi: 10.1042/bj0330389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLYNN F., MAIZELS M. Cation control in human erythrocytes. J Physiol. 1949 Dec;110(3-4):301–318. doi: 10.1113/jphysiol.1949.sp004440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funder J., Wieth J. O. Effects of some monovalent anions on fluxes of Na and K, and on glucose metabolism of ouabain treated human red cells. Acta Physiol Scand. 1967 Oct-Nov;71(2):168–185. doi: 10.1111/j.1748-1716.1967.tb03723.x. [DOI] [PubMed] [Google Scholar]
- GLYNN I. M. Sodium and potassium movements in human red cells. J Physiol. 1956 Nov 28;134(2):278–310. doi: 10.1113/jphysiol.1956.sp005643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrahan P. J., Glynn I. M. Measurement of 24Na and 42K with a liquid-scintillation counting system without added scintillator. J Physiol. 1966 Oct;186(2):55P–56P. [PubMed] [Google Scholar]
- Garrahan P. J., Glynn I. M. The sensitivity of the sodium pump to external sodium. J Physiol. 1967 Sep;192(1):175–188. doi: 10.1113/jphysiol.1967.sp008295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gary-Bobo C. M., Solomon A. K. Properties of hemoglobin solutions in red cells. J Gen Physiol. 1968 Nov;52(5):825–853. doi: 10.1085/jgp.52.5.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Lew V. L., Lüthi U. Reversal of the potassium entry mechanism in red cells, with and without reversal of the entire pump cycle. J Physiol. 1970 Apr;207(2):371–391. doi: 10.1113/jphysiol.1970.sp009067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRIS E. J., MAIZELS M. Distribution of ions in suspensions of human erythrocytes. J Physiol. 1952 Sep;118(1):40–53. doi: 10.1113/jphysiol.1952.sp004771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. The potassium permeability of a giant nerve fibre. J Physiol. 1955 Apr 28;128(1):61–88. doi: 10.1113/jphysiol.1955.sp005291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman J. F. The red cell membrane and the transport of sodium and potassium. Am J Med. 1966 Nov;41(5):666–680. doi: 10.1016/0002-9343(66)90029-5. [DOI] [PubMed] [Google Scholar]
- Horowicz P., Gage P. W., Eisenberg R. S. The role of the electrochemical gradient in determining potassium fluxes in frog striated muscle. J Gen Physiol. 1968 May;51(5 Suppl):193S+–193S+. [PubMed] [Google Scholar]
- Jay A. W., Burton A. C. Direct measurement of potential difference across the human red blood cell membrane. Biophys J. 1969 Feb;9(2):115–121. doi: 10.1016/S0006-3495(69)86372-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaCelle P. L., Rothsteto A. The passive permeability of the red blood cell in cations. J Gen Physiol. 1966 Sep;50(1):171–188. doi: 10.1085/jgp.50.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lant A. F., Priestland R. N., Whittam R. The coupling of downhill ion movements associated with reversal of the sodium pump in human red cells. J Physiol. 1970 Apr;207(2):291–301. doi: 10.1113/jphysiol.1970.sp009062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassen U. V., Sten-Knudsen O. Direct measurements of membrane potential and membrane resistance of human red cells. J Physiol. 1968 Apr;195(3):681–696. doi: 10.1113/jphysiol.1968.sp008482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubowitz H., Whittam R. Ion movements in human red cells independent of the sodium pump. J Physiol. 1969 May;202(1):111–131. doi: 10.1113/jphysiol.1969.sp008798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizels M. The permeation of erythrocytes by cations. Biochem J. 1935 Aug;29(8):1970–1982. doi: 10.1042/bj0291970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POST R. L., JOLLY P. C. The linkage of sodium, potassium, and ammonium active transport across the human erythrocyte membrane. Biochim Biophys Acta. 1957 Jul;25(1):118–128. doi: 10.1016/0006-3002(57)90426-2. [DOI] [PubMed] [Google Scholar]
- Priestland R. N., Whittam R. The influence of external sodium ions on the sodium pump in erythrocytes. Biochem J. 1968 Sep;109(3):369–374. doi: 10.1042/bj1090369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero P. J., Whittam R. The control by internal calcium of membrane permeability to sodium and potassium. J Physiol. 1971 May;214(3):481–507. doi: 10.1113/jphysiol.1971.sp009445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANDERSON P. H. Potentiometric determination of chloride in biological fluids. Biochem J. 1952 Nov;52(3):502–505. doi: 10.1042/bj0520502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VIDAVER G. A. MUCATE INHIBITION OF GLYCINE ENTRY INTO PIGEON RED CELLS. Biochemistry. 1964 Jun;3:799–803. doi: 10.1021/bi00894a012. [DOI] [PubMed] [Google Scholar]
- VIDAVER G. A. SOME TESTS OF THE HYPOTHESIS THAT THE SODIUM-ION GRADIENT FURNISHES THE ENERGY FOR GLYCINE-ACTIVE TRANSPORT BY PIGEON RED CELLS. Biochemistry. 1964 Jun;3:803–808. doi: 10.1021/bi00894a013. [DOI] [PubMed] [Google Scholar]
- Whittam R., Ager M. E. The connexion between active cation transport and metabolism in erythrocytes. Biochem J. 1965 Oct;97(1):214–227. doi: 10.1042/bj0970214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittam R., Wiley J. S. Potassium transport and nucleoside metabolism in human red cells. J Physiol. 1967 Aug;191(3):633–652. doi: 10.1113/jphysiol.1967.sp008272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieth J. O. Effect of some monovalent anions on chloride and sulphate permeability of human red cells. J Physiol. 1970 May;207(3):581–609. doi: 10.1113/jphysiol.1970.sp009082. [DOI] [PMC free article] [PubMed] [Google Scholar]


