Abstract
1. The total carbonic anhydrase activity in some guinea-pig tissues has been measured using a pH-stat procedure. Stomach, gall bladder, proximal colon and caecum all possess more carbonic anhydrase activity per unit amount of protein than does whole blood.
2. The carbonic anhydrase activity of the small intestine is low. Reasons are given for supposing that activity found there is not entirely due to contamination by whole blood, and it is suggested that in this tissue the enzyme may be localized in some cell type other than the columnar absorbing cells.
3. Evidence is presented which indicates that heavy metals interfere with the activity of the enzyme as measured in tissue homogenates.
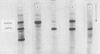
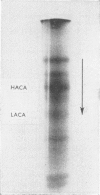
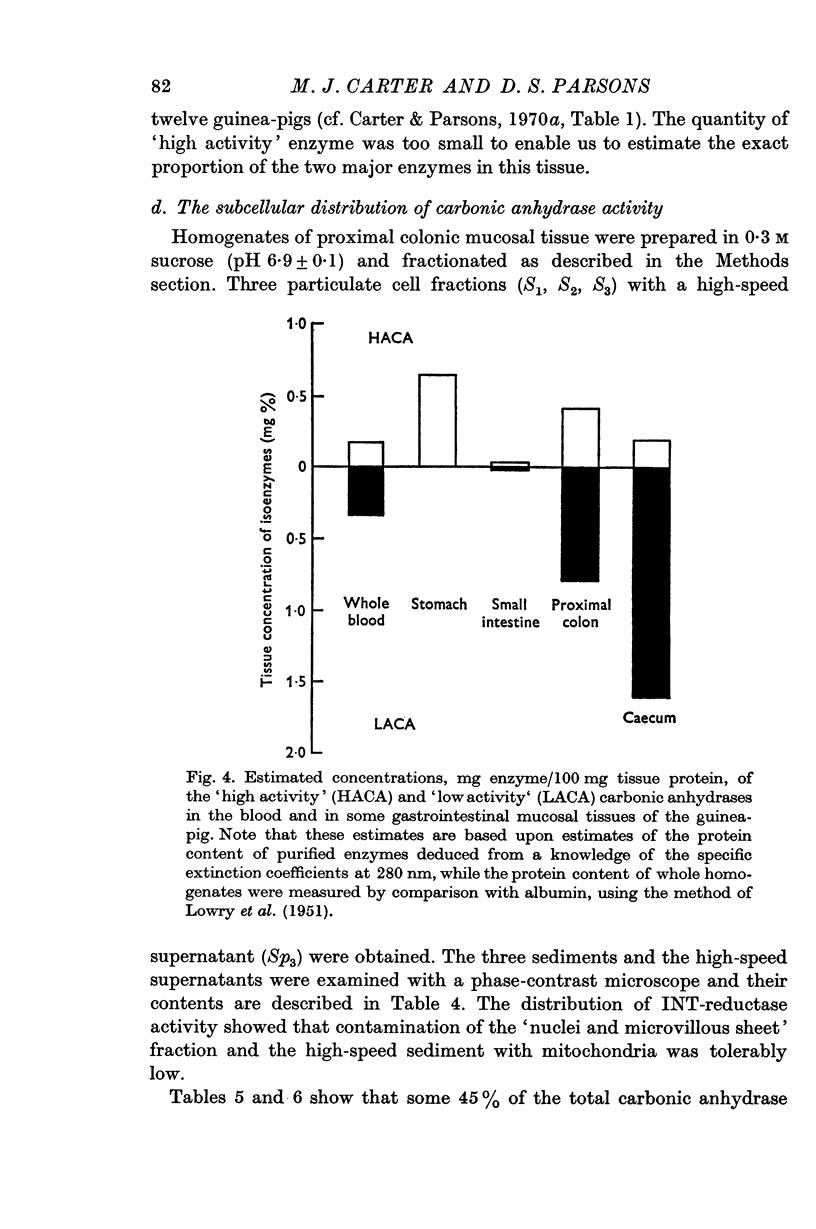
4. The distribution and concentration of the two major isoenzymes of carbonic anhydrase have been measured in different tissues. Blood and proximal colon contain both isoenzymes in comparable concentrations, the ratio of the concentration of the `low activity' isoenzyme to that of the `high activity' being about 2. The gastric mucosa contains much `high activity' carbonic anhydrase, but only a negligible amount of the `low activity' isoenzyme. In the caecal mucosa, the `low activity' isoenzyme is predominant, the ratio of its concentration to that of the `high activity' isoenzyme being about 9. It is also found that more than 1·5% of the protein in the caecal mucosa is accounted for as carbonic anhydrase enzymes.
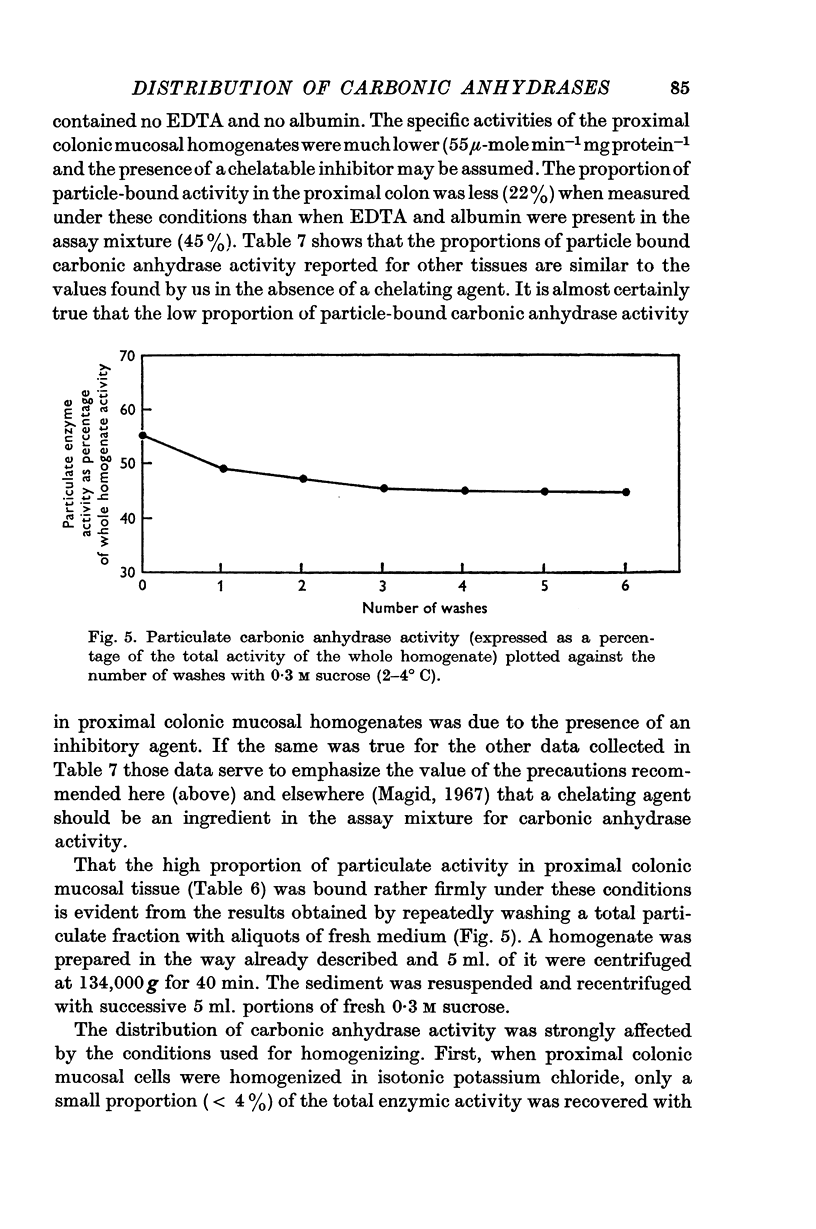
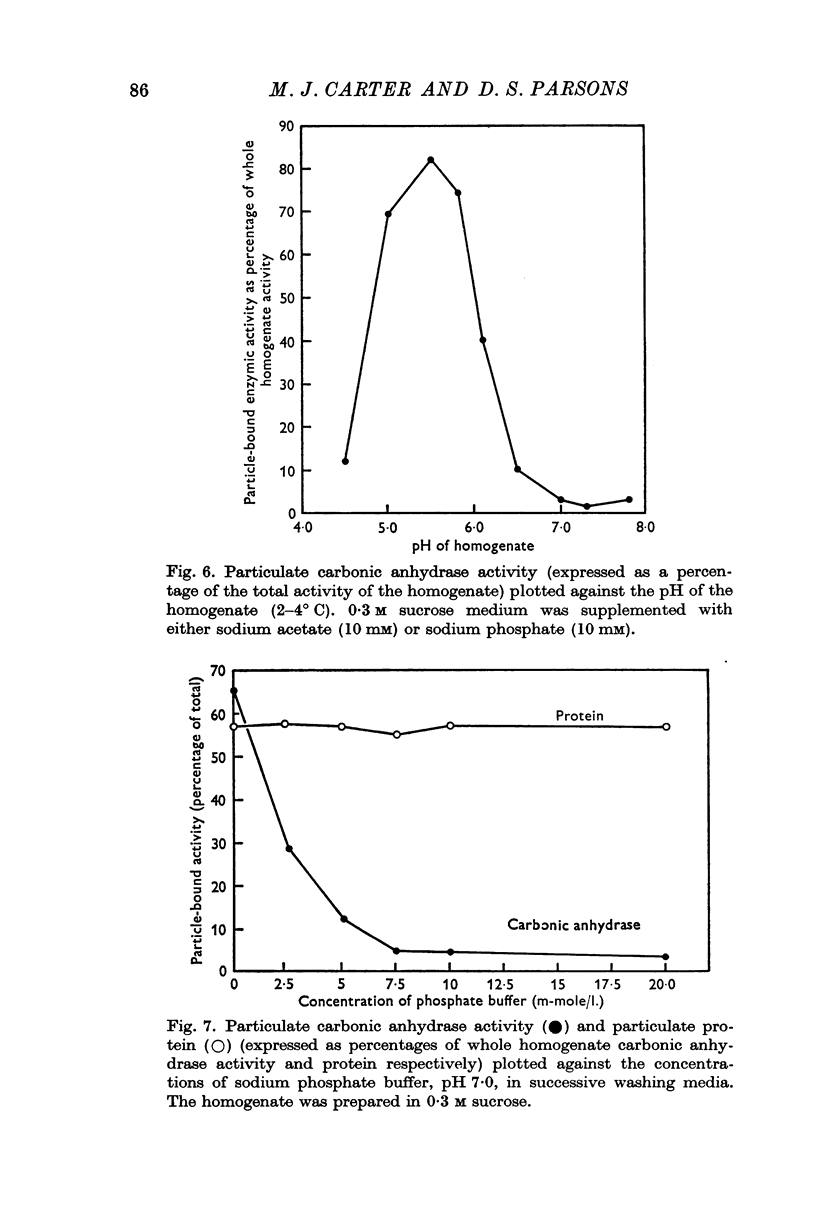
5. It is found that some 45% of the total carbonic anhydrase activity of sucrose homogenates of the guinea-pig colon is bound to particles. The activity is located mainly in the nuclear and microvillous fraction and in the `high-speed supernatant' fraction. The form of enzyme bound is largely of the `high activity' variety. When the tissue is homogenized in potassium chloride solutions less than 4% of the total activity is recovered in particulate fractions. The amount of activity which is bound to particulate fractions increases as the ionic strength or pH of the homogenate is lowered.
6. The findings are discussed in relation to the possible physiological roles of the isoenzymes in tissues other than blood. Possible relationships between the presence of the enzymes and the metabolism and transport of ammonium and fatty acids are considered.
Full text
PDF



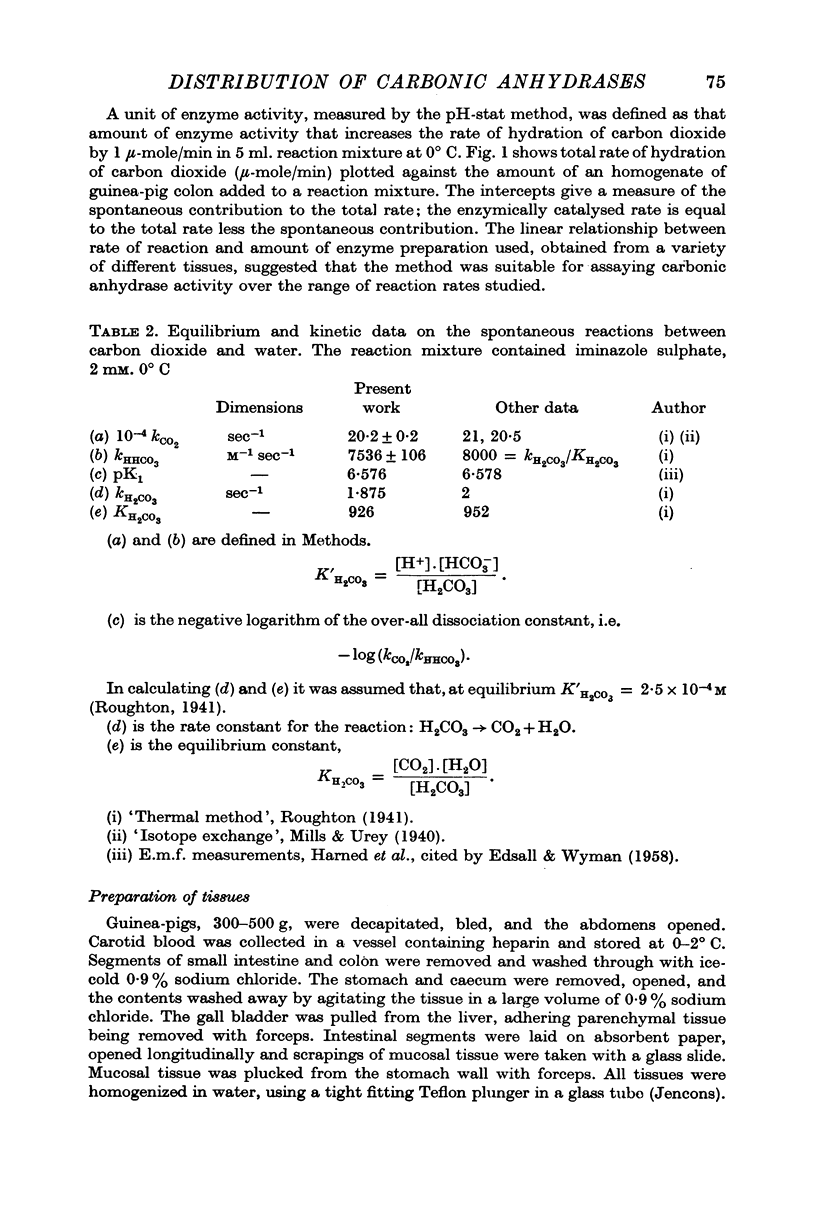

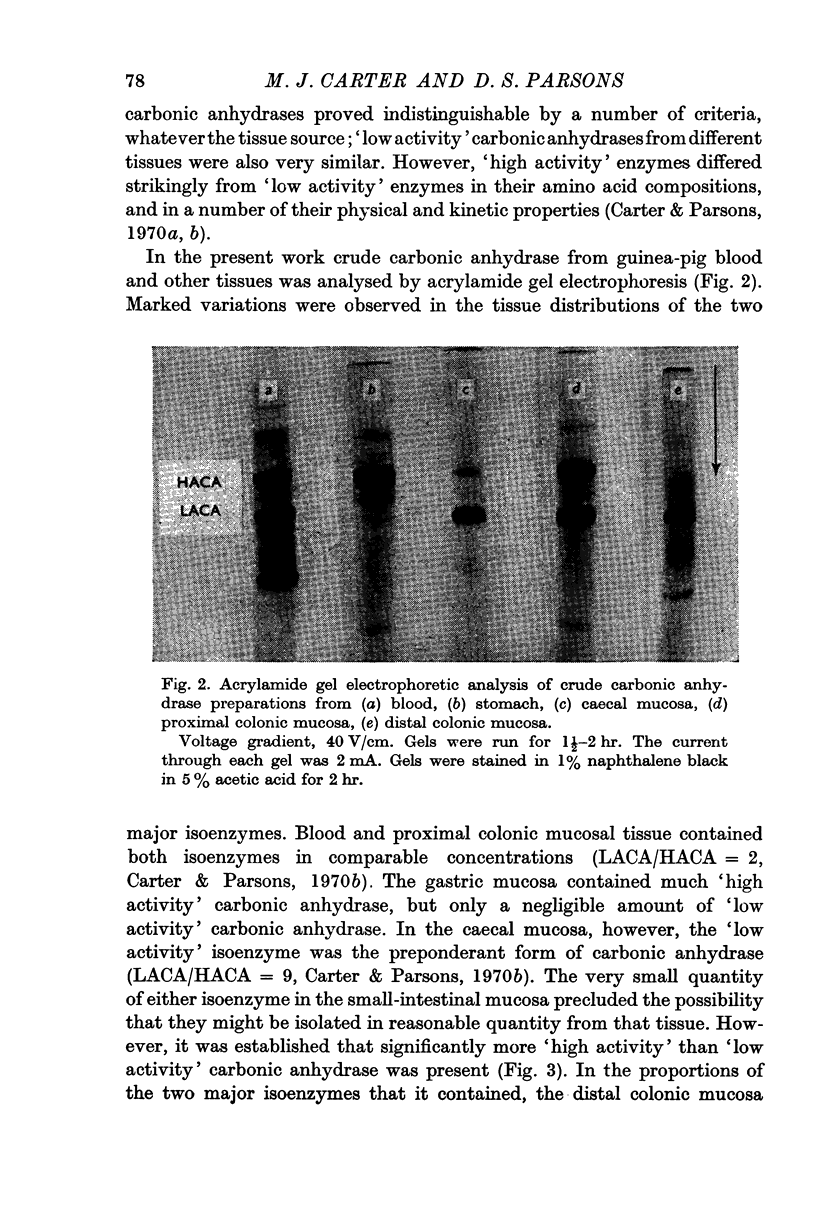












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aafjes J. H. Carbonic anhydrase in the wall of the forestomachs of cows. Br Vet J. 1967 Jun;123(6):252–256. doi: 10.1016/s0007-1935(17)39956-6. [DOI] [PubMed] [Google Scholar]
- Armstrong J. M., Myers D. V., Verpoorte J. A., Edsall J. T. Purification and properties of human erythrocyte carbonic anhydrases. J Biol Chem. 1966 Nov 10;241(21):5137–5149. [PubMed] [Google Scholar]
- BONTING S. L., CARAVAGGIO L. L., CANADY M. R., HAWKINS N. M. STUDIES ON SODIUM-POTASSIUM-ACTIVATED ADENOSINETRIPHOSPHATASE. XI. THE SALT GLAND OF THE HERRING GULL. Arch Biochem Biophys. 1964 Jul 20;106:49–56. doi: 10.1016/0003-9861(64)90155-9. [DOI] [PubMed] [Google Scholar]
- Bernstein R. S., Nevalainen T., Schraer R., Schraer H. Intracellular distribution and role of carbonic anhydrase in the avian (Gallus domesticus) shell gland mucosa. Biochim Biophys Acta. 1968 Jun 4;159(2):367–376. doi: 10.1016/0005-2744(68)90085-5. [DOI] [PubMed] [Google Scholar]
- Byvoet P., Gotti A. Isolation and properties of carbonic anhydrase from dog kidney and erythrocytes. Mol Pharmacol. 1967 Mar;3(2):142–152. [PubMed] [Google Scholar]
- Carter M. J., Parsons D. S. Carbonic anhydrase activity of mucosa of small intestine and colon. Nature. 1968 Jul 13;219(5150):176–177. doi: 10.1038/219176a0. [DOI] [PubMed] [Google Scholar]
- Carter M. J., Parsons D. S. The carbonic anhydrases of some guinea-pig tissues. Biochim Biophys Acta. 1970 Apr 22;206(1):190–192. doi: 10.1016/0005-2744(70)90099-9. [DOI] [PubMed] [Google Scholar]
- Carter M. J., Parsons D. S. The purification and properties of carbonic anhydrases from guinea-pig erythrocytes and mucosae of the gastrointestinal tract. Biochem J. 1970 Dec;120(4):797–808. doi: 10.1042/bj1200797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DATTA P. K., SHEPARD T. H., 2nd Intracellular localization of carbonic anhydrase in rat liver and kidney tissues. Arch Biochem Biophys. 1959 Mar;81(1):124–129. doi: 10.1016/0003-9861(59)90182-1. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Edmonds C. J. Transport of sodium and secretion of potassium and bicarbonate by the colon of normal and sodium-depleted rats. J Physiol. 1967 Dec;193(3):589–602. doi: 10.1113/jphysiol.1967.sp008380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edsall J. T. Multiple molecular forms of carbonic anhydrase in erythrocytes. Ann N Y Acad Sci. 1968 Jun 14;151(1):41–63. doi: 10.1111/j.1749-6632.1968.tb11877.x. [DOI] [PubMed] [Google Scholar]
- Furth A. J. Purification and properties of horse erythrocyte carbonic anhydrases. J Biol Chem. 1968 Sep 25;243(18):4832–4841. [PubMed] [Google Scholar]
- Hansson H. P. Demonstration of carbonic anhydrase by means of fluorescent antibodies in human erythrocytes. Life Sci. 1965 May;4(9):965–968. doi: 10.1016/0024-3205(65)90198-0. [DOI] [PubMed] [Google Scholar]
- Hansson H. P. Histochemical demonstration of carbonic anhydrase activity in some epithelia noted for active transport. Acta Physiol Scand. 1968 Aug;73(4):427–434. doi: 10.1111/j.1365-201x.1968.tb10882.x. [DOI] [PubMed] [Google Scholar]
- Hogben C. A. Gastric secretion of hydrochloric acid. Introduction: the natural history of the isolated bullfrog gastric mucosa. Fed Proc. 1965 Nov-Dec;24(6):1353–1359. [PubMed] [Google Scholar]
- KARLER R., WOODBURY D. M. Intracellular distribution of carbonic anhydrase. Biochem J. 1960 Jun;75:538–543. doi: 10.1042/bj0750538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KINNEY V. R., CODE C. F. CANINE ILEAL CHLORIDE ABSORPTION: EFFECT OF CARBONIC ANHYDRASE INHIBITOR ON TRANSPORT. Am J Physiol. 1964 Nov;207:998–1004. doi: 10.1152/ajplegacy.1964.207.5.998. [DOI] [PubMed] [Google Scholar]
- Keilin D., Mann T. Carbonic anhydrase. Purification and nature of the enzyme. Biochem J. 1940 Sep;34(8-9):1163–1176. doi: 10.1042/bj0341163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen L. K., Korhonen E., Hyyppä M. Histochemical demonstration of carbonic anhydrase activity in the alimentary canal. Histochemie. 1966;6(2):168–172. doi: 10.1007/BF00308189. [DOI] [PubMed] [Google Scholar]
- Krebs H. A., Roughton F. J. Carbonic anhydrase as a tool in studying the mechanism of reactions involving H(2)CO(3), CO(2) or HCO(3)'. Biochem J. 1948;43(4):550–555. doi: 10.1042/bj0430550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINDSKOG S. Purification and properties of bovine erythrocyte carbonic anhydrase. Biochim Biophys Acta. 1960 Apr 8;39:218–226. doi: 10.1016/0006-3002(60)90156-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LUTWAK-MANN C. Carbonic anhydrase in the female reproductive tract; occurrence, distribution and hormonal dependence. J Endocrinol. 1955 Oct;13(1):26–38. doi: 10.1677/joe.0.0130026. [DOI] [PubMed] [Google Scholar]
- MAETZ J., GARCIAROMEU F. THE MECHANISM OF SODIUM AND CHLORIDE UPTAKE BY THE GILLS OF A FRESH-WATER FISH, CARASSIUS AURATUS. II. EVIDENCE FOR NH4 ION/NA ION AND HCO3 ION/C1 ION EXCHANGES. J Gen Physiol. 1964 Jul;47:1209–1227. doi: 10.1085/jgp.47.6.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALMSTROM B. G. The interaction of purified enolase with its activating metal-ions. Arch Biochem Biophys. 1953 Oct;46(2):345–363. doi: 10.1016/0003-9861(53)90207-0. [DOI] [PubMed] [Google Scholar]
- Magid E. The activity of carbonic anhydrases B and C from human erythrocytes and the inhibition of the enzymes fby copper. Scand J Haematol. 1967;4(4):257–270. doi: 10.1111/j.1600-0609.1967.tb01627.x. [DOI] [PubMed] [Google Scholar]
- McIntosh J. E. Carbonic anhydrase isoenzymes in the erythrocytes and dorsolateral prostate of the rat. Biochem J. 1969 Sep;114(3):463–476. doi: 10.1042/bj1140463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldrum N. U., Roughton F. J. Carbonic anhydrase. Its preparation and properties. J Physiol. 1933 Dec 5;80(2):113–142. doi: 10.1113/jphysiol.1933.sp003077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell C. D., Mitchell W. B., Hanahan D. J. Enzyme and hemoglobin retention in human erythrocyte stroma. Biochim Biophys Acta. 1965 Jul 8;104(2):348–358. doi: 10.1016/0304-4165(65)90340-5. [DOI] [PubMed] [Google Scholar]
- Mossberg S. M. Ammonia absorption in hamster ileum: effect of pH and total CO2 on transport in everted sacs. Am J Physiol. 1967 Nov;213(5):1327–1330. doi: 10.1152/ajplegacy.1967.213.5.1327. [DOI] [PubMed] [Google Scholar]
- PENNINGTON R. J. Biochemistry of dystrophic muscle. Mitochondrial succinate-tetrazolium reductase and adenosine triphosphatase. Biochem J. 1961 Sep;80:649–654. doi: 10.1042/bj0800649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHILPOT J. S., STANIER J. E. The choice of the suspension medium for rat-liver-cell nuclei. Biochem J. 1956 Jun;63(2):214–223. doi: 10.1042/bj0630214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RECTOR F. C., Jr, CARTER N. W., SELDIN D. W. THE MECHANISM OF BICARBONATE REABSORPTION IN THE PROXIMAL AND DISTAL TUBULES OF THE KIDNEY. J Clin Invest. 1965 Feb;44:278–290. doi: 10.1172/JCI105142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards P., Metcalfe-Gibson A., Ward E. E., Wrong O., Houghton B. J. Utilisation of ammonia nitrogen for protein synthesis in man, and the effect of protein restriction and uraemia. Lancet. 1967 Oct 21;2(7521):845–849. doi: 10.1016/s0140-6736(67)92588-3. [DOI] [PubMed] [Google Scholar]
- Roughton F. J., Booth V. H. The effect of substrate concentration, pH and other factors upon the activity of carbonic anhydrase. Biochem J. 1946;40(2):319–330. doi: 10.1042/bj0400319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEN M., DRANCE S. M., WOODFORD V. R. Separation of bovine lens carbonic anhydrase into two components. Can J Biochem Physiol. 1963 May;41:1235–1241. [PubMed] [Google Scholar]
- SHEPARD T. H. Carbonic anhydrase activity in early developing chick embryos. J Embryol Exp Morphol. 1962 Jun;10:191–201. [PubMed] [Google Scholar]
- SILEN W., HARPER H. A., MAWDSLEY D. L., WEIRICH W. L. Effect of antibacterial agents on ammonia production within the intestine. Proc Soc Exp Biol Med. 1955 Jan;88(1):138–140. doi: 10.3181/00379727-88-21516. [DOI] [PubMed] [Google Scholar]
- Severinghaus J. W., Hamilton F. N., Cotev S. Carbonic acid production and the role of carbonic anhydrase in decarboxylation in brain. Biochem J. 1969 Oct;114(4):703–705. doi: 10.1042/bj1140703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swallow J. H., Code C. F. Intestinal transmucosal fluxes of bicarbonate. Am J Physiol. 1967 Mar;212(3):717–723. doi: 10.1152/ajplegacy.1967.212.3.717. [DOI] [PubMed] [Google Scholar]
- Turbeck B. O., Foder B. Studies on a carbonic anhydrase from the midgut epithelium of larvae of lepidoptera. Biochim Biophys Acta. 1970 Jul 15;212(1):139–149. doi: 10.1016/0005-2744(70)90187-7. [DOI] [PubMed] [Google Scholar]
- Wistrand P. J., Rao S. N. Immunologic and kinetic properties of carbonic anhydrases from various tissues. Biochim Biophys Acta. 1968 Jan 22;154(1):130–144. doi: 10.1016/0005-2795(68)90265-1. [DOI] [PubMed] [Google Scholar]