Abstract
1. The pattern of depression of Ia IPSPs by volleys in recurrent motor axon collaterals was investigated in motoneurones supplying hind-limb muscles in the cat. The test IPSPs were evoked by stimulation of dorsal roots and the conditioning antidromic volleys by stimulation of motor fibres in different peripheral muscle nerves.
2. In all motor nuclei investigated the strongest depression of Ia IPSPs is evoked from motor fibres to muscles whose Ia afferents produce the IPSPs. For example, the Ia IPSP from the knee extensor recorded in motoneurones to a knee flexor is most effectively depressed by antidromic stimulation of motor fibres to the knee extensor.
3. The origin of recurrent inhibition of α-motoneurones and of Ia inhibitory interneurones with the same Ia input display a striking similarity. This suggests that the same population of Renshaw cells mediates effects to motoneurones and to Ia inhibitory interneurones.
4. The functional significance of impulses in motor axon collaterals was discussed and it was suggested that they have an important role in the control of the excitatory as well as inhibitory Ia actions to motoneurones. The recurrent inhibition may limit the Ia effects to excitation of homonymous motoneurones, which would provide optimal conditions for control of individual muscles via the γ-loop.
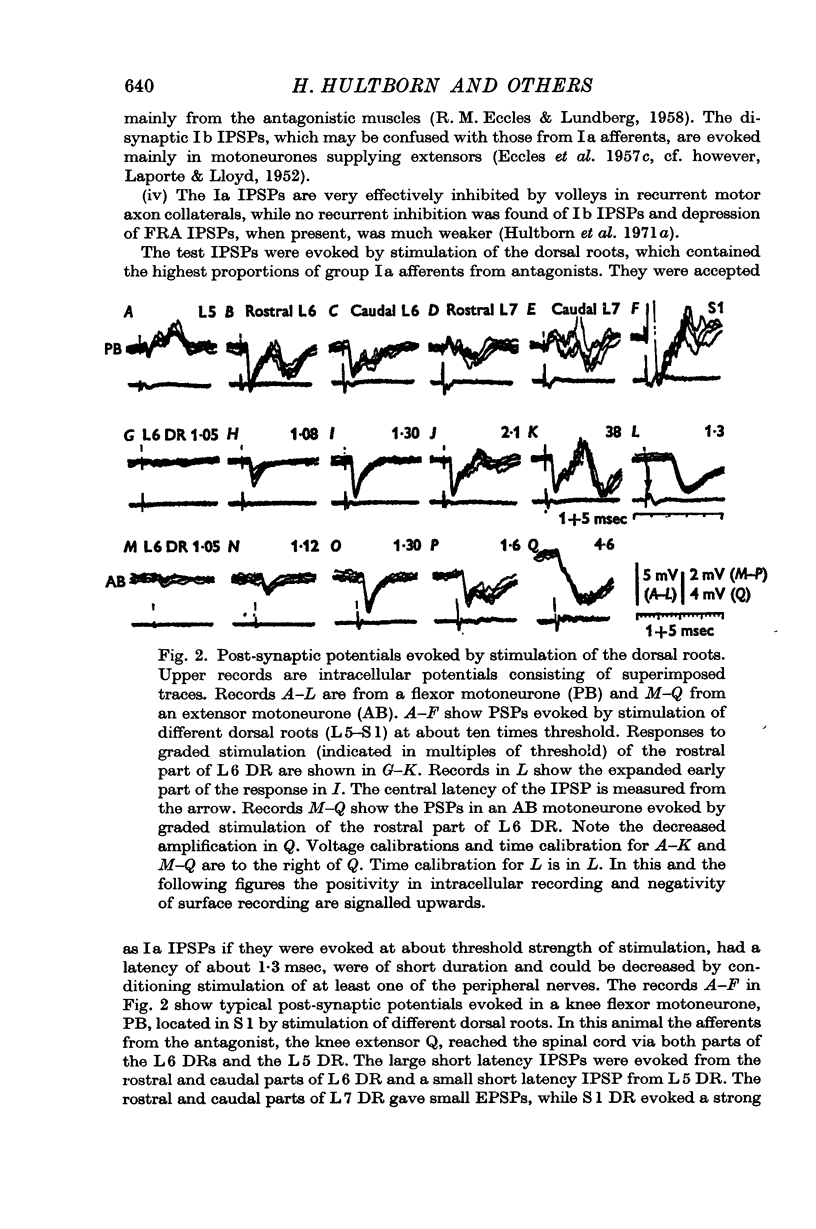
Full text
PDF


























Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andén N. E., Jukes M. G., Lundberg A., Vyklický L. The effect of DOPA on the spinal cord. 1. Influence on transmission from primary afferents. Acta Physiol Scand. 1966 Jul-Aug;67(3):373–386. doi: 10.1111/j.1748-1716.1966.tb03324.x. [DOI] [PubMed] [Google Scholar]
- BROOKS V. B., WILSON V. J. Recurrent inhibition in the cat's spinal cord. J Physiol. 1959 May 19;146(2):380–391. doi: 10.1113/jphysiol.1959.sp006199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmans J., Burke R., Lundberg A. Inhibition of transmission in the recurrent inhibitory pathway to motoneurones. Brain Res. 1969 May;13(3):600–602. doi: 10.1016/0006-8993(69)90270-4. [DOI] [PubMed] [Google Scholar]
- CURTIS D. R., PHILLIS J. W., WATKINS J. C. Cholinergic and non-cholinergic transmission in the mammalian spinal cord. J Physiol. 1961 Sep;158:296–323. doi: 10.1113/jphysiol.1961.sp006770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis D. R., Ryall R. W. The synaptic excitation of Renshaw cells. Exp Brain Res. 1966;2(1):81–96. doi: 10.1007/BF00234362. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., IGGO A., ITO M. Distribution of recurrent inhibition among motoneurones. J Physiol. 1961 Dec;159:479–499. doi: 10.1113/jphysiol.1961.sp006822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. Synaptic actions on motoneurones caused by impulses in Golgi tendon organ afferents. J Physiol. 1957 Sep 30;138(2):227–252. doi: 10.1113/jphysiol.1957.sp005849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. Synaptic actions on motoneurones in relation to the two components of the group I muscle afferent volley. J Physiol. 1957 May 23;136(3):527–546. doi: 10.1113/jphysiol.1957.sp005778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. The convergence of monosynaptic excitatory afferents on to many different species of alpha motoneurones. J Physiol. 1957 Jun 18;137(1):22–50. doi: 10.1113/jphysiol.1957.sp005794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., FATT P., KOKETSU K. Cholinergic and inhibitory synapses in a pathway from motor-axon collaterals to motoneurones. J Physiol. 1954 Dec 10;126(3):524–562. doi: 10.1113/jphysiol.1954.sp005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., FATT P., LANDGREN S. Central pathway for direct inhibitory action of impulses in largest afferent nerve fibres to muscle. J Neurophysiol. 1956 Jan;19(1):75–98. doi: 10.1152/jn.1956.19.1.75. [DOI] [PubMed] [Google Scholar]
- ECCLES R. M., LUNDBERG A. Integrative pattern of Ia synaptic actions on motoneurones of hip and knee muscles. J Physiol. 1958 Dec 4;144(2):271–298. doi: 10.1113/jphysiol.1958.sp006101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engberg I., Lundberg A. An electromyographic analysis of muscular activity in the hindlimb of the cat during unrestrained locomotion. Acta Physiol Scand. 1969 Apr;75(4):614–630. doi: 10.1111/j.1748-1716.1969.tb04415.x. [DOI] [PubMed] [Google Scholar]
- FRANK K., FUORTES M. G. Unitary activity of spinal interneurones of cats. J Physiol. 1956 Feb 28;131(2):424–435. doi: 10.1113/jphysiol.1956.sp005472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILL P. K., KUNO M. PROPERTIES OF PHRENIC MOTONEURONES. J Physiol. 1963 Sep;168:258–273. doi: 10.1113/jphysiol.1963.sp007191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granit R., Haase J., Rutledge L. T. Recurrent inhibition in relation to frequency of firing and limitation of discharge rate of extensor motoneurones. J Physiol. 1960 Dec;154(2):308–328. doi: 10.1113/jphysiol.1960.sp006581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAASE J., van der MEULEN J. Effects of supraspinal stimulation on Renshaw cells belonging to extensor motoneurones. J Neurophysiol. 1961 Sep;24:510–520. doi: 10.1152/jn.1961.24.5.510. [DOI] [PubMed] [Google Scholar]
- Hultborn H., Jankowska E., Lindström S. Recurrent inhibition from motor axon collaterals of transmission in the Ia inhibitory pathway to motoneurones. J Physiol. 1971 Jul;215(3):591–612. doi: 10.1113/jphysiol.1971.sp009487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H., Jankowska E., Lindström S. Recurrent inhibition of interneurones monosynaptically activated from group Ia afferents. J Physiol. 1971 Jul;215(3):613–636. doi: 10.1113/jphysiol.1971.sp009488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E., Jukes M. G., Lund S., Lundberg A. The effect of DOPA on the spinal cord. 5. Reciprocal organization of pathways transmitting excitatory action to alpha motoneurones of flexors and extensors. Acta Physiol Scand. 1967 Jul-Aug;70(3):369–388. doi: 10.1111/j.1748-1716.1967.tb03636.x. [DOI] [PubMed] [Google Scholar]
- KOIZUMI K., USHIYAMA J., BROOKS C. M. A study of reticular formation action on spinal interneurons and motoneurons. Jpn J Physiol. 1959 Sep 15;9:282–303. doi: 10.2170/jjphysiol.9.282. [DOI] [PubMed] [Google Scholar]
- LAPORTE Y., LLOYD D. P. C. Nature and significance of the reflex connections established by large afferent fibers of muscular origin. Am J Physiol. 1952 Jun;169(3):609–621. doi: 10.1152/ajplegacy.1952.169.3.609. [DOI] [PubMed] [Google Scholar]
- MacLean J. B., Leffman H. Supraspinal control of Renshaw cells. Exp Neurol. 1967 May;18(1):94–104. doi: 10.1016/0014-4886(67)90091-x. [DOI] [PubMed] [Google Scholar]
- ROMANES G. J. The motor cell columns of the lumbo-sacral spinal cord of the cat. J Comp Neurol. 1951 Apr;94(2):313–363. doi: 10.1002/cne.900940209. [DOI] [PubMed] [Google Scholar]
- SEARS T. A. SOME PROPERTIES AND REFLEX CONNEXIONS OF RESPIRATORY MOTONEURONES OF THE CAT'S THORACIC SPINAL CORD. J Physiol. 1964 Dec;175:386–403. doi: 10.1113/jphysiol.1964.sp007523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington C. S. Flexion-reflex of the limb, crossed extension-reflex, and reflex stepping and standing. J Physiol. 1910 Apr 26;40(1-2):28–121. doi: 10.1113/jphysiol.1910.sp001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C., Wilson V. J. Recurrent interactions between motoneurons of known location in the cervical cord of the cat. J Neurophysiol. 1967 Jul;30(4):661–674. doi: 10.1152/jn.1967.30.4.661. [DOI] [PubMed] [Google Scholar]
- WILSON V. J., TALBOT W. H., DIECKE F. P. Distribution of recurrent facilitation and inhibition in cat spinal cord. J Neurophysiol. 1960 Mar;23:144–153. doi: 10.1152/jn.1960.23.2.144. [DOI] [PubMed] [Google Scholar]
- WILSON V. J., TALBOT W. H., KATO M. INHIBITORY CONVERGENCE UPON RENSHAW CELLS. J Neurophysiol. 1964 Nov;27:1063–1079. doi: 10.1152/jn.1964.27.6.1063. [DOI] [PubMed] [Google Scholar]
- WILSON V. J., TALBOT W. H. Recurrent conditioning in the cat spinal cord. Differential effect of meprobamate on recurrent facilitation and inhibition. J Gen Physiol. 1960 Jan;43:495–502. doi: 10.1085/jgp.43.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]


