Abstract
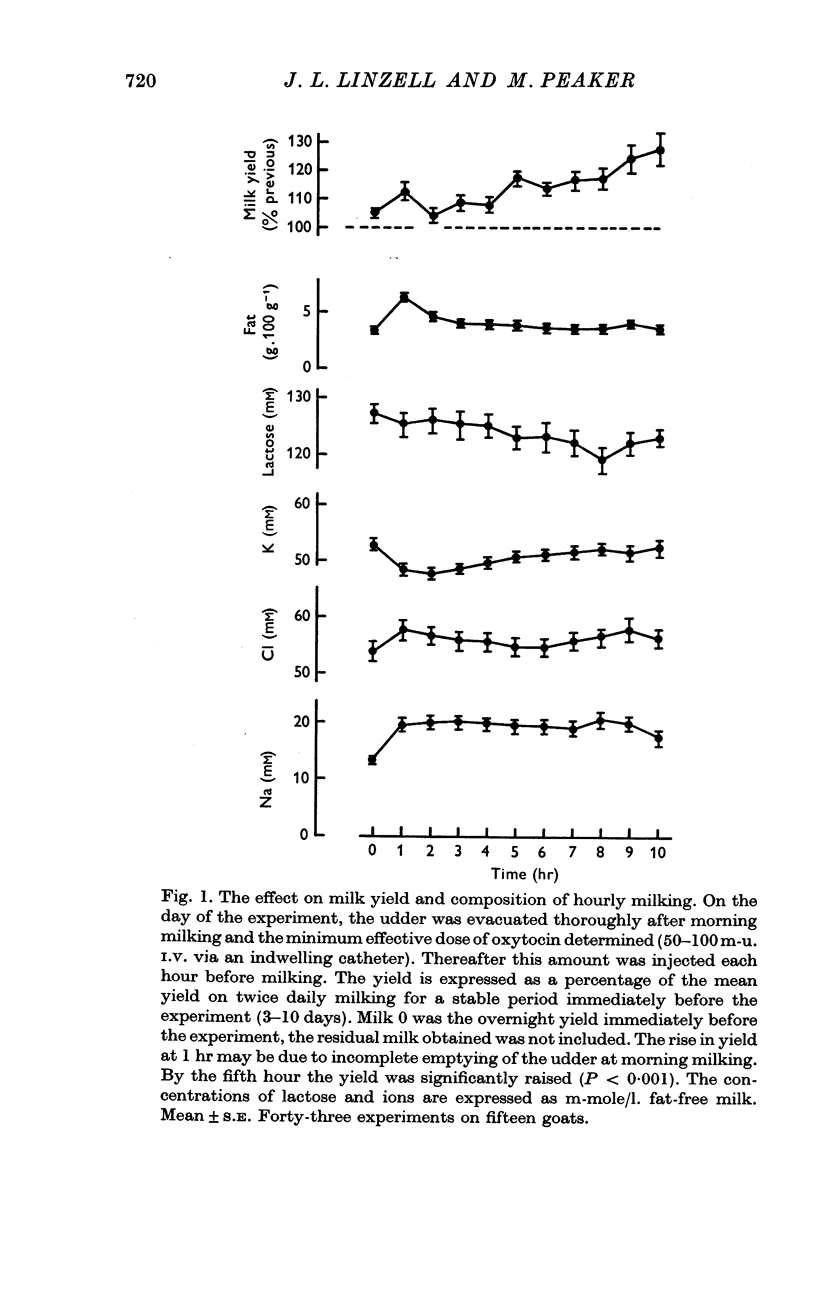
1. When goats were milked each hour after being given a dose of synthetic oxytocin within the range thought to be released by the pituitary, there was a progressive rise in milk yield becoming statistically significant by 5 hr. The effect was reduced if the milk was not removed from the gland each hour.
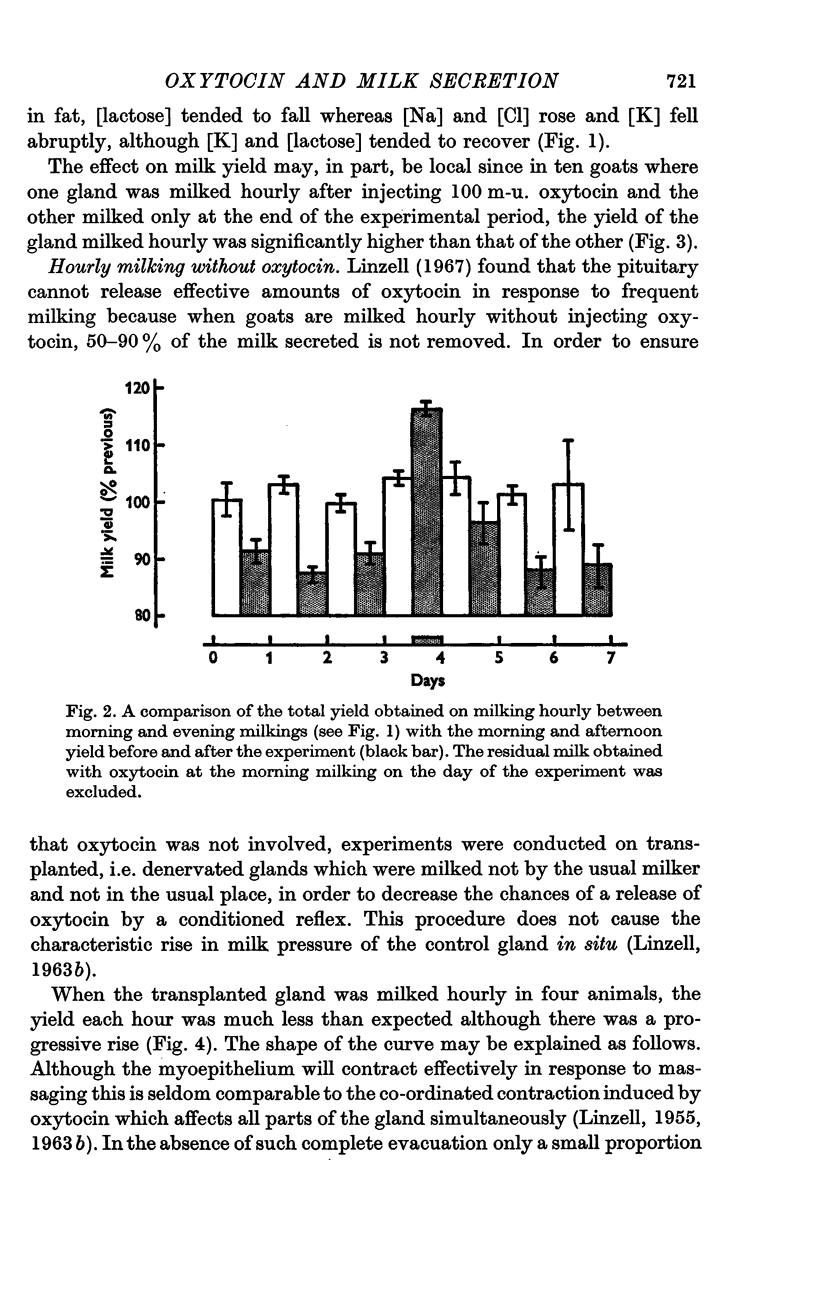
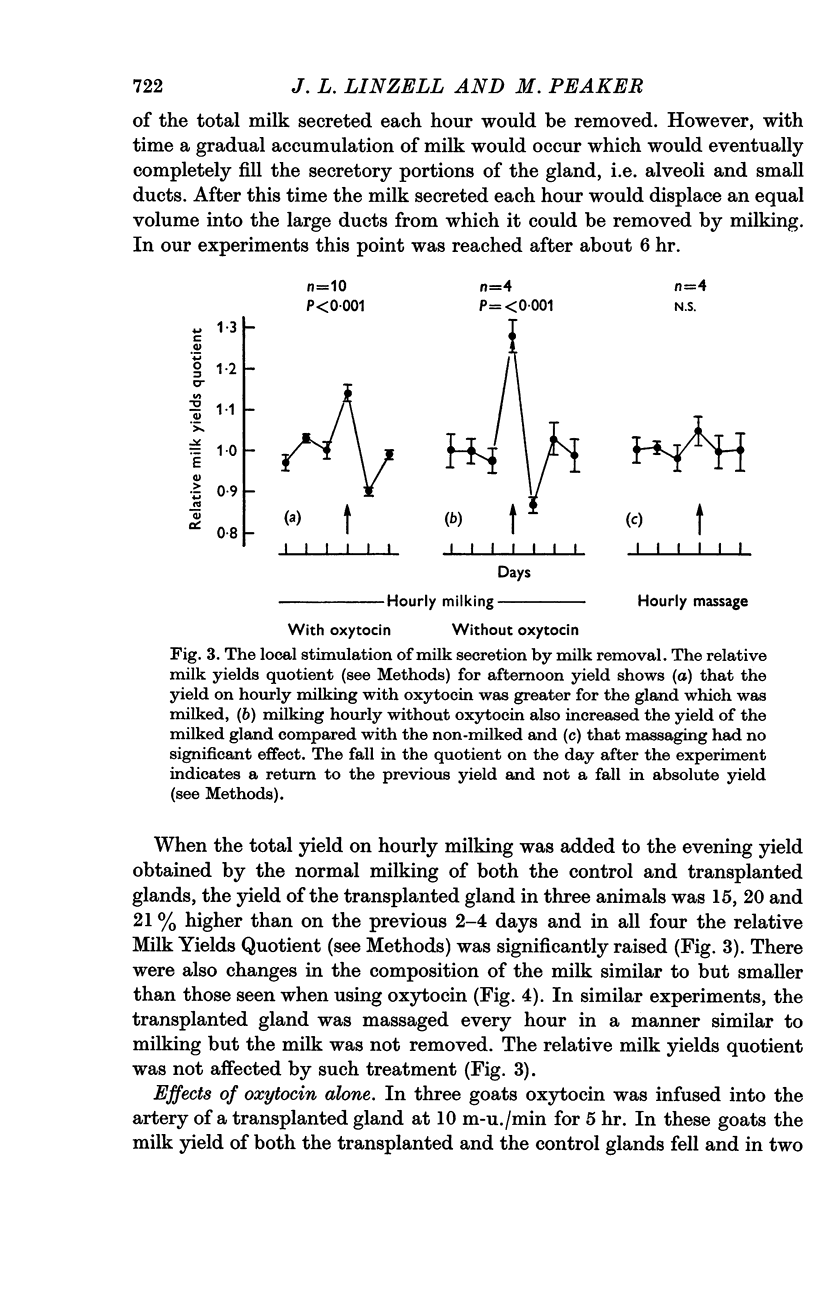
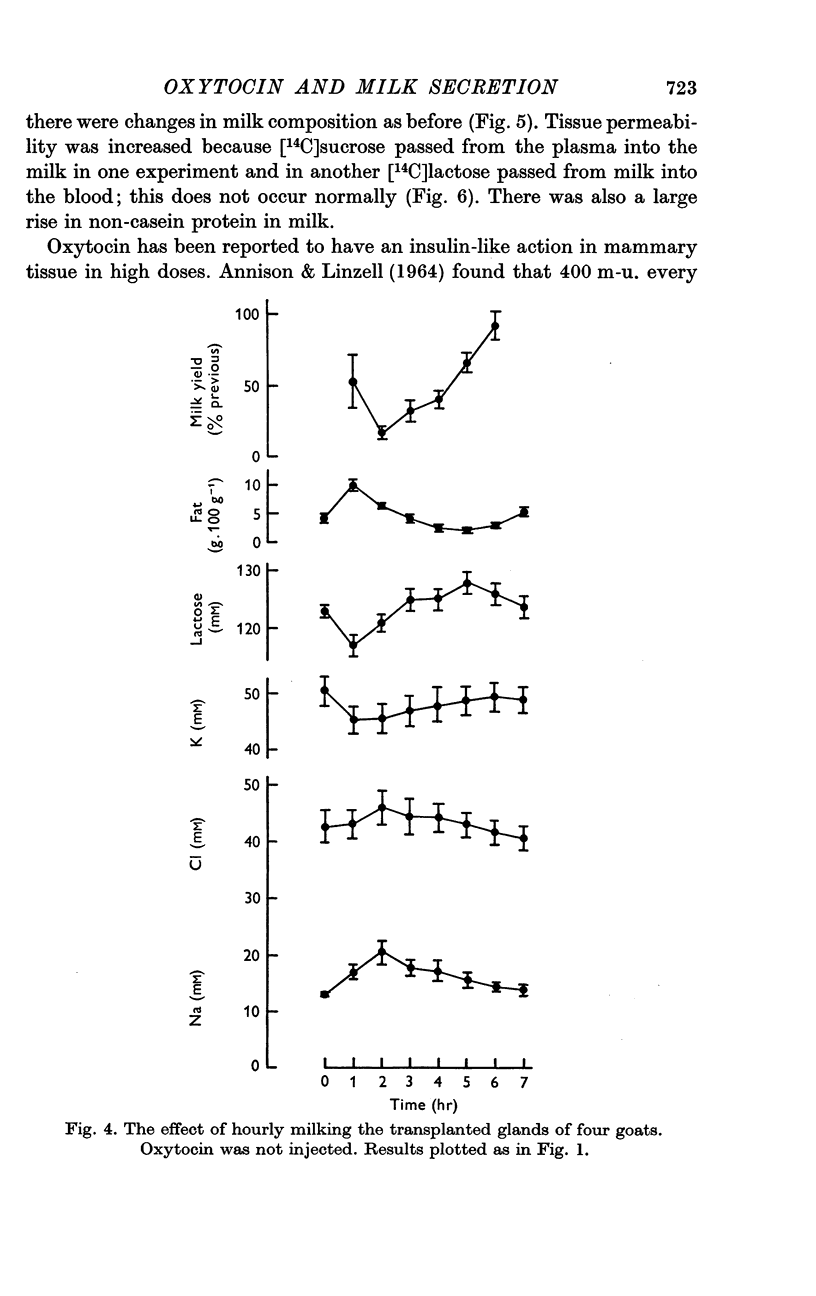
2. Milking transplanted glands each hour without injecting oxytocin also increased milk yield. The yield of the unmilked glands on the same animals was not affected. Massaging the transplanted glands had no effect on the milk yield.
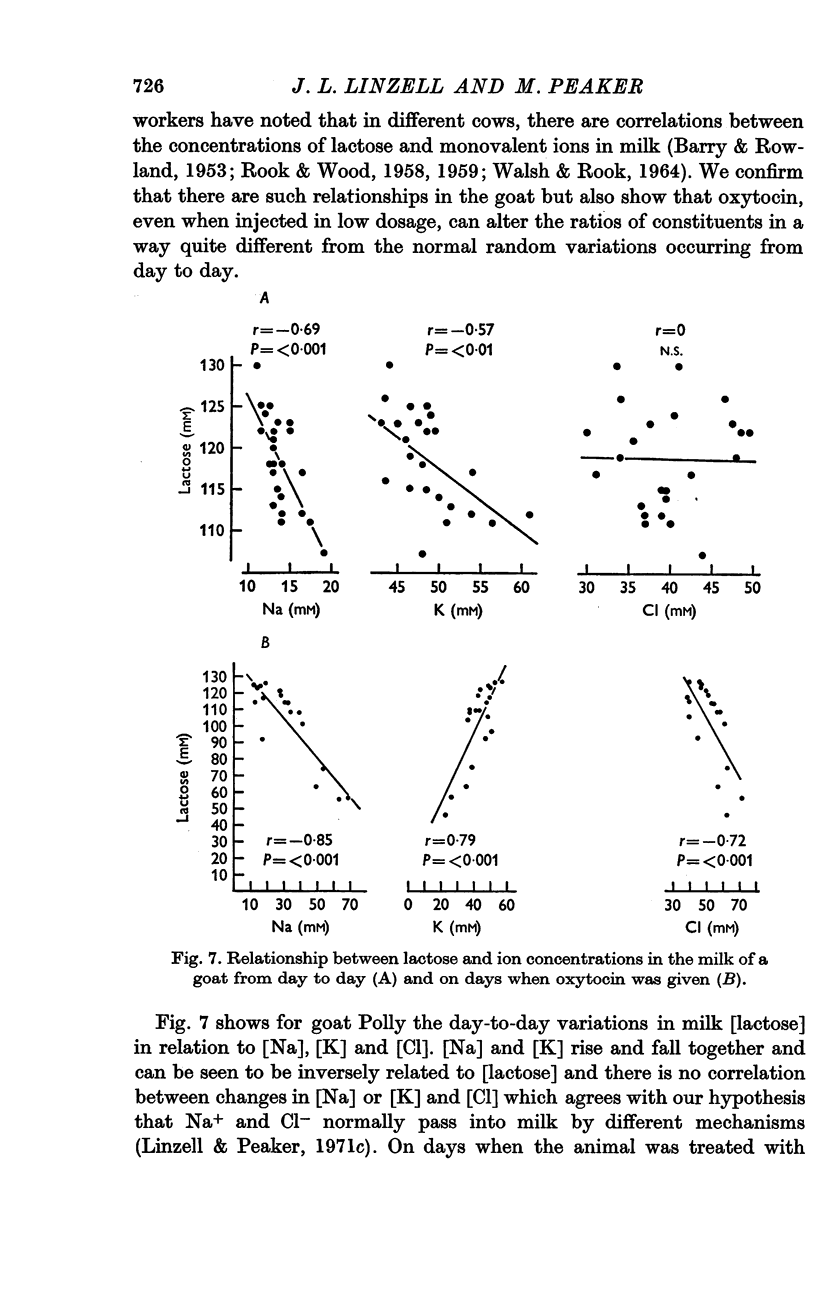
3. Oxytocin treatment and, to a lesser extent, frequent milking without oxytocin, altered milk composition. [Na], [Cl] and [non-casein protein] increased; [K] and [lactose] decreased.
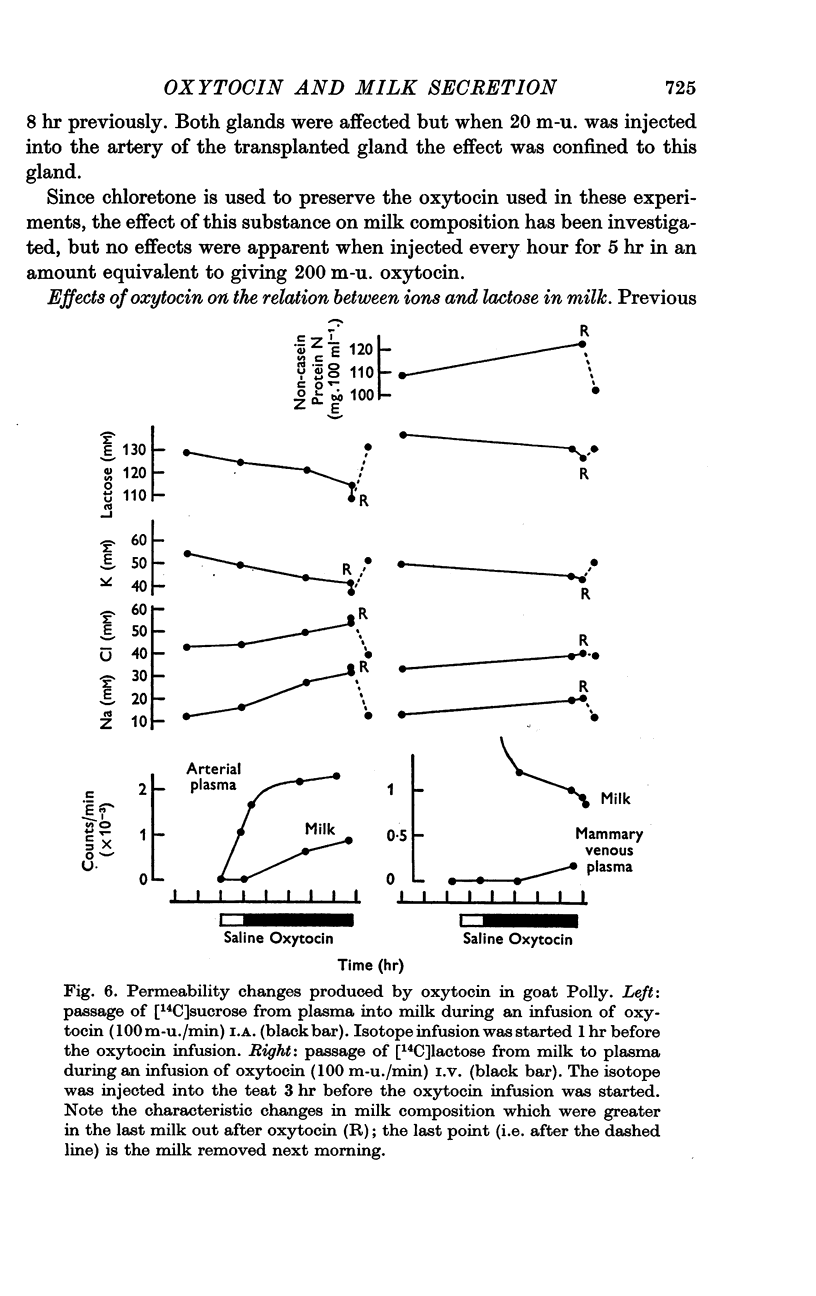
4. Oxytocin infusions permitted the leakage of [14C]lactose from milk to plasma and [14C]sucrose from plasma to milk.
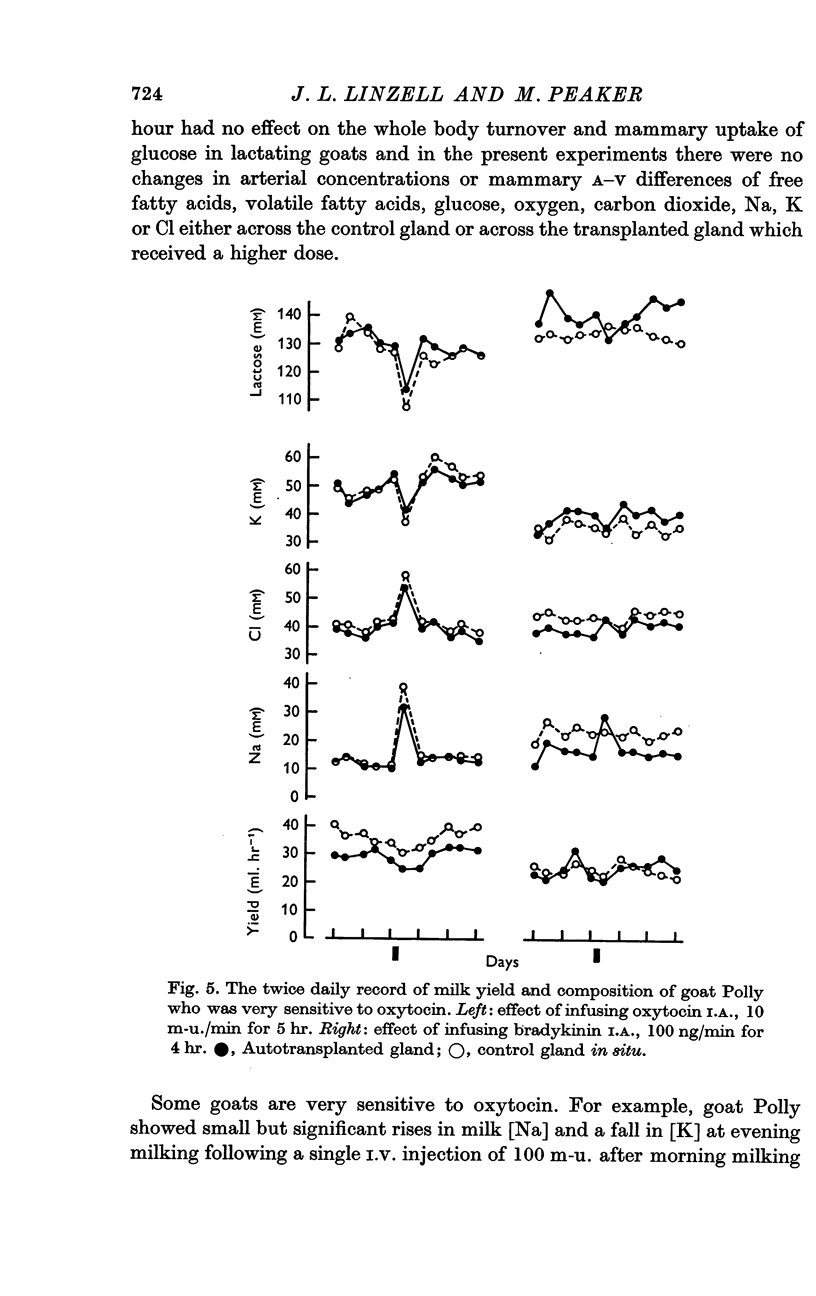
5. In some goats very small doses of oxytocin caused changes in milk composition and in one such animal these changes were mimicked by the close arterial infusion of bradykinin.
6. Reasons are given for believing that the changes in composition are incidental to the main action of oxytocin in expelling milk and could be caused by a small number of leaks between the tight junctions connecting secretory cells.
7. The increase in the rate of milk secretion following milk removal is probably of greater physiological significance than the small changes in milk composition and supports Levy's idea of a local negative feed-back via a chemical component of milk.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANNISON E. F., LINZELL J. L. THE OXIDATION AND UTILIZATION OF GLUCOSE AND ACETATE BY THE MAMMARY GLAND OF THE GOAT IN RELATION TO THEIR OVER-ALL METABOLISM AND MILK FORMATION. J Physiol. 1964 Dec;175:372–385. doi: 10.1113/jphysiol.1964.sp007522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARRY J. M., ROWLAND S. J. Variations in the ionic and lactose concentrations of milk. Biochem J. 1953 Jul;54(4):575–578. doi: 10.1042/bj0540575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENSON G. K., FOLLEY S. J. The effect of oxytocin on mammary gland involution in the rat. J Endocrinol. 1957 Dec;16(2):189–201. doi: 10.1677/joe.0.0160189. [DOI] [PubMed] [Google Scholar]
- Bryant G. D., Linzell J. L., Greenwood F. C. Plasma prolactin in goats measured by radioimmunoassay: the effects of teat stimulation, mating behavior, stress, fasting and of oxytocin, insulin and glucose injections. Hormones. 1970;1(1):26–35. doi: 10.1159/000178171. [DOI] [PubMed] [Google Scholar]
- CROSS B. A. Sympathetico-adrenal inhibition of the neurohypophysial milk-ejection mechanism. J Endocrinol. 1953 Jan;9(1):7–18. [PubMed] [Google Scholar]
- DENAMUR R. Action de doses répétées d'ocytocine sur la sécretion du lait chez la chèvre. C R Seances Soc Biol Fil. 1953 Jan;147(1-2):88–92. [PubMed] [Google Scholar]
- DENAMUR R., MARTINET J. [Action of oxytocin on milk secretion in the sheep]. Ann Endocrinol (Paris) 1961 Sep-Oct;22:777–781. [PubMed] [Google Scholar]
- HARRIS G. W. The central nervous system, neurohypophysis and milk ejection. Proc R Soc Lond B Biol Sci. 1958 Dec 17;149(936):336–353. doi: 10.1098/rspb.1958.0074. [DOI] [PubMed] [Google Scholar]
- LINZELL J. L. Mammary-gland blood flow and oxygen, glucose and volatile fatty acid uptake in the conscious goat. J Physiol. 1960 Oct;153:492–509. doi: 10.1113/jphysiol.1960.sp006550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINZELL J. L. Physiology of the mammary glands. Physiol Rev. 1959 Jul;39(3):534–576. doi: 10.1152/physrev.1959.39.3.534. [DOI] [PubMed] [Google Scholar]
- LINZELL J. L. Some observations on the contractile tissue of the mammary glands. J Physiol. 1955 Nov 28;130(2):257–267. doi: 10.1113/jphysiol.1955.sp005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzell J. L., Peaker M. Intracellular concentrations of sodium, potassium and chloride in the lactating mammary gland and their relation to the secretory mechanism. J Physiol. 1971 Aug;216(3):683–700. doi: 10.1113/jphysiol.1971.sp009547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzell J. L., Peaker M. The permeability of mammary ducts. J Physiol. 1971 Aug;216(3):701–716. doi: 10.1113/jphysiol.1971.sp009548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzell J. L. The effect of very frequent milking and of oxytocin on the yield and composition of milk in fed and fasted goats. J Physiol. 1967 May;190(2):333–346. doi: 10.1113/jphysiol.1967.sp008212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell A. L., Rothera A. C. The action of pituitrin on the secretion of milk. J Physiol. 1915 Aug 31;49(6):483–491. doi: 10.1113/jphysiol.1915.sp001723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morag M., Brick D. The stimulation and inhibition of milk secretion by oxytocin in the rat. Life Sci. 1969 Feb 1;8(3):143–150. doi: 10.1016/0024-3205(69)90087-3. [DOI] [PubMed] [Google Scholar]
- Peaker M. Intracellular concentrations of sodium, potassium and chloride in the salt-gland of the domestic goose and their relation to the secretory mechanism. J Physiol. 1971 Mar;213(2):399–410. doi: 10.1113/jphysiol.1971.sp009389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGERS T. A., KLEIBER M. Milk fat synthesis from acetate in mammary gland of the cow. Proc Soc Exp Biol Med. 1957 Apr;94(4):705–708. doi: 10.3181/00379727-94-23059. [DOI] [PubMed] [Google Scholar]
- ROOK J. A., WOOD M. Interrelationships of the concentrations of sodium, potassium, lactose and water in milk. Nature. 1958 May 3;181(4618):1284–1285. doi: 10.1038/1811284a0. [DOI] [PubMed] [Google Scholar]
- ROOK J. A., WOOD M. Potassium and lactose in milk in relation to the physiology of milk secretion. Nature. 1959 Aug 22;184(Suppl 9):647–648. doi: 10.1038/184647b0. [DOI] [PubMed] [Google Scholar]
- Reynaert H., Peeters G., Verbeke R., Houvenaghel A. Further studies of the physiology of plasma kinins and kallikreins in the udder of ruminants. Arch Int Pharmacodyn Ther. 1968 Dec;176(2):473–475. [PubMed] [Google Scholar]
- Reynolds M., Linzell J. L., Rasmussen F. Comparison of four methods for measuring mammary blood flow in conscious goats. Am J Physiol. 1968 Jun;214(6):1415–1424. doi: 10.1152/ajplegacy.1968.214.6.1415. [DOI] [PubMed] [Google Scholar]
- SELDINGER S. I. Catheter replacement of the needle in percutaneous arteriography; a new technique. Acta radiol. 1953 May;39(5):368–376. doi: 10.3109/00016925309136722. [DOI] [PubMed] [Google Scholar]
- WALSH J. P., ROOK J. A. RATIO OF POTASSIUM TO LACTOSE IN COWS' MILK AND ITS GENETIC BASIS. Nature. 1964 Oct 24;204:353–355. doi: 10.1038/204353a0. [DOI] [PubMed] [Google Scholar]
- Wooding F. B., Peaker LINZELL J. L. Theories of milk secretion: evidence from e electron microscopic examination of milk. Nature. 1970 May 23;226(5247):762–764. doi: 10.1038/226762a0. [DOI] [PubMed] [Google Scholar]


