Abstract
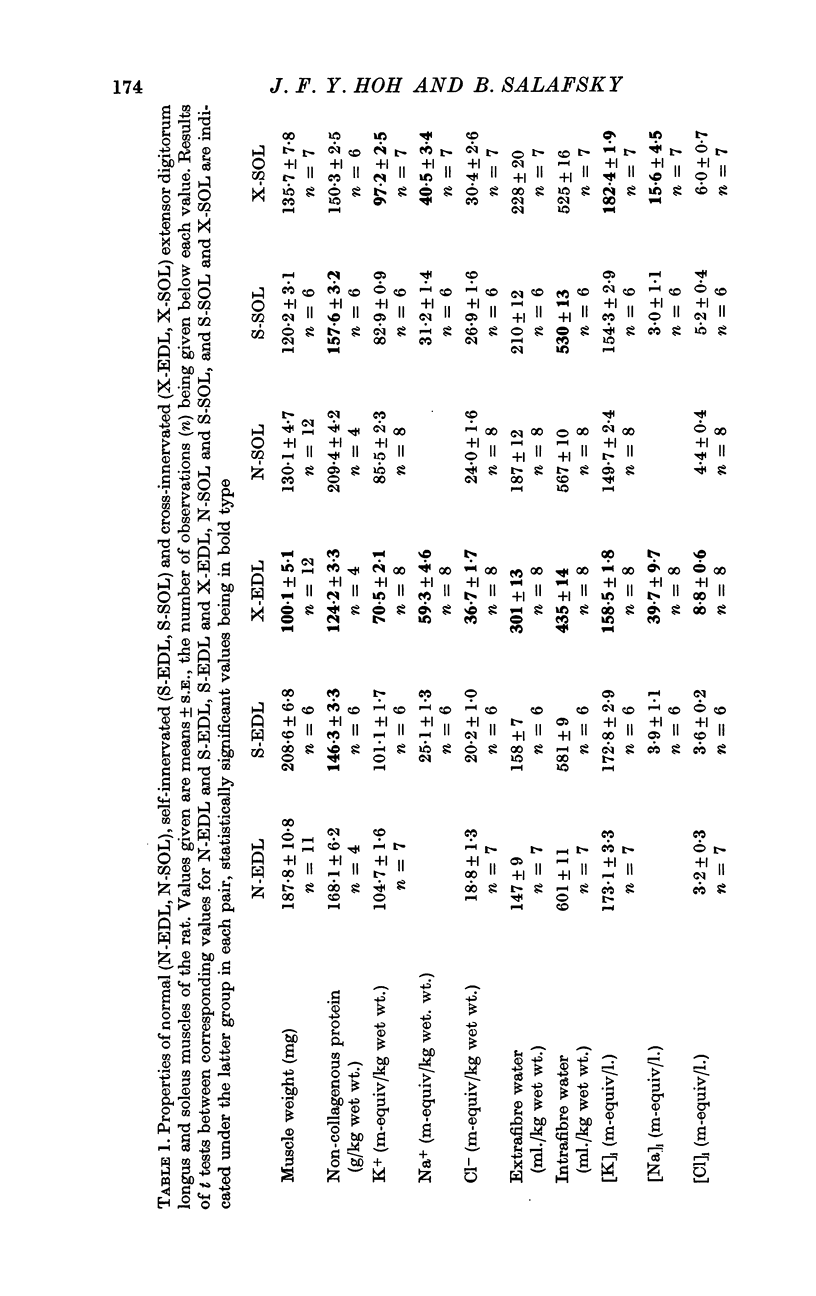
1. Electrolytes of normal, self-innervated and cross-innervated extensor digitorum longus and soleus muscles of rats have been determined.
2. [K]i was 173 m-equiv/l. for both normal and self-innervated extensor digitorum longus. In cross-innervated extensor digitorum longus it was reduced to 159 m-equiv/l.
3. For normal and self-innervated soleus, [K]i was 150 m-equiv/l. and 154 m-equiv/l. respectively. In cross-innervated soleus it was increased to 182 m-equiv/l.
4. The content and distribution of most other electrolytes of cross-innervated soleus, as well as its weight, were not significantly different from those of controls. On the other hand, cross-innervated extensor digitorum longus weighed about half as much as controls and contained markedly elevated Na+, Cl- and extrafibre water and reduced non-collagenous protein and intrafibre water.
5. It is concluded that [K]i of fast-twitch and slow-twitch muscle fibres are under neural regulation. Possible mechanisms for this regulation are discussed.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albuquerque E. X., Thesleff S. Influence of phospholipase C on some electrical properties of the skeletal muscle membrane. J Physiol. 1967 May;190(1):123–137. doi: 10.1113/jphysiol.1967.sp008197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAKER N., BLAHD W. H., HART P. Concentrations of K and Na in skeletal muscle of mice with a hereditary myopathy (Dystrophia muscularis). Am J Physiol. 1958 Jun;193(3):530–533. doi: 10.1152/ajplegacy.1958.193.3.530. [DOI] [PubMed] [Google Scholar]
- BLAXTER K. L., WOOD W. A. Composition of the tissues of normal and dystrophic calves. Br J Nutr. 1952;6(2):144–163. doi: 10.1079/bjn19520015. [DOI] [PubMed] [Google Scholar]
- CONWAY E. J. Nature and significance of concentration relations of potassium and sodium ions in skeletal muscle. Physiol Rev. 1957 Jan;37(1):84–132. doi: 10.1152/physrev.1957.37.1.84. [DOI] [PubMed] [Google Scholar]
- Close R. Dynamic properties of fast and slow skeletal muscles of the rat after nerve cross-union. J Physiol. 1969 Oct;204(2):331–346. doi: 10.1113/jphysiol.1969.sp008916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close R., Hoh J. F. Post-tetanic potentiation of twitch contractions of cross-innervated rat fast and slow muscles. Nature. 1969 Jan 11;221(5176):179–181. doi: 10.1038/221179a0. [DOI] [PubMed] [Google Scholar]
- Dockry M., Kernan R. P., Tangney A. Active transport of sodium and potassium in mammalian skeletal muscle and its modification by nerve and by cholinergic and adrenergic agents. J Physiol. 1966 Sep;186(1):187–200. doi: 10.1113/jphysiol.1966.sp008028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EICHELBERGER L., AKESON W. H., ROMA M. Effects of denervation on the histochemical characterization of skeletal muscle during growth. Am J Physiol. 1956 May;185(2):287–298. doi: 10.1152/ajplegacy.1956.185.2.287. [DOI] [PubMed] [Google Scholar]
- FLEAR C. T., FLORENCE I. A rapid and micro method for the analysis of skeletal muscle for water, sodium, potassium, chloride and fat. Clin Chim Acta. 1961 Jan;6:129–135. doi: 10.1016/0009-8981(61)90046-8. [DOI] [PubMed] [Google Scholar]
- Fedorov V. V. Osobennosti biopotentsialov volokon bystrykh i medlennykh skeletnykh myshts krysy. Fiziol Zh SSSR Im I M Sechenova. 1969 May;55(5):588–596. [PubMed] [Google Scholar]
- Guth L. "Trophic" influences of nerve on muscle. Physiol Rev. 1968 Oct;48(4):645–687. doi: 10.1152/physrev.1968.48.4.645. [DOI] [PubMed] [Google Scholar]
- HORVATH B., BERG L., CUMMINGS D. J., SHY G. M. Muscular dystrophy: cation concentrations in residual muscle. J Appl Physiol. 1955 Jul;8(1):22–30. doi: 10.1152/jappl.1955.8.1.22. [DOI] [PubMed] [Google Scholar]
- Kernan R. P. Accumulation of caesium and rubidium in vivo by red and white muscles of the rat. J Physiol. 1969 Sep;204(1):195–205. doi: 10.1113/jphysiol.1969.sp008908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi N., Yonemura K. The extracellular space in red and white muscles of the rat. Jpn J Physiol. 1967 Dec 15;17(6):698–707. doi: 10.2170/jjphysiol.17.698. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lafferty K. J., Jones M. A. Reactions of the graft versus host (GVH) type. Aust J Exp Biol Med Sci. 1969 Feb;47(1):17–54. doi: 10.1038/icb.1969.3. [DOI] [PubMed] [Google Scholar]
- MANERY J. F. Water and electrolyte metabolism. Physiol Rev. 1954 Apr;34(2):334–417. doi: 10.1152/physrev.1954.34.2.334. [DOI] [PubMed] [Google Scholar]
- Miledi R., Stefani E., Zelená J. Neural control of acetylcholine-sensitivity in rat muscle fibers. Nature. 1968 Nov 2;220(5166):497–498. doi: 10.1038/220497a0. [DOI] [PubMed] [Google Scholar]
- Miledi R., Zelená J. Sensitivity to acetylcholine in rat slow muscle. Nature. 1966 May 21;210(5038):855–856. doi: 10.1038/210855a0. [DOI] [PubMed] [Google Scholar]
- Prewitt M. A., Salafsky B. Enzymic and histochemical changes in fast and slow muscles after cross innervation. Am J Physiol. 1970 Jan;218(1):69–74. doi: 10.1152/ajplegacy.1970.218.1.69. [DOI] [PubMed] [Google Scholar]
- SRETER F. A., WOO G. CELL WATER, SODIUM, AND POTASSIUM IN RED AND WHITE MAMMALIAN MUSCLES. Am J Physiol. 1963 Dec;205:1290–1294. doi: 10.1152/ajplegacy.1963.205.6.1290. [DOI] [PubMed] [Google Scholar]
- YOUNG H. L., YOUNG W., EDELMAN I. S. Electrolyte and lipid composition of skeletal and cardiac muscle in mice with hereditary muscular dystrophy. Am J Physiol. 1959 Aug;197:487–490. doi: 10.1152/ajplegacy.1959.197.2.487. [DOI] [PubMed] [Google Scholar]
- Yonemura K. Resting and action potentials in red and white muscles of the rat. Jpn J Physiol. 1967 Dec 15;17(6):708–719. doi: 10.2170/jjphysiol.17.708. [DOI] [PubMed] [Google Scholar]



