Abstract
1. Action potentials have been recorded extracellularly from individual preganglionic nerve terminals in the lumbar sympathetic chain of frogs (cf. Koketsu & Nishi, 1968).
2. The action potentials were unaffected by changes in external Mg2+ or Ca2+ concentration, but were attenuated or even abolished by acetylcholine or carbachol. The effect on the presynaptic action potential was transient, in contrast to the blockade of synaptic transmission caused by these drugs, which as is well known persists indefinitely in their presence.
3. In the presence of tubocurarine (5 × 10-5 M), acetylcholine and carbachol had no effect on the presynaptic action potential.
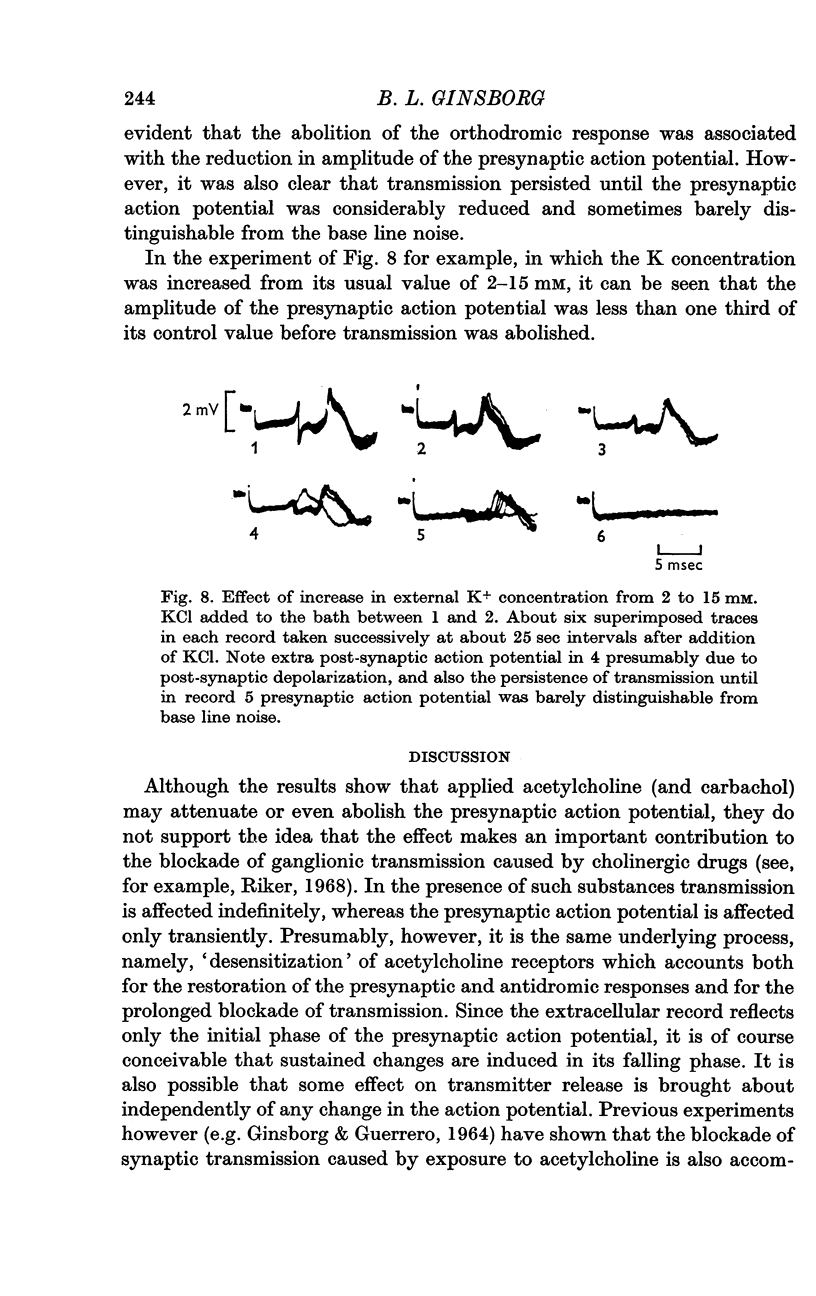
4. The action potentials were reduced by an increase in external K+ concentration and abolished at between 9 and 16 mM K+. Synaptic transmission persisted until the amplitude of the action potential was reduced to less than one third of its control value.
5. It is concluded that although the presynaptic nerve is endowed with acetylcholine receptors, they are not the source of the long lasting blockade of synaptic transmission caused by cholinergic substances.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLACKMAN J. G., GINSBORG B. L., RAY C. Some effects of changes in ionic concentration on the action potential of sympathetic ganglion cells in the frog. J Physiol. 1963 Jul;167:374–388. doi: 10.1113/jphysiol.1963.sp007156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACKMAN J. G., GINSBORG B. L., RAY C. Synaptic transmission in the sympathetic ganglion of the frog. J Physiol. 1963 Jul;167:355–373. doi: 10.1113/jphysiol.1963.sp007155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Jones K. B., Halliwell J. V., Quilliam J. P. Evidence against a presynaptic action of acetylcholine during ganglionic transmission. Nature. 1970 Jun 6;226(5249):958–959. doi: 10.1038/226958a0. [DOI] [PubMed] [Google Scholar]
- GINSBORG B. L., GUERRERO S. ON THE ACTION OF DEPOLARIZING DRUGS ON SYMPATHETIC GANGLION CELLS OF THE FROG. J Physiol. 1964 Aug;172:189–206. doi: 10.1113/jphysiol.1964.sp007412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. PROPAGATION OF ELECTRIC ACTIVITY IN MOTOR NERVE TERMINALS. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:453–482. doi: 10.1098/rspb.1965.0015. [DOI] [PubMed] [Google Scholar]
- KOELLE G. B. A new general concept of the neurohumoral functions of acetylcholine and acetylcholinesterase. J Pharm Pharmacol. 1962 Feb;14:65–90. doi: 10.1111/j.2042-7158.1962.tb11057.x. [DOI] [PubMed] [Google Scholar]
- Koketsu K., Nishi S. Cholinergic receptors at sympathetic preganglionic nerve terminals. J Physiol. 1968 May;196(2):293–310. doi: 10.1113/jphysiol.1968.sp008508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISHI S., KOKETSU K. Electrical properties and activities of single sympathetic neurons in frogs. J Cell Comp Physiol. 1960 Feb;55:15–30. doi: 10.1002/jcp.1030550104. [DOI] [PubMed] [Google Scholar]
- Riker W. K. Ganglion cell depolarization and transmission block by ACh: independent events. J Pharmacol Exp Ther. 1968 Feb;159(2):345–352. [PubMed] [Google Scholar]
- Tosaka T., Chichibu S., Libet B. Intracellular analysis of slow inhibitors and excitatory postsynaptic potentials in sympathetic ganglia of the frog. J Neurophysiol. 1968 May;31(3):396–409. doi: 10.1152/jn.1968.31.3.396. [DOI] [PubMed] [Google Scholar]



