Abstract
Ultrafine particles (UFPs; aerodynamic diameter < 100 nm) may contribute to the respiratory and cardiovascular morbidity and mortality associated with particulate air pollution. We tested the hypothesis that inhalation of carbon UFPs has vascular effects in healthy and asthmatic subjects, detectable as alterations in blood leukocyte expression of adhesion molecules. Healthy subjects inhaled filtered air and freshly generated elemental carbon particles (count median diameter ~ 25 nm, geometric standard deviation ~ 1.6), for 2 hr, in three separate protocols: 10 μg/m3 at rest, 10 and 25 μg/m3 with exercise, and 50 μg/m3 with exercise. In a fourth protocol, subjects with asthma inhaled air and 10 μg/m3 UFPs with exercise. Peripheral venous blood was obtained before and at intervals after exposure, and leukocyte expression of surface markers was quantitated using multiparameter flow cytometry. In healthy subjects, particle exposure with exercise reduced expression of adhesion molecules CD54 and CD18 on monocytes and CD18 and CD49d on granulocytes. There were also concentration-related reductions in blood monocytes, basophils, and eosinophils and increased lymphocyte expression of the activation marker CD25. In subjects with asthma, exposure with exercise to 10 μg/m3 UFPs reduced expression of CD11b on monocytes and eosinophils and CD54 on granulocytes. Particle exposure also reduced the percentage of CD4+ T cells, basophils, and eosinophils. Inhalation of elemental carbon UFPs alters peripheral blood leukocyte distribution and expression of adhesion molecules, in a pattern consistent with increased retention of leukocytes in the pulmonary vascular bed.
Keywords: blood leukocytes, human, monocytes, ultrafine particles
Exposure to particulate matter (PM) air pollution is associated with increased respiratory and cardiovascular morbidity and mortality (Peters et al. 2000, 2001a; Pope et al. 2004). Plausible mechanisms explaining the cardiovascular effects of particle exposure have not been clearly defined (Utell et al. 2002). However, recent studies provide evidence that PM exposure is associated with systemic inflammation and changes in vascular function that have been implicated in the pathophysiology of cardiovascular disease, providing clues to possible mechanisms. PM exposure has been associated with increased systolic blood pressure (Ibald-Mulli et al. 2001), plasma viscosity (Peters et al. 1997a), C-reactive protein (Peters et al. 2001b), fibrinogen (Pekkanen et al. 2000), and release of leukocytes from the bone marrow (Mukae et al. 2001; Tan et al. 2000). Increases in ambient concentrations of PM were associated with increased blood leukocyte and platelet counts, as well as fibrinogen (Schwartz 2001). Brook et al. (2002) found evidence for systemic vasoconstriction in resting human subjects exposed to concentrated ambient air particles and ozone.
Ultrafine particles (UFPs), defined as particles with a diameter < 100 nm, have been hypothesized as contributors to cardiovascular effects of PM (Seaton et al. 1995) because, compared with fine particles at similar mass concentrations, they have greater pulmonary deposition efficiency (Chalupa et al. 2004; Daigle et al. 2003), induce more pulmonary inflammation (Li et al. 1999; Oberdörster et al. 1995), have enhanced oxidant capacity (Brown et al. 2001; Li et al. 2003), have a higher propensity to penetrate the epithelium and reach interstitial sites (Stearns et al. 1994), and may even enter the systemic circulation in humans (Nemmar et al. 2002; Oberdörster et al. 2002).
Relatively few epidemiologic studies have examined the health effects of UFP exposure because most ambient air monitoring measures particle mass, and there is relatively poor correlation between particle mass (dominated by fine particles) and particle number (dominated by UFPs). However, a recent study in Erfurt, Germany, found associations between ambient UFPs and mortality (Wichmann et al. 2000). In a study of patients with stable coronary artery disease (Pekkanen et al. 2002), investigators performed repeated exercise tests concurrent with monitoring of ambient particle mass and number counts. Significant independent effects were found for both fine particles and UFPs on the degree of ST-segment depression on the electrocardiogram during exercise.
Asthma, a disease characterized by airway inflammation, confers an increased risk for PM health effects (Atkinson et al. 2001; Lipsett et al. 1997; Tolbert et al. 2000). There is evidence for activation of lung leukocytes and pulmonary vascular endothelium in subjects with asthma, particularly during exacerbations (Ohkawara et al. 1995). Activation of T-lymphocytes with production of “type 2” inflammatory cytokines drives the recruitment and retention of eosinophils in the airway, which contribute to the chronic epithelial injury characteristic of this disease (Corrigan and Kay 1990; Wilson et al. 1992). Treatment with inhaled corticosteroids reduces expression of activation markers CD25 and human leukocyte antigen (HLA)-DR in lung lymphocytes and also reduces HLA-DR expression in blood lymphocytes (Wilson et al. 1994). In asthma, blood CD4+ T cells express increased mRNA for interleukin (IL)-4, IL-5, and granulocyte macrophage colony stimulating factor, and IL-5 mRNA expression correlates with asthma severity and eosinophilia (Corrigan et al. 1995). Allergen challenge in subjects with asthma causes a reduction in blood CD4+ T cells (Walker et al. 1992) and an increase in airway CD4+ cells (Virchow et al. 1995). UFP exposure may worsen asthma by further shifting lymphocyte responses to the type 2 phenotype, by further activating resident lymphocytes, by increasing the likelihood that lymphocytes will encounter antigen, and/or by increasing penetration of allergen through an injured epithelium.
We have initiated controlled exposure studies with carbon UFPs in humans, as a surrogate for environmental UFPs, demonstrating that UFPs have a high pulmonary deposition efficiency in healthy subjects (Daigle et al. 2003), which is further increased in subjects with asthma (Chalupa et al. 2004). Exposure to 50 μg/m3 carbon UFPs caused a reduction in the pulmonary diffusing capacity for carbon monoxide (Pietropaoli et al. 2004b) associated with reductions in the systemic vascular response to increased flow (Pietropaoli et al. 2004a), without significant effects on symptoms, airway inflammation, lung function, or markers of blood coagulation (Pietropaoli et al. 2004c). We hypothesized that inhalation of UFPs alters vascular function, detectable as alterations in blood leukocyte distribution, activation, and expression of adhesion molecules. We further hypothesized that people with asthma, who have airway and systemic inflammation at baseline as well as enhanced UFP deposition, have enhanced susceptibility to these vascular effects. In this article we present detailed analyses of venous blood leukocytes from subjects participating in four separate studies involving carbon UFP exposure: three protocols with varying exposure concentrations in healthy subjects, and one protocol with asthmatic subjects. Some data in this article have been presented previously in abstract form (Frampton et al. 2004).
Materials and Methods
Subjects.
Written, informed consent was obtained from all subjects, and the studies were approved by the Research Subjects Review Board of the University of Rochester. Fifty-six never-smoking subjects 18–40 years of age (40 healthy and 16 with asthma) participated and were paid a stipend. Subjects were not studied within 6 weeks of a respiratory infection. Healthy subjects were required to have normal spirometry, a normal 12-lead electrocardiogram, and no history of chronic respiratory disease.
Inclusion criteria for subjects with asthma have been reported previously (Chalupa et al. 2004). These criteria included a consistent clinical history, and either a significant bronchodilator response or airway hyper-responsiveness to methacholine. The severity was consistent with mild intermittent to moderate persistent asthma (National Institutes of Health 1997). Subjects with forced expiratory volume in 1 sec (FEV1) < 70% of predicted at baseline screening, or with > 20% reduction in FEV1 after the screening exercise, were excluded.
Study design.
Each study used a crossover design in which each subject was exposed to filtered air and to UFPs, so that each subject served as his or her own control. Within each study, the order of air/UFP exposure was randomized, and the randomization was blocked by order of presentation and sex, so that equal numbers of men and women inhaled air first or UFPs first. Exposures were blinded to both subjects and investigators.
Table 1 provides details of each study protocol. The first, UPREST, involved 12 (six female) subjects exposed at rest to approximately 10 μg/m3 UFPs or filtered air for 2 hr. The second study protocol, UPDOSE, involved 12 subjects (six female) with three 2-hr exposures with exercise for each subject: approximately 10 μg/m3 UFPs, approximately 25 μg/m3 UFPs, and filtered air. Subjects exercised on a bicycle ergometer for 15 min of each half hour at an intensity adjusted to increase the minute ventilation to approximately 20 L/min/m2 body surface area. For safety reasons, the order of exposure was randomized in a restricted fashion, so that each subject received the 10-μg/m3 exposure before the 25-μg/m3. The third protocol, UP50, involved 16 healthy subjects (eight female) exposed to approximately 50 μg/m3 UFPs and air for 2 hr, with intermittent exercise as in the UPDOSE protocol. The final protocol, UPASTHMA, involved 16 subjects with asthma (eight female) exposed to approximately 10 μg/m3 UFPs and air for 2 hr, with intermittent exercise as in the UPDOSE protocol. All exposures were separated by at least 2 weeks.
Table 1.
Study design (mean ± SD).
| UPREST | UPDOSE | UP50 | UPASTHMA | |
|---|---|---|---|---|
| No. of subjects | 12 | 12 | 16 | 16 |
| Subject age (years) | 30.1 ± 8.9 | 26.9 ± 5.8 | 26.9 ± 6.5 | 23.0 ± 2.7 |
| FEV1 (% predicted) | 103.8 ± 8.0 | 106.3 ± 16.6 | 102.8 ± 9.5 | 97.6 ± 5.0 |
| Nominala particle mass (μg/m3) | 0, 10 | 0, 10, 25 | 0, 50 | 0, 10 |
| Rest/exercise | Rest | Intermittent exercise | Intermittent exercise | Intermittent exercise |
The target mass concentration of UFPs for each protocol.
Exposures to either filtered air or UFPs were administered by mouthpiece (with nose clip) for 2 hr, interrupted by a 10-min break after the first hour. Before and at 0, 3.5, and 21 hr after exposure, blood pressure, heart rate, and oxygen saturation by pulse oximetry were measured, and blood was drawn from an antecubital vein. For UP50 and UPASTHMA, measurements were also obtained 45 hr after exposure.
Exposure system.
The rationale and design of the exposure facility have been described in detail elsewhere (Chalupa et al. 2002). Briefly, particles [count median diameter ~ 25 nm, geometric standard deviation ~ 1.6] were generated in an argon atmosphere using an electric spark discharge between two graphite electrodes, and then deionized and diluted with filtered air to the desired concentration. Particle number, mass, and size distribution were monitored on both the inspiratory and expiratory sides of the subject. Electronic integration of a pneumotachograph signal provided tidal volume, respiratory frequency, and minute ventilation measurements. Air for the control exposures, and for dilution of the particles, was passed through charcoal and high-efficiency particle filters and was essentially free of particles (0–10 particles/cm3).
Blood leukocyte immunofluorescence analysis.
Fresh heparinized whole blood was stained with three monoclonal antibodies: the marker of interest (Table 2) conjugated to fluorescein isothiocyanate, CD14 conjugated to phycoerythrin, and CD45 conjugated to pericidin chlorophyll protein. This permitted determination of the relative expression of adhesion molecules and other markers separately on polymorphonuclear leukocytes (PMNs), eosinophils, lymphocytes, and monocytes. The appropriate isotype control antibodies were run with each experiment to assist in appropriate gate setting. The adhesion markers shown in Table 2 were measured in each of the study protocols, except for CD18, which was measured in UP50 and UPASTHMA only.
Table 2.
Leukocyte markers measured in each protocol.
| Cluster designation | Name | Source (clone) | Description |
|---|---|---|---|
| CD3 | BD Biosciencea (SK7) | Marker of T-lymphocytes | |
| CD4 | BD Bioscience (SK3) | Marker of T-helper lymphocytes | |
| CD8 | BD Bioscience (SK1) | Marker of T-cytotoxic lymphocytes | |
| CD11a | Leukocyte function antigen-1 | GenTrakb (38) or Coulterc (25.3.1) | Part of β2 integrin adhesion molecule complex |
| CD11b | Mac-1 | Ancelld (ICRF44) | Subunit of complement receptor 3, part of β2 integrin adhesion molecule complex |
| CD18e | Pharmigena (6.7) or BD Bioscience (L130) | Part of β2 adhesion molecule complex with CD11a and CD11b | |
| CD25 | Tac | BD Bioscience (2A3) | Epitope of IL-2 receptor, activation marker on lymphocytes |
| CD49d | Very late antigen-α4 | Serotecf (44H6) | Part of β1 integrin adhesion molecule complex |
| CD54 | Intercellular adhesion molecule-1 | Southern Biotechnologyg (15.2) | Adhesion molecule |
| CD62L | L-selectin | Coulter (DREG56) or Pharmigen (DREG56) | Adhesion molecule |
San Jose, CA.
Plymouth Meeting, PA.
Miami, FL.
Bayport, MN.
Measured in UP50 and UPASTHMA only.
Raleigh, NC.
Birmingham, AL.
Red blood cells were lysed and cells were analyzed on a FACScan flow cytometer (BD Bioscience, San Jose, CA) equipped with a 15-mW argon ion laser emitting at 488 nm. Ten thousand events were collected from each sample in list mode. Standardized fluorescent microbeads (Quantium 24P and 25P; Bangs Laboratories, Fishers, IN) were run with each experiment to convert mean channel numbers to molecules of equivalent soluble fluorochrome (MESF) (Gavras et al. 1994). This provided a correction for minor day-to-day instrument variations in fluorescence detection.
Total and differential blood leukocyte and platelet counts were performed in the clinical laboratories of Strong Memorial Hospital, using an automated analyzer (Celldyne 4000; Abott Laboratories, Santa Clara, CA).
Data handling and statistical methods.
Data were entered on a desktop computer using Microsoft Excel and analyzed using SAS (SAS Institute Inc., Cary, NC).
UPREST, UPASTHMA, and UP50 used a standard, two-period crossover design in which each subject received both particles and air. Equal numbers of males and females were included. The order of presentation was randomized separately for each sex, with half of each group of subjects receiving each of the two possible orders. UPDOSE used a three-period crossover design in which each subject received air and both 10-μg/m3 and 25-μg/m3 concentrations of particles. There were then three possible exposure sequences, depending on where in the sequence the air exposure was placed. Equal numbers of subjects were randomly assigned to each sequence.
Repeated-measures analysis of variance (ANOVA) was used (Wallenstein and Fisher 1977), with order of presentation as a between-subjects factor, with exposure and time as within-subject factors. The analysis included tests for period and carryover effects, although the latter were expected to be minimal because of the nature of the exposures and the length of the washout period. In cases where carryover effects were significant, first-period data were examined separately (Jones and Kenward 1989). Each ANOVA included an examination of residuals as a check on the required assumptions of normally distributed errors with constant variance. If these assumptions were not satisfied, data transformations (e.g., square-root transformation for cell counts) were considered. A p-value of 0.05 was required for statistical significance. Data are shown as mean ± SE, unless otherwise indicated.
Results
Exposure data and subject characteristics.
Table 3 shows the exposure parameters and subject characteristics for each protocol. Most of the subjects with asthma were atopic (15 of 16), and most (11 of 16) were not on inhaled steroids, long-acting bronchodilators, or leukotriene inhibitors. All subjects completed every exposure; men and women did not differ in the achieved minute ventilation, adjusted for body surface area. There were no significant effects of UFP exposure on ventilatory parameters or pulmonary function; these results, and UFP deposition, have been published previously (Daigle et al. 2003).
Table 3.
Exposure parameters (mean ± SD).
| UPREST | UPDOSE | UPDOSE | UP50 | UPASTHMA | |
|---|---|---|---|---|---|
| Nominal particle mass (μg/m3) | 10 | 10 | 25 | 50 | 10 |
| Measured particle mass (μg/m3) | 10.00 ± 2.14 | 13.87 ± 4.02 | 28.46 ± 5.13 | 49.97 ± 3.88 | 11.08 ± 3.11 |
| Particle number (× 106 particles/cm3) | 1.88 ± 0.09 | 2.04 ± 0.07 | 6.96 ± 0.10 | 10.79 ± 1.66 | 2.20 ± 0.10 |
| CMD (nm) | 27.3 ± 2.5 | 25.2 ± 1.7 | 26.5 ± 1.5 | 27.9 ± 2.2 | 23.1 ± 1.6 |
| GSD | 1.62 ± 0.02 | 1.60 ± 0.02 | 1.60 ± 0.02 | 1.65 ± 0.02 | 1.64 ± 0.01 |
Abbreviations: CMD, count median diameter; GSD, geometric standard deviation.
The UPREST protocol, with exposures at rest to 10 μg/m3 UFPs, showed no convincing differences between particle and air exposure for leukocyte expression of adhesion molecules or total and differential leukocyte counts. There were rare statistically significant comparisons, but the significance levels were modest, and the data did not suggest a consistent biologic response. Overall, exposure to 10 μg/m3 UFPs at rest had no significant effects on blood leukocytes.
Findings from the three studies involving exercise are described below.
Blood leukocyte expression of adhesion molecules.
In these studies, quantitative surface expression of molecules that mediate leukocyte-endothelial interactions served as an indirect indicator of exposure effects on pulmonary vascular endothelial function. The use of flow cytometry with calibrated fluorescent beads allowed quantitation of small changes in surface marker density. Adhesion molecule expression for monocytes and PMNs in the three protocols involving exercise is shown in Tables 4–6.
Table 4.
Adhesion molecule expression on monocytes and PMNs, UPDOSE protocol (mean ± SE, MESF).
| Exposure (μg/m3) | Baseline | 0 hr | 3.5 hr | 21 hr | ANOVA | |
|---|---|---|---|---|---|---|
| Monocytes | ||||||
| CD11a | Air | 64,429 ± 2,072 | 62,483 ± 2,140 | 62,571 ± 1,689 | 65,682 ± 2,435 | |
| UFP 10 | 63,818 ± 4,109 | 59,900 ± 2,493 | 59,190 ± 3,063 | 65,249 ± 2,518 | ||
| UFP 25 | 62,835 ± 2,644 | 56,207 ± 5,436 | 59,635 ± 2,404 | 63,008 ± 2,126 | ||
| CD11b | Air | 19,034 ± 986 | 19,497 ± 997 | 21,076 ± 1,653 | 20,901 ± 1,912 | |
| UFP 10 | 17,632 ± 990 | 17,287 ± 1,171 | 18,335 ± 1,501 | 19,391 ± 1,185 | ||
| UFP 25 | 19,056 ± 1,214 | 17,769 ± 922 | 22,059 ± 4,697 | 22,669 ± 3,357 | ||
| CD49d | Air | 14,222 ± 1,000 | 13,562 ± 854 | 13,717 ± 880 | 13,989 ± 964 | |
| UFP 10 | 13,634 ± 1,029 | 12,587 ± 694 | 12,946 ± 706 | 13,059 ± 797 | ||
| UFP 25 | 13,590 ± 839 | 12,779 ± 574 | 12,372 ± 683 | 13,542 ± 935 | ||
| CD54 | Air | 12,188 ± 319 | 13,096 ± 519 | 13,908 ± 645 | 13,307 ± 823 | Exposure |
| UFP 10 | 12,541 ± 469 | 12,470 ± 583 | 12,855 ± 592 | 13,110 ± 781 | p = 0.001 | |
| UFP 25 | 13,717 ± 686 | 12,591 ± 584 | 13,533 ± 856 | 14,482 ± 991 | ||
| CD62L | Air | 43,970 ± 3,212 | 34,937 ± 3,519 | 37,600 ± 3,391 | 37,399 ± 3,716 | Exposure × sex |
| UFP 10 | 38,953 ± 3,465 | 30,281 ± 2,510 | 32,409 ± 1,719 | 36,356 ± 3,207 | p = 0.006 | |
| UFP 25 | 41,357 ± 4,453 | 33,134 ± 2,940 | 34,676 ± 3,234 | 39,168 ± 4,196 | ||
| PMNs | ||||||
| CD11a | Air | 28,637 ± 1,073 | 28,613 ± 1,228 | 28,793 ± 1,183 | 28,867 ± 1,503 | |
| UFP 10 | 29,124 ± 1,073 | 26,216 ± 1,160 | 26,260 ± 985 | 27,620 ± 923 | ||
| UFP 25 | 28,444 ± 1,397 | 27,939 ± 1,151 | 27,817 ± 1,137 | 27,157 ± 1,411 | ||
| CD11b | Air | 18,467 ± 1,117 | 18,837 ± 1,223 | 21,427 ± 3,186 | 21,189 ± 2,383 | |
| UFP 10 | 16,728 ± 907 | 15,997 ± 1,175 | 16,049 ± 1,112 | 21,169 ± 2,394 | ||
| UFP 25 | 19,778 ± 2,671 | 15,671 ± 1,179 | 20,461 ± 3,457 | 18,653 ± 1,760 | ||
| CD49d | Air | 7,422 ± 593 | 6,572 ± 542 | 6,404 ± 498 | 6,098 ± 686 | Exposure × sex |
| UFP 10 | 7,007 ± 561 | 6,172 ± 559 | 6,173 ± 423 | 6,340 ± 650 | p = 0.007 | |
| UFP 25 | 6,681 ± 465 | 6,031 ± 442 | 5,677 ± 446 | 5,925 ± 470 | ||
| CD54 | Air | 4,792 ± 279 | 4,500 ± 280 | 4,586 ± 246 | 4,457 ± 243 | |
| UFP 10 | 4,953 ± 271 | 4,292 ± 242 | 4,608 ± 424 | 4,435 ± 213 | ||
| UFP 25 | 4,771 ± 321 | 4,084 ± 216 | 4,122 ± 215 | 4,417 ± 230 | ||
| CD62L | Air | 66,179 ± 3,910 | 59,419 ± 4,413 | 64,867 ± 4,303 | 59,671 ± 5,970 | |
| UFP 10 | 60,976 ± 4,340 | 57,202 ± 4,515 | 56,621 ± 4,636 | 60,626 ± 4,180 | ||
| UFP 25 | 66,145 ± 4,231 | 60,044 ± 5,434 | 59,625 ± 4,296 | 61,184 ± 4,054 | ||
Table 6.
Adhesion molecule expression on monocytes and PMNs, UPASTHMA protocol (mean ± SE, MESF).
| Exposure | Baseline | 0 hr | 3.5 hr | 21 hr | 45 hr | ANOVA | |
|---|---|---|---|---|---|---|---|
| Monocytes | |||||||
| CD11a | Air | 21,179 ± 4,120 | 20,442 ± 3,989 | 19,336 ± 4,042 | 21,126 ± 5,569 | 21,407 ± 5,550 | |
| UFP | 32,102 ± 7,076 | 30,277 ± 6,791 | 29,592 ± 6,630 | 30,468 ± 6,809 | 29,751 ± 6,640 | ||
| CD11b | Air | 25,022 ± 2,822 | 31,626 ± 5,969 | 26,553 ± 3,319 | 26,345 ± 3,456 | 27,703 ± 3,228 | Exposure |
| UFP | 26,958 ± 4,112 | 25,452 ± 4,611 | 25,742 ± 4,241 | 24,498 ± 4,199 | 25,814 ± 3,502 | p = 0.029 | |
| CD18 | Air | 85,586 ± 6,773 | 87,234 ± 8,882 | 82,899 ± 6,465 | 82,697 ± 7,370 | 85,455 ± 7,819 | |
| UFP | 84,999 ± 7,252 | 81,131 ± 7,931 | 81,297 ± 9,950 | 82,028 ± 6,767 | 77,346 ± 7,334 | ||
| CD49d | Air | 17,172 ± 731 | 16,739 ± 925 | 16,013 ± 616 | 16,627 ± 837 | 16,856 ± 771 | |
| UFP | 18,378 ± 865 | 16,967 ± 873 | 17,138 ± 919 | 17,715 ± 877 | 17,327 ± 879 | ||
| CD54 | Air | 19,102 ± 1,386 | 19,432 ± 1,430 | 18,285 ± 1,248 | 19,043 ± 1,410 | 19,281 ± 1,319 | |
| UFP | 20,673 ± 2,009 | 20,438 ± 2,088 | 19,861 ± 1,934 | 20,014 ± 1,853 | 19,284 ± 1,491 | ||
| CD62L | Air | 45,571 ± 2,571 | 39,446 ± 2,652 | 41,214 ± 2,703 | 45,100 ± 2,847 | 44,329 ± 2,870 | |
| UFP | 51,939 ± 5,305 | 43,483 ± 4,955 | 42,198 ± 3,954 | 46,105 ± 4,023 | 45,608 ± 4,271 | ||
| PMNs | |||||||
| CD11a | Air | 10,540 ± 1,775 | 10,010 ± 1,771 | 10,107 ± 1,837 | 10,986 ± 2,830 | 11,199 ± 2,953 | |
| UFP | 14,562 ± 2,749 | 14,161 ± 2,679 | 13,790 ± 2,780 | 13,765 ± 2,727 | 13,710 ± 2,652 | ||
| CD11b | Air | 24,078 ± 2,783 | 26,353 ± 3,578 | 25,211 ± 2,533 | 25,199 ± 2,072 | 30,893 ± 4,350 | |
| UFP | 23,819 ± 2,343 | 22,792 ± 3,224 | 25,376 ± 2,984 | 22,085 ± 2,479 | 22,781 ± 1,886 | ||
| CD18 | Air | 48,861 ± 3,054 | 47,564 ± 3,026 | 45,449 ± 2,457 | 45,303 ± 2,719 | 50,312 ± 5,429 | |
| UFP | 46,982 ± 2,925 | 44,465 ± 2,676 | 43,512 ± 3,174 | 44,599 ± 2,862 | 43,470 ± 3,006 | ||
| CD49d | Air | 5,342 ± 211 | 5,122 ± 228 | 5,090 ± 162 | 4,805 ± 248 | 4,923 ± 185 | |
| UFP | 5,499 ± 315 | 4,964 ± 212 | 4,887 ± 210 | 4,783 ± 234 | 4,950 ± 241 | ||
| CD54 | Air | 5,631 ± 230 | 5,348 ± 236 | 5,234 ± 222 | 5,433 ± 277 | 5,635 ± 239 | Exposure × time |
| UFP | 6,262 ± 451 | 5,759 ± 453 | 5,604 ± 458 | 5,535 ± 399 | 5,660 ± 398 | p = 0.031 | |
| CD62L | Air | 78,859 ± 3,812 | 69,825 ± 3,978 | 71,796 ± 3,691 | 72,829 ± 4,711 | 72,429 ± 4,184 | Exposure × sex |
| UFP | 79,315 ± 6,332 | 75,646 ± 6,405 | 70,468 ± 4,961 | 74,971 ± 5,500 | 74,541 ± 5,925 | p = 0.011 | |
UPDOSE.
UFP exposure caused a concentration-related reduction in monocyte expression of CD54 [intercellular adhesion molecule-1 (ICAM-1) (exposure effect, p = 0.0012); Figure 1]. Expression increased after exposure to filtered air and decreased with 25 μg/m3 UFPs, with differences resolving by 21 hr after exposure. Expression of CD62L showed a significant exposure–sex interaction (p = 0.0006; data not shown), with expression increasing in females but decreasing in males relative to air exposure. However, these findings lacked a clear concentration response.
Figure 1.
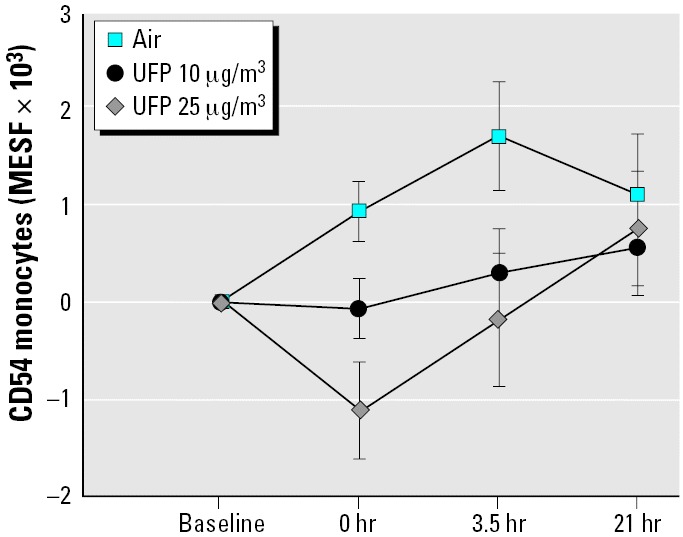
Changes in monocyte expression of CD54 (ICAM-1), UPDOSE protocol. In this and following figures, data are shown as mean ± SE changes from baseline. Nominal UFP exposure concentrations are shown in μg/m3. Exposure, p = 0.012.
UP50.
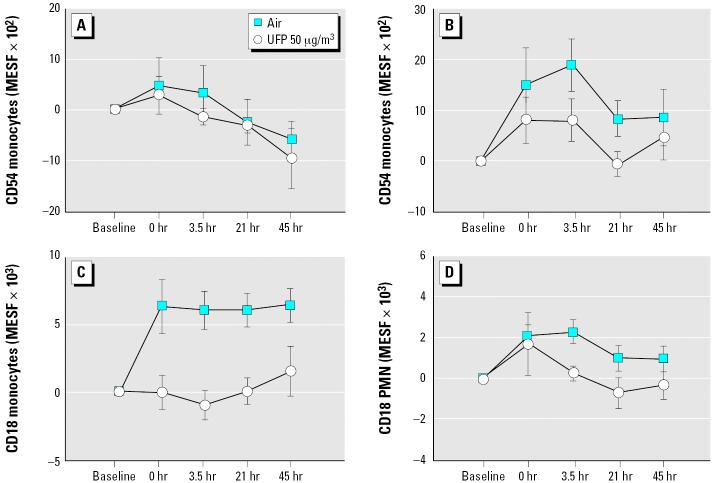
Exposure to 50 μg/m3 UFPs also reduced expression of CD54 on monocytes (Figure 2A,B), but to a greater extent in males (exposure–sex interaction, p = 0.025). The percentage of monocytes expressing CD54 was also reduced (p = 0.035; data not shown). UFP exposure persistently blunted the air-related increase in CD18 expression on monocytes (p = 0.0002; Figure 2C). Expression of CD18 was also reduced on PMNs (Figure 2D), and ANOVA indicated significantly increased CD11a expression on PMNs (exposure–time interaction, p = 0.037; data not shown).
Figure 2.
Changes in leukocyte expression of adhesion molecules, UP50 protocol. (A) Monocyte expression of CD54, females. UFP × sex, p = 0.025. (B) Monocyte expression of CD54, males. UFP × sex, p = 0.025. (C) Monocyte expression of CD18. UFP, p = 0.0002. (D) PMN expression of CD18. UFP, p = 0.023.
UPASTHMA.
As expected, we found baseline differences between healthy and asthmatic subjects in leukocyte expression of adhesion molecules; these data are shown in Table 7. For example, monocyte expression of CD11b, CD54, and CD62L was higher in subjects with asthma than in healthy subjects.
Table 7.
Blood leukocyte marker expression at baseline that differed between asthmatic and healthy subjects (mean ± SE, MESF).
| Healthya | Asthma | p-Value | |
|---|---|---|---|
| Lymphocytes | |||
| CD11a | 41,710 ± 1,844 | 14,575 ± 4,161 | < 0.001 |
| CD11b | 1,460 ± 67 | 1,784 ± 107 | 0.017 |
| CD49d | 8,168 ± 335 | 10,486 ± 324 | < 0.001 |
| CD54 | 2,381 ± 69 | 2,964 ± 155 | 0.003 |
| Monocytes | |||
| CD11a | 64,155 ± 4,041 | 26,220 ± 5,260 | < 0.001 |
| CD11b | 17,944 ± 915 | 25,047 ± 2,751 | 0.025 |
| CD49d | 13,556 ± 915 | 17,089 ± 642 | 0.005 |
| CD54 | 12,314 ± 401 | 17,942 ± 1,065 | < 0.001 |
| PMNs | |||
| CD11a | 28,358 ± 904 | 12,753 ± 2,276 | < 0.001 |
| CD11b | 16,868 ± 1,055 | 24,178 ± 2,705 | 0.021 |
| CD49d | 7,189 ± 545 | 5,292 ± 282 | 0.007 |
| CD62L | 63,591 ± 4,614 | 80,656 ± 5,954 | 0.032 |
Includes subjects from UPREST and UPDOSE (n = 24). Source of some immunofluorescence markers differed for UP50, resulting in changes in baseline values, so these healthy subjects were not included.
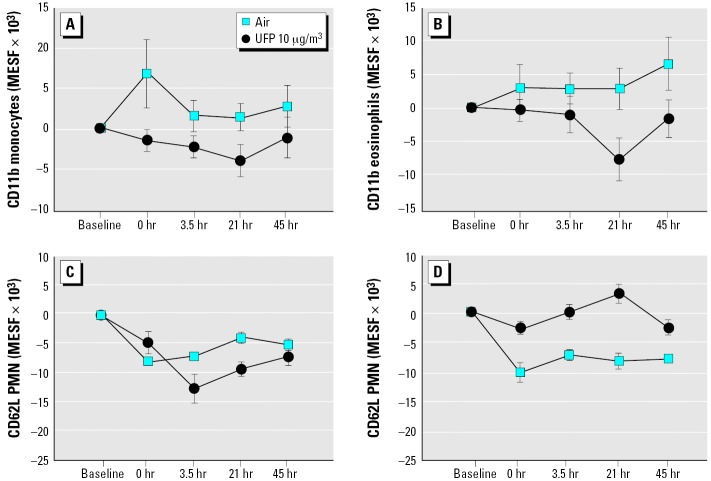
In subjects with asthma, exposure to 10 μg/m3 UFPs reduced expression of CD11b on blood monocytes (p = 0.029; Figure 3A) and also reduced expression on eosinophils (p = 0.015; Figure 3B). Expression of CD62L on PMNs increased in males but not females (exposure–sex interaction, p = 0.011; Figure 3C,D). Expression of CD54 on PMNs decreased, with the greatest difference from control at 45 hr after exposure (exposure–time interaction, p = 0.031; data not shown).
Figure 3.
Changes in leukocyte expression of adhesion molecules, UPASTHMA protocol. (A) Monocyte expression of CD11b. UFP, p = 0.029. (B) Eosinophil expression of CD11b. UFP, p = 0.015. (C) PMN expression of CD62L, females. UFP × sex, p = 0.011. (D) PMN expression of CD62L, males. UFP × sex, p = 0.011.
Lymphocyte subsets and activation.
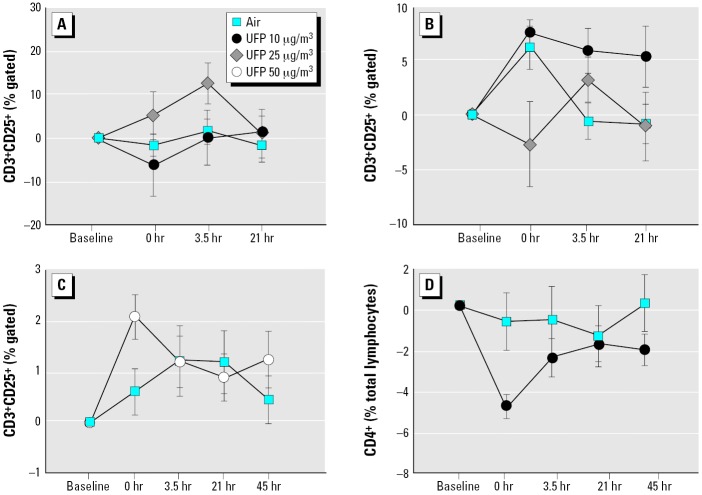
There was evidence for increased activated T cells after UFP exposure in healthy subjects. In UPDOSE, CD25 expression on CD3+ T cells increased in females, but not in males, early after exposure to 25 μg/m3 UFPs (exposure–sex interaction, p = 0.002; Figure 4A,B). In UP50, exposure to 50 μg/m3 increased CD25 expression on T cells 0 hr after exposure (p = 0.001 by paired t-test at 0 hr after exposure; p = 0.085 by ANOVA; Figure 4C). There were no other changes in lymphocyte subsets in the healthy subjects.
Figure 4.
Changes in blood T-lymphocyte subsets. (A) UPDOSE protocol, percentage of CD25+ cells within the T-cell (CD3+) gate, females. UFP × sex, p = 0.0024. (B) UPDOSE protocol, CD3+CD25+ T cells, males. UFP × sex, p = 0.0024. (C) UP50 protocol, CD3+CD25+ T cells, all subjects. UFP × time, p = 0.085. (D) UPASTHMA protocol, CD4+ T cells, all subjects. UFP × time, p = 0.021.
In UPASTHMA, CD4+ T cells decreased immediately after exposure to UFPs, compared with air (exposure–time interaction, p = 0.021; Figure 2D). There were no significant effects on other lymphocyte subsets or CD25 expression. However, the percentage of T-lymphocytes expressing the activation marker CD25 was higher in asthmatic subjects than in healthy subjects before exposure (UPASTHMA, 33.0 ± 3.3%, vs. UPDOSE, 27.0 ± 2.5%; p = 0.04).
Overall, the data suggest that UFP exposure induces activation (healthy subjects) or sequestration (subjects with asthma) of T-lymphocytes.
Blood leukocyte and platelet counts.
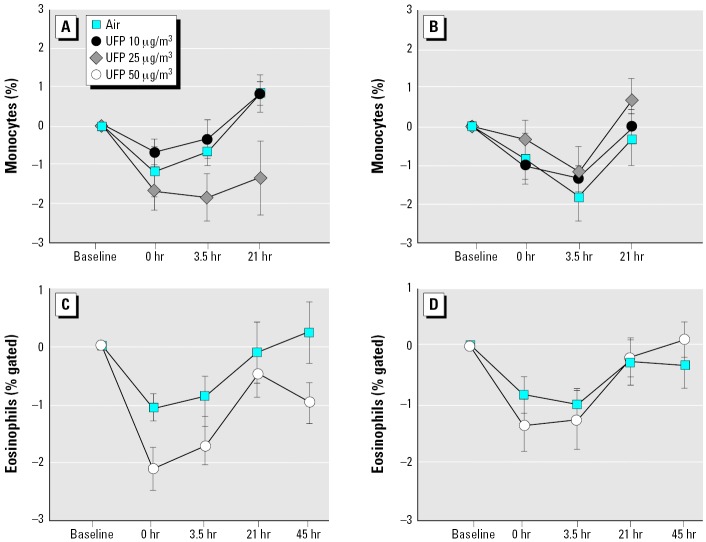
In each of the protocols involving exercise (UPDOSE, UP50, and UPASTHMA), consistent postexposure increases were seen in the total leukocyte count and the percentage of PMNs, with decreases in the percentage of eosinophils and monocytes. In the UPDOSE protocol, ANOVA showed a significant exposure–sex interaction for an effect on the percentage of blood monocytes (p = 0.0015). As shown in Figure 5A,B, in females monocytes decreased after exposure to 25 μg/m3 and did not return to baseline at 21 hr after exposure. This observation was confirmed when monocyte numbers were analyzed by flow cytometry, using light scatter and CD14 expression (overall effect of UFPs, p = 0.035; exposure–sex interaction, p = 0.002). A significant decrease in blood basophils in females was also seen with both UFP concentrations (exposure–sex interaction, p = 0.015; data not shown).
Figure 5.
Changes in percentage of blood leukocytes with exposure to UFPs. (A) UPDOSE protocol, monocytes, females. UFP × sex, p = 0.0015. (B) UPDOSE protocol, monocytes, males. UFP × sex, p = 0.0015. (C) UP50 protocol, eosinophils, females. UFP × time × sex, p = 0.01. (D) UP50 protocol, eosinophils, males. UFP × time × sex, p = 0.01.
Exposure to 50 μg/m3 UFPs caused small reductions in the percentage of eosinophils, with a larger effect in females (Figure 5C,D). There were no significant effects on the percentage of blood monocytes, PMNs, or basophils in this protocol.
In subjects with asthma exposed to 10 μg/m3 UFPs, basophils decreased in both men and women at 0 and 3.5 hr after exposure to UFPs, compared with air exposure (exposure–time interaction, p = 0.02; data not shown). The percentage of blood eosinophils as determined by flow cytometry decreased 0 and 3.5 hr after exposure, with greater reductions after UFP exposure than after air (p = 0.049).
UFP exposure did not change platelet counts in any of the exposure protocols.
These data suggest that exposure to UFPs with exercise causes small changes in blood leukocyte differential counts in both healthy and asthmatic subjects.
Discussion
The objective of these studies was to determine whether inhalation of carbon UFPs has vascular effects in healthy subjects, and in subjects with asthma. We postulated that changes in blood leukocyte phenotype and expression of adhesion molecules would serve as a “window” on vascular inflammatory effects after inhalation challenge. Although the specific findings differed among the protocols, all three protocols with exercise showed UFP-associated reductions in expression of adhesion molecules on leukocytes, mainly ICAM-1 (CD54) and the β2 integrins CD11b and CD18. There were significant differences between healthy and asthmatic subjects in leukocyte expression of adhesion molecules, when measured before exposure (Table 7). For example, blood monocytes from subjects with asthma showed decreased expression of CD11a and increased expression of CD11b, CD49d, and CD54 relative to healthy subjects. This may reflect relative activation or priming of circulating leukocytes as a consequence of airway inflammation. In subjects with asthma, inhalation of UFPs reduced expression of CD11b on monocytes and eosinophils (Figure 3) and reduced CD54 expression on PMNs (Table 6).
In addition, the data suggested subtle reductions relative to air exposure in the percentage of blood monocytes, eosinophils, and basophils. There was evidence for activation of CD4+ T-lymphocytes in healthy subjects and transient reductions in CD4+ T-cell numbers in asthmatic subjects. Sex interactions were seen for some of these changes. A summary of these findings is shown in Table 8.
Table 8.
Summary of UFP exposure effects.
| Protocol | Adhesion molecules | Lymphocyte subsets and activation | Leukocyte counts |
|---|---|---|---|
| UPREST (n = 12) | No convincing effects (see text) | No effects | No effects |
| UPDOSE (n = 12) | Decreased monocyte CD54
Decreased PMN CD49d (males) |
Increased CD25+ T cells (females) | Decreased monocytes and basophils (females) |
| UP50 (n = 16) | Decreased monocyte CD18 and CD54 (males) | Increased CD25+ T cells | Decreased eosinophils |
| Decreased PMN CD18 and increased CD11a | |||
| UPASTHMA (n = 16) | Decreased monocyte CD11b
Decreased PMN CD54 and increased CD62L (males) Decreased eosinophil CD11b |
Decreased CD4+ T cells and basophils | Decreased eosinophils |
The findings provide evidence that inhalation of elemental carbon UFPs, with intermittent exercise, causes phenotypic alterations in blood leukocytes at concentrations as low as approximately 10 μg/m3 or approximately 2 × 106 particles/cm3. However, the overall nature and direction of the changes do not suggest increased systemic inflammation. This is consistent with the lack of evidence for airway or systemic inflammation that we have reported previously for these studies (Pietropaoli et al. 2004a, 2004c).
The reductions in leukocyte subsets and adhesion molecule expression seen in these studies suggest the possibility of leukocyte sequestration or margination in response to UFP exposure. The relative reductions in monocyte, basophil, and eosinophil percentages may result from slightly prolonged transit time through the pulmonary circulation after exposure to UFPs, possibly as a consequence of pulmonary vasoconstriction. The reductions in expression of the adhesion molecules CD54, CD11b, and CD18 are consistent with this hypothesis. Blood leukocytes normally marginate in the lung, requiring several seconds to transit the pulmonary circulation (Doerschuk 2003). PMNs are larger than pulmonary capillaries and must deform in order to transit. The integrins CD11a and CD11b are expressed as dimers with CD18 and mediate blood leukocyte recruitment to areas of inflammation and injury via specific receptors on vascular endothelial cells. Activation of monocytes and PMNs increases expression of CD11b and CD18 and decreases cell deformability through actin polymerization (Anderson et al. 2001), slowing transit time. Exercise increases pulmonary blood flow and decreases leukocyte transit time through the pulmonary circulation, leading to mobilization of the pulmonary leukocyte pool into the systemic vascular pool. Van Eeden et al. (1999) have shown that maximal exercise increases the blood leukocyte count and also increases expression of CD11b on peripheral blood PMNs, suggesting that cells expressing higher levels of CD11b preferentially marginate in the pulmonary circulation and are “flushed out” with exercise. Thus, our data are consistent with, but do not prove, increased retention of leukocytes expressing higher levels of adhesion molecules in the pulmonary vascular bed in response to UFP exposure.
Pulmonary vasoconstriction in response to UFP exposure would be expected to delay leukocyte transit through the lung. We have reported (Pietropaoli et al. 2004b) that, in the UP50 protocol, UFP exposure caused reductions in the diffusing capacity for carbon monoxide, without effects on the forced vital capacity, consistent with reduced vascular perfusion or reduced ventilation/perfusion matching. We also reported preliminary findings (Pietropaoli et al. 2004a) of subtle but significant effects on systemic flow-mediated vascular dilatation, and a decrease in blood nitrate levels, suggesting the vascular effects may result from decreased nitric oxide availability. Batalha et al. (2002) have shown pulmonary vaso-constriction in rats exposed to concentrated ambient fine particles.
Alternative mechanisms for reductions in leukocyte and their surface markers include a) direct effects of UFPs on blood leukocytes, reducing surface marker expression through shedding, redistribution, or internalization; b) indirect effects of mediators released by vascular endothelium, such as nitric oxide, which has anti-inflammatory properties (Lefer 1997), reduces endothelial expression of adhesion molecules via inhibition of nuclear factor κB activation, and reduces monocyte adhesion to endothelium (De Caterina et al. 1995); c) adsorption of soluble cytokines, such as transforming growth factor-β, onto the surface of the particles, reducing inflammatory effects (Kim et al. 2003); d) recruitment of immature leukocytes from the bone marrow in response to UFP inhalation, as has been suggested in previous studies of fine particle exposure (Tan et al. 2000); and e) selective toxicity of UFPs for activated blood leukocytes, inducing apoptosis of specific cell subsets.
The two protocols with exercise in healthy subjects showed increased expression of CD25 on blood T-lymphocytes, and subjects with asthma showed a transient reduction in CD4+ lymphocytes after UFP exposure. CD25 is the α-chain of the IL-2 receptor; IL-2 promotes lymphocyte proliferation. We found that lymphocyte CD25 expression was higher in subjects with asthma than in healthy subjects, confirming previous observations that people with asthma have a higher percentage of circulating activated T-lymphocytes (Corrigan and Kay 1990), which may explain why UFP exposure did not increase it further in these subjects. The rapid and transient nature of the reduction in CD4+ T cells suggests redistribution or margination of cells, as postulated above for other blood leukocytes.
The changes in response to carbon UFP exposure reported in these studies were generally small and would not be expected to adversely affect healthy and mildly asthmatic subjects similar to those studied. However, ambient UFPs contain reactive organic species and transition metals that may induce greater effects than those we observed. People with severely compromised cardiovascular status may experience adverse effects from even small changes in vascular homeostasis. Furthermore, prolonged, repeated exposures may hasten the progression of atherosclerosis, as has been suggested in an epidemiology study of fine particle exposure (Künzli et al. 2005).
The UFP number concentrations used in these studies are higher than UFP background concentrations but are relevant to episodic levels seen in specific situations. UFPs are always present in ambient air, with background urban levels in the range of 40,000–50,000 particles/cm3 or estimated mass concentrations of 3–4 μg/m3 (Peters et al. 1997b). Episodic increases have been documented to 300,000 particles/cm3, or estimated to approximately 50 μg/m3 UFPs as an hourly average (Brand et al. 1991, 1992). Particle numbers inside a vehicle on a major highway reached 107 particles/cm3 (Kittelson et al. 2001), comparable with the highest concentrations used in our studies.
Although not specifically powered to detect sex differences, these studies were designed to include an analysis of sex interactions with the effects of UFP exposure. In the UPDOSE protocol, females showed greater decreases in blood monocytes (Figure 5A) and basophils and greater increases lymphocyte CD25 expression (Figure 4A) compared with males. Females also showed decreased eosinophils in the UP50 protocol (Figure 5C). In UPASTHMA, expression of L-selectin (CD62L) on PMNs was increased in males (Figure 3B). It is possible that males and females differ in their cardiovascular responses to particle exposure. There are known sex differences in leukocyte function and cardiovascular responses, based in part on hormonal influences. For example, females have a higher percentage of CD4+ T cells and a higher CD4+:CD8+ ratio than do males. Stimulated blood monocytes from females produce more prostaglandin E2 (Leslie and Dubey 1994) and less tumor necrosis factor-α and IL-6 (Schwarz et al. 2000) than those from males. There are also sex differences in endothelial function and antioxidant defenses that may affect vascular response to inhaled challenge. However, we do not feel that these studies have convincingly established or excluded significant sex differences in responses to carbon UFPs.
There are limitations to this study. First, our particles were laboratory-generated elemental carbon, without significant organic species, metals, oxides, nitrates, or sulfates. The findings of these studies may not be representative of exposure to ambient particles, which are a mix of ultrafine, fine, and coarse particles, with reactive organic species, metals, and oxidants in addition to elemental carbon. These and other chemical species may enhance pulmonary and vascular effects. Second, each protocol involved a fairly large number of measurements, and some statistically significant changes may have been chance related. Our approach was to consider results that showed consistency within and across protocols and to discount findings of isolated statistical significance that were not supported by other data. The observations of UFP effects on leukocyte distribution and surface marker expression meet those criteria.
Conclusions
Overall, the findings from these studies provide evidence that inhalation of carbon UFPs, with exercise, reduces peripheral blood monocytes, eosinophils, and basophils and reduces expression of some adhesion molecules on monocytes and PMNs. When considered in light of other evidence, the leukocyte changes may be a consequence of endothelial activation or vasoconstriction in the pulmonary and/or systemic circulation.
Table 5.
Adhesion molecule expression on monocytes and PMNs, UP50 protocol (mean ± SE, MESF).
| Exposure | Baseline | 0 hr | 3.5 hr | 21 hr | 45 hr | ANOVA | |
|---|---|---|---|---|---|---|---|
| Monocytes | |||||||
| CD11a | Air | 65,882 ± 3,277 | 66,463 ± 2,934 | 65,658 ± 2,963 | 69,888 ± 2,853 | 71,292 ± 2,885 | |
| UFP | 69,090 ± 3,146 | 68,680 ± 2,935 | 66,222 ± 2,696 | 69,813 ± 2,835 | 71,773 ± 3,132 | ||
| CD11b | Air | 16,840 ± 899 | 20,104 ± 905 | 19,938 ± 835 | 18,728 ± 1,092 | 18,364 ± 993 | |
| UFP | 18,365 ± 1,153 | 19,733 ± 1,206 | 18,531 ± 952 | 18,389 ± 932 | 18,369 ± 815 | ||
| CD18 | Air | 62,675 ± 2,948 | 68,897 ± 2,942 | 67,872 ± 2,780 | 68,661 ± 2,749 | 68,963 ± 3,187 | Exposure |
| UFP | 67,246 ± 2,751 | 67,175 ± 2,582 | 66,277 ± 2,488 | 67,307 ± 2,768 | 68,754 ± 3,052 | p = 0.0002 | |
| CD49d | Air | 16,334 ± 939 | 16,588 ± 859 | 17,371 ± 954 | 16,951 ± 9,571 | 17,126 ± 1,079 | |
| UFP | 16,643 ± 938 | 16,445 ± 874 | 17,182 ± 965 | 17,282 ± 909 | 17,484 ± 1,167 | ||
| CD54 | Air | 9,637 ± 1,431 | 10,654 ± 1,668 | 11,198 ± 1,728 | 9,969 ± 1,639 | 9,827 ± 1,687 | Exposure × sex |
| UFP | 10,526 ± 1,715 | 11,095 ± 1,782 | 10,889 ± 1,871 | 10,352 ± 1,791 | 10,339 ± 1,811 | p = 0.025 | |
| CD62L | Air | 58,551 ± 3,188 | 50,197 ± 3,410 | 48,580 ± 3,027 | 9,699 ± 1,557 | 59,189 ± 2,271 | |
| UFP | 57,666 ± 3,519 | 49,307 ± 3,261 | 50,241 ± 2,848 | 56,880 ± 3,515 | 58,283 ± 3,020 | ||
| PMNs | |||||||
| CD11a | Air | 30,921 ± 851 | 30,934 ± 862 | 31,339 ± 960 | 31,683 ± 944 | 31,712 ± 937 | Exposure × time |
| UFP | 31,569 ± 1,014 | 32,158 ± 1,055 | 31,652 ± 912 | 31,751 ± 927 | 32,130 ± 921 | p = 0.037 | |
| CD11b | Air | 16,406 ± 628 | 18,053 ± 934 | 17,262 ± 678 | 17,355 ± 869 | 17,525 ± 848 | |
| UFP | 16,678 ± 830 | 19,155 ± 1,953 | 17,076 ± 777 | 18,014 ± 713 | 17,545 ± 694 | ||
| CD18 | Air | 34,919 ± 1,335 | 36,961 ± 1,352 | 36,486 ± 1,286 | 35,907 ± 1,226 | 35,868 ± 1,450 | Exposure |
| UFP | 36,010 ± 1,032 | 37,687 ± 1,810 | 36,255 ± 1,060 | 35,316 ± 983 | 35,682 ± 1,087 | p = 0.023 | |
| CD49d | Air | 6,455 ± 412 | 6,345 ± 264 | 6,399 ± 279 | 6,145 ± 204 | 6,070 ± 203 | |
| UFP | 6,186 ± 335 | 6,252 ± 330 | 6,362 ± 340 | 6,284 ± 305 | 6,114 ± 258 | ||
| CD54 | Air | 8,182 ± 584 | 8,339 ± 484 | 8,973 ± 552 | 8,114 ± 415 | 8,072 ± 383 | |
| UFP | 8,524 ± 427 | 9,071 ± 545 | 8,668 ± 458 | 8,501 ± 402 | 8,446 ± 389 | ||
| CD62L | Air | 87,437 ± 4,510 | 88,596 ± 3,485 | 88,617 ± 4,056 | 87,244 ± 3,362 | 89,489 ± 2,648 | |
| UFP | 92,053 ± 4,760 | 89,783 ± 4,262 | 90,736 ± 4,227 | 89,363 ± 3,898 | 94,055 ± 4,598 | ||
Footnotes
This work was supported by contract 98-19 from the Health Effects Institute (HEI); U.S. Environmental Protection Agency (EPA) assistance agreements R826781-01 and R827354-01; grants RO1 ES011853, RR00044, and ES01247 from the National Institutes of Health; and grant 4913-ERTER-ES-99 from the New York State Energy Research and Development Authority.
Some of the research described in this article was conducted under contract to the HEI, an organization jointly funded by the U.S. EPA (assistance agreement X-812059) and automotive manufacturers. The contents of this article do not necessarily reflect the views of the HEI, nor do they necessarily reflect the policies of the U.S. EPA or of automotive manufacturers.
References
- Anderson GJ, Roswit WT, Holtzman MJ, Hogg JC, Van Eeden SF. Effect of mechanical deformation of neutrophils on their CD18/ICAM-1-dependent adhesion. J Appl Physiol. 2001;91:1084–1090. doi: 10.1152/jappl.2001.91.3.1084. [DOI] [PubMed] [Google Scholar]
- Atkinson RW, Anderson HR, Sunyer J, Ayres J, Baccini M, Vonk JM, et al. Acute effects of particulate air pollution on respiratory admissions. Results from APHEA 2 project. Am J Respir Crit Care Med. 2001;164:1860–1866. doi: 10.1164/ajrccm.164.10.2010138. [DOI] [PubMed] [Google Scholar]
- Batalha JR, Saldiva PH, Clarke RW, Coull BA, Stearns RC, Lawrence J, et al. Concentrated ambient air particles induce vasoconstriction of small pulmonary arteries in rats. Environ Health Perspect. 2002;110:1191–1197. doi: 10.1289/ehp.021101191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand P, Gebhart J, Below M, Georgi B, Heyder J. Characterization of environmental aerosols on Heligoland Island. Atmos Environ. 1991;25A:581–585. [Google Scholar]
- Brand P, Ruob K, Gebhart J. Performance of a mobile aerosol spectrometer for an in situ characterization of environmental aerosols in Frankfurt City. Atmos Environ. 1992;26A:2451–2457. [Google Scholar]
- Brook RD, Brook JR, Urch B, Vincent R, Rajagopalan S, Silverman F. Inhalation of fine particulate air pollution and ozone causes acute arterial vasoconstriction in healthy adults. Circulation. 2002;105:1534–1536. doi: 10.1161/01.cir.0000013838.94747.64. [DOI] [PubMed] [Google Scholar]
- Brown DM, Wilson MR, MacNee W, Stone V, Donaldson K. Size-dependent proinflammatory effects of ultrafine polystyrene particles: a role for surface area and oxidative stress in the enhanced activity of ultrafines. Toxicol Appl Pharmacol. 2001;175:191–199. doi: 10.1006/taap.2001.9240. [DOI] [PubMed] [Google Scholar]
- Chalupa DC, Morrow PE, Oberdörster G, Utell MJ, Frampton MW. Ultrafine particle deposition in subjects with asthma. Environ Health Perspect. 2004;112:879–882. doi: 10.1289/ehp.6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalupa DF, Gibb FR, Morrow PE, Oberdörster G, Riesenfeld E, Gelein R, et al. 2002. A facility for controlled human exposures to ultrafine particles. In: Crucial Issues in Inhalation Research—Mechanistic, Clinical and Epidemiologic (Heinrich U, Mohr U, eds). Washington, DC:ILSI Press, 241–253.
- Corrigan CJ, Hamid Q, North J, Barkans J, Moqbel R, Durham S, et al. Peripheral blood CD4 but not CD8 T-lymphocytes in patients with exacerbation of asthma transcribe and translate messenger RNA encoding cytokines which prolong eosinophil survival in the context of a Th2-type pattern: effect of glucocorticoid therapy. Am J Respir Cell Mol Biol. 1995;12:567–578. doi: 10.1165/ajrcmb.12.5.7742019. [DOI] [PubMed] [Google Scholar]
- Corrigan CJ, Kay AB. CD4 T-lymphocyte activation in acute severe asthma. Am Rev Respir Dis. 1990;141:970–977. doi: 10.1164/ajrccm/141.4_Pt_1.. [DOI] [PubMed] [Google Scholar]
- Daigle CC, Chalupa DC, Gibb FR, Morrow PE, Oberdörster G, Utell MJ, et al. Ultrafine particle deposition in humans during rest and exercise. Inhal Toxicol. 2003;15:539–552. doi: 10.1080/08958370304468. [DOI] [PubMed] [Google Scholar]
- De Caterina R, Libby P, Peng HB, Thannickal VJ, Rajavashisth TB, Gimbrone MAJ, et al. Nitric oxide decreases cytokine-induced endothelial activation. J Clin Invest. 1995;96:60–68. doi: 10.1172/JCI118074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerschuk CM. 2003. Neutrophil emigration in the lungs. In: Therapeutic Targets in Airway Inflammation (Eissa T, Huston DP, eds). New York:Marcel Dekker, 249–280.
- Frampton MW, Stewart JC, Oberdörster G, Pietropaoli AP, Morrow PE, Chalupa D, et al. Inhalation of carbon ultrafine particles decreases expression of CD18 and CD11a on blood leukocytes [Abstract] Am J Respir Crit Care Med. 2004;169:A280. [Google Scholar]
- Gavras JB, Frampton MW, Ryan DH, Levy PC, Looney RJ, Cox C, et al. Expression of membrane antigens on human alveolar macrophages after exposure to nitrogen dioxide. Inhal Toxicol. 1994;6:633–646. [Google Scholar]
- Ibald-Mulli A, Stieber J, Wichmann HE, Koenig W, Peters A. Effects of air pollution on blood pressure: a population-based approach. Am J Public Health. 2001;91:571–577. doi: 10.2105/ajph.91.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B, Kenward MG. 1989. Design and Analysis of Crossover Trials. New York:Chapman and Hall.
- Kim H, Liu X, Kobayashi T, Kohyama T, Wen F-Q, Romberger DJ, et al. Ultrafine carbon black particles inhibit human lung fibroblast-mediated collagen gel contraction. Am J Respir Cell Mol Biol. 2003;28:111–121. doi: 10.1165/rcmb.4796. [DOI] [PubMed] [Google Scholar]
- Kittelson DB, Watts WF, Johnson JP. 2001. Fine Particle (nanoparticle) Emissions on Minnesota Highways. Mn/DOT Report No. 2001-12. St. Paul, MN:Minnesota Department of Transportation.
- Künzli N, Jerrett M, Mack WJ, Beckerman B, LaBree L, Gilliland F, et al. Ambient air pollution and atherosclerosis in Los Angeles. Environ Health Perspect. 2005;113:201–206. doi: 10.1289/ehp.7523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefer AM. Nitric oxide: nature’s naturally occurring leukocyte inhibitor. Circulation. 1997;95:553–554. doi: 10.1161/01.cir.95.3.553. [DOI] [PubMed] [Google Scholar]
- Leslie CA, Dubey DP. Increased PGE2 from human monocytes isolated in the luteal phase of the menstrual cycle. Implications for immunity? Prostaglandins. 1994;47:41–54. doi: 10.1016/0090-6980(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Li N, Sioutas C, Cho A, Schmitz D, Misra C, Sempf J, et al. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ Health Perspect. 2003;111:455–460. doi: 10.1289/ehp.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XY, Brown D, Smith S, MacNee W, Donaldson K. Short-term inflammatory responses following intratracheal instillation of fine and ultrafine carbon black in rats. Inhal Toxicol. 1999;11:709–731. doi: 10.1080/089583799196826. [DOI] [PubMed] [Google Scholar]
- Lipsett M, Hurley S, Ostro B. Air pollution and emergency room visits for asthma in Santa Clara County, California. Environ Health Perspect. 1997;105:216–222. doi: 10.1289/ehp.97105216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukae H, Vincent R, Quinlan K, English D, Hards J, Hogg JC, et al. The effect of repeated exposure to particulate air pollution (PM10) on the bone marrow. Am J Respir Crit Care Med. 2001;163:201–209. doi: 10.1164/ajrccm.163.1.2002039. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health 1997. Expert Panel Report 2, Guidelines for the Diagnosis and Management of Asthma. NIH Publication No. 97-4051. Bethesda, MD:National Institutes of Health, U.S. Department of Health and Human Services.
- Nemmar A, Hoet PH, Vanquickenborne B, Dinsdale D, Thomeer M, Hoylaerts MF, et al. Passage of inhaled particles into the blood circulation in humans. Circulation. 2002;105:411–414. doi: 10.1161/hc0402.104118. [DOI] [PubMed] [Google Scholar]
- Oberdörster G, Gelein RM, Ferin J, Weiss B. Association of particulate air pollution and acute mortality: involvement of ultrafine particles? Inhal Toxicol. 1995;7:111–124. doi: 10.3109/08958379509014275. [DOI] [PubMed] [Google Scholar]
- Oberdörster G, Sharp Z, Attudorei V, Elder A, Gelein R, Lunts A, et al. Extrapulmonary translocation of ultrafine carbon particles following whole-body inhalation exposure of rats. J Toxicol Environ Health A. 2002;65:1531–1543. doi: 10.1080/00984100290071658. [DOI] [PubMed] [Google Scholar]
- Ohkawara Y, Yamauchi K, Maruyama N, Hoshi H, Ohno I, Honma M, et al. In situ expression of the cell adhesion molecules in bronchial tissues from asthmatics with air flow limitation: in vivo evidence of VCAM-1/VLA-4 interaction in selective eosinophil infiltration. Am J Respir Cell Mol Biol. 1995;12:4–12. doi: 10.1165/ajrcmb.12.1.7529029. [DOI] [PubMed] [Google Scholar]
- Pekkanen J, Brunner EJ, Anderson HR, Tiitanen P, Atkinson RW. Daily concentrations of air pollution and plasma fibrinogen in London. Occup Environ Med. 2000;57:818–822. doi: 10.1136/oem.57.12.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekkanen J, Peters A, Hoek G, Tiittanen P, Brunekreef B, de Hartog J, et al. Particulate air pollution and risk of ST-segment depression during repeated submaximal exercise tests among subjects with coronary heart disease. The exposure and risk assessment for fine and ultrafine particles in ambient air (ULTRA) study. Circulation. 2002;106:933–938. doi: 10.1161/01.cir.0000027561.41736.3c. [DOI] [PubMed] [Google Scholar]
- Peters A, Dockery DW, Muller JE, Mittleman MA. Increased particulate air pollution and the triggering of myocardial infarction. Circulation. 2001a;103:2810–2815. doi: 10.1161/01.cir.103.23.2810. [DOI] [PubMed] [Google Scholar]
- Peters A, Doring A, Wichmann H-E, Koenig W. Increased plasma viscosity during an air pollution episode: a link to mortality? Lancet. 1997a;349:1582–1587. doi: 10.1016/S0140-6736(97)01211-7. [DOI] [PubMed] [Google Scholar]
- Peters A, Frohlich M, Doring A, Immervoll T, Wichmann HE, Hutchinson WL, et al. Particulate air pollution is associated with an acute phase response in men; results from the MONICA-Augsburg Study. Eur Heart J. 2001b;22:1198–1204. doi: 10.1053/euhj.2000.2483. [DOI] [PubMed] [Google Scholar]
- Peters A, Liu E, Verrier RL, Schwartz J, Gold DR, Mittleman M, et al. Air pollution and incidence of cardiac arrhythmia. Epidemiology. 2000;11:11–17. doi: 10.1097/00001648-200001000-00005. [DOI] [PubMed] [Google Scholar]
- Peters A, Wichmann HE, Tuch T, Heinrich J, Heyder J. Respiratory effects are associated with the number of ultra-fine particles. Am J Respir Crit Care Med. 1997b;155:1376–1383. doi: 10.1164/ajrccm.155.4.9105082. [DOI] [PubMed] [Google Scholar]
- Pietropaoli AP, Delehanty JM, Perkins PT, Utell MJ, Oberdörster G, Hyde RW, et al. Venous nitrate, nitrite, and forearm blood flow after carbon ultrafine particle exposure in healthy human subjects [Abstract] Am J Respir Crit Care Med. 2004a;169:A883. [Google Scholar]
- Pietropaoli AP, Frampton MW, Hyde RW, Morrow PE, Oberdörster G, Cox C, et al. Pulmonary function, diffusing capacity and inflammation in healthy and asthmatic subjects exposed to ultrafine particles. Inhal Toxicol. 2004b;16(suppl 1):59–72. doi: 10.1080/08958370490443079. [DOI] [PubMed] [Google Scholar]
- Pietropaoli AP, Frampton MW, Oberdörster G, Cox C, Huang LS, Marder V, et al. 2004c. Blood markers of coagulation and inflammation in healthy human subjects exposed to carbon ultrafine particles. In: Effects of Air Contaminants on the Respiratory Tract—Interpretations from Molecular to Meta analysis (Heinrich U, ed). Stuttgart:INIS Monographs, Fraunhofer IRB Verlag, 181–194.
- Pope CA, III, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, et al. Cardiovascular mortality and long-term exposure to particulate air pollution. Epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109:71–77. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- Schwartz J. Air pollution and blood markers of cardiovascular risk. Environ Health Perspect. 2001;109(suppl 3):405–409. doi: 10.1289/ehp.01109s3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz E, Schafer C, Bode JC, Bode C. Influence of the menstrual cycle on the LPS-induced cytokine response of monocytes. Cytokine. 2000;12:413–416. doi: 10.1006/cyto.1999.0570. [DOI] [PubMed] [Google Scholar]
- Seaton A, MacNee W, Donaldson K, Godden D. Particulate air pollution and acute health effects. Lancet. 1995;345:176–178. doi: 10.1016/s0140-6736(95)90173-6. [DOI] [PubMed] [Google Scholar]
- Stearns RC, Murthy GGK, Skornik W, Hatch V, Katler M, Godleski JJ. 1994. Detection of ultrafine copper oxide particles in the lungs of hamsters by electron spectroscopic imaging. In: Proceedings of ICEM 13-PARIS, 1994 (Jouffrey B, Colliex C, eds). Paris:Les Editions de Physique, 763–764.
- Tan WC, Qiu D, Liam BL, Ng TP, Lee SH, van Eeden SF, et al. The human bone marrow response to acute air pollution caused by forest fires. Am J Respir Crit Care Med. 2000;161:1213–1217. doi: 10.1164/ajrccm.161.4.9904084. [DOI] [PubMed] [Google Scholar]
- Tolbert PE, Mulholland JA, MacIntosh DL, Xu F, Daniels D, Devine OJ, et al. Air quality and pediatric emergency room visits for asthma in Atlanta, Georgia, USA. Am J Epidemiol. 2000;151:798–810. doi: 10.1093/oxfordjournals.aje.a010280. [DOI] [PubMed] [Google Scholar]
- Utell MJ, Frampton MW, Zareba W, Devlin RB, Cascio WE. Cardiovascular effects associated with air pollution: potential mechanisms and methods of testing. Inhal Toxicol. 2002;14:1231–1247. doi: 10.1080/08958370290084881. [DOI] [PubMed] [Google Scholar]
- van Eeden SF, Granton J, Hards JM, Moore B, Hogg JC. Expression of the cell adhesion molecules on leukocytes that demarginate during acute maximal exercise. J Appl Physiol. 1999;86:970–976. doi: 10.1152/jappl.1999.86.3.970. [DOI] [PubMed] [Google Scholar]
- Virchow JCJ, Walker C, Hafner D, Kortsik C, Werner P, Matthys H, et al. T cells and cytokines in bronchoalveolar lavage fluid after segmental allergen provocation in atopic asthma. Am J Respir Crit Care Med. 1995;151:960–968. doi: 10.1164/ajrccm/151.4.960. [DOI] [PubMed] [Google Scholar]
- Walker C, Bode E, Boer L, Hansel TT, Blaser K, Virchow J-CJ. Allergic and nonallergic asthmatics have distinct patterns of T-cell activation and cytokine production in peripheral blood and bronchoalveolar lavage. Am Rev Respir Dis. 1992;146:109–115. doi: 10.1164/ajrccm/146.1.109. [DOI] [PubMed] [Google Scholar]
- Wallenstein S, Fisher AC. Analysis of the two-period repeated measurements crossover design with application to clinical trials. Biometrics. 1977;30:261–269. [PubMed] [Google Scholar]
- Wichmann H-E, Spix C, Tuch T, Wölke G, Peters A, Heinrich J, et al. Daily mortality and fine and ultrafine particles in Erfurt, Germany. Part I: Role of particle number and particle mass. Health Eff Inst Res Rep. 2000;98:1–86. [PubMed] [Google Scholar]
- Wilson JW, Djukanovic R, Howarth PH, Holgate ST. Lymphocyte activation in bronchoalveolar lavage and peripheral blood in atopic asthma. Am Rev Respir Dis. 1992;145:958–960. doi: 10.1164/ajrccm/145.4_Pt_1.958. [DOI] [PubMed] [Google Scholar]
- Wilson JW, Djukanovic R, Howarth PH, Holgate ST. Inhaled beclomethasone dipropionate downregulates airway lymphocyte activation in atopic asthma. Am J Respir Crit Care Med. 1994;149:86–90. doi: 10.1164/ajrccm.149.1.8111605. [DOI] [PubMed] [Google Scholar]