Abstract
The ability to regulate volume is an important property of most, if not all cells. In epithelial cells, amongst others, cell volume-activated chloride channels are central to this response. The molecular identities of these channels are not yet known. Expression of the human multidrug resistance P-glycoprotein (P-gp) has been associated with cell volume-regulated chloride currents, although the nature of this association is the subject of debate. Recent data indicate that P-gp acts by regulating the activation of an endogenous channel protein. In this review, evidence associating P-gp with cell volume-activated chloride currents, and the possible mechanisms by which this might be achieved, are discussed.
Full text
PDF
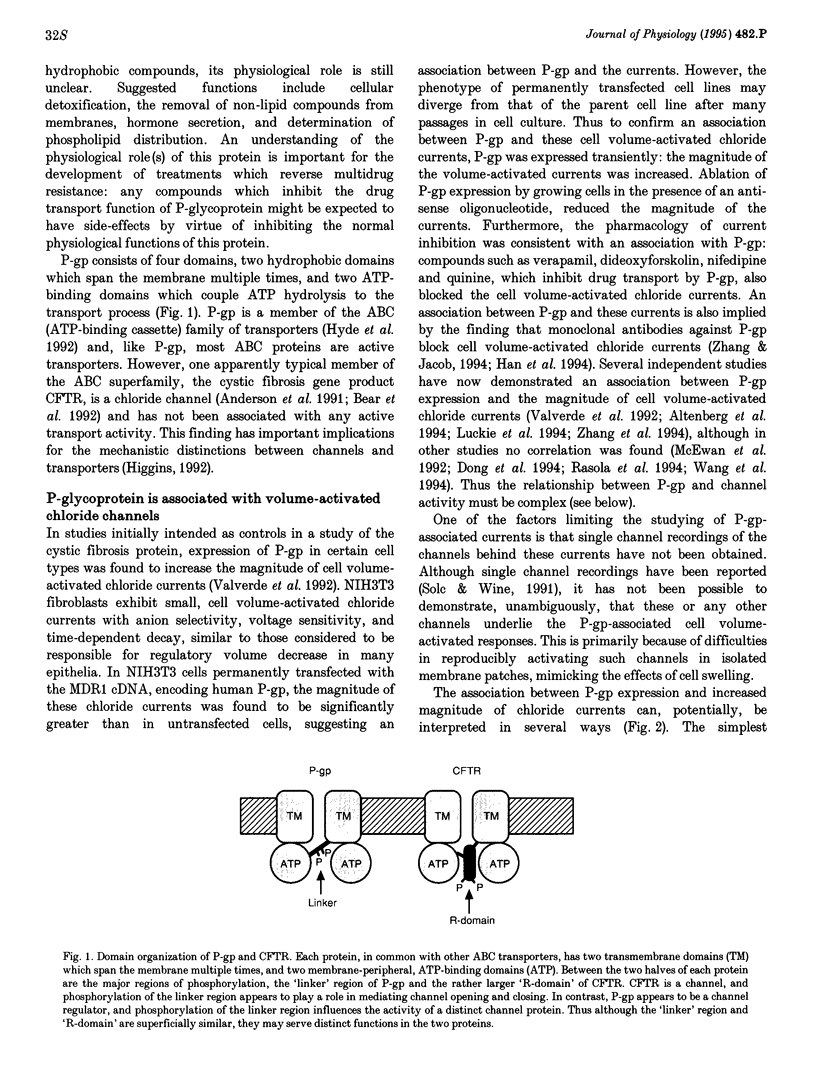
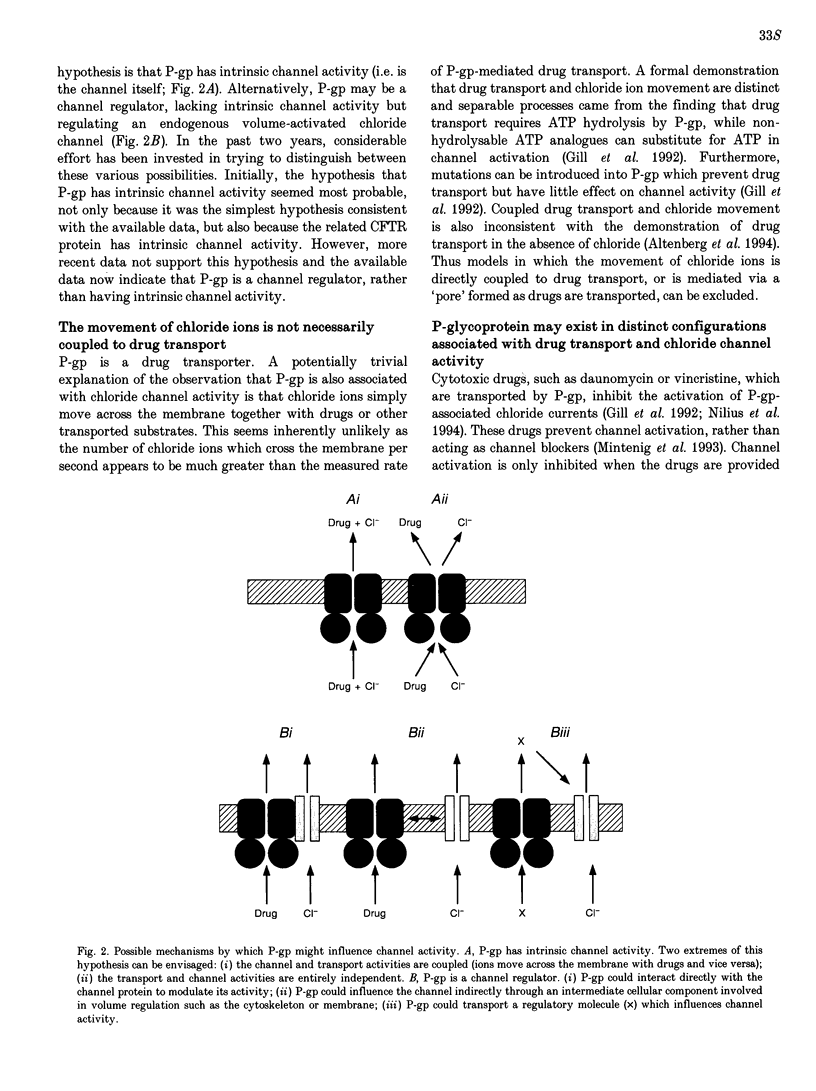



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman M. J., Wickman K. D., Clapham D. E. Hypotonicity activates a native chloride current in Xenopus oocytes. J Gen Physiol. 1994 Feb;103(2):153–179. doi: 10.1085/jgp.103.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altenberg G. A., Deitmer J. W., Glass D. C., Reuss L. P-glycoprotein-associated Cl- currents are activated by cell swelling but do not contribute to cell volume regulation. Cancer Res. 1994 Feb 1;54(3):618–622. [PubMed] [Google Scholar]
- Altenberg G. A., Vanoye C. G., Han E. S., Deitmer J. W., Reuss L. Relationships between rhodamine 123 transport, cell volume, and ion-channel function of P-glycoprotein. J Biol Chem. 1994 Mar 11;269(10):7145–7149. [PubMed] [Google Scholar]
- Anderson M. P., Gregory R. J., Thompson S., Souza D. W., Paul S., Mulligan R. C., Smith A. E., Welsh M. J. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science. 1991 Jul 12;253(5016):202–205. doi: 10.1126/science.1712984. [DOI] [PubMed] [Google Scholar]
- Bear C. E., Li C. H., Kartner N., Bridges R. J., Jensen T. J., Ramjeesingh M., Riordan J. R. Purification and functional reconstitution of the cystic fibrosis transmembrane conductance regulator (CFTR). Cell. 1992 Feb 21;68(4):809–818. doi: 10.1016/0092-8674(92)90155-6. [DOI] [PubMed] [Google Scholar]
- Breuer W., Slotki I. N., Ausiello D. A., Cabantchik I. Z. Induction of multidrug resistance downregulates the expression of CFTR in colon epithelial cells. Am J Physiol. 1993 Dec;265(6 Pt 1):C1711–C1715. doi: 10.1152/ajpcell.1993.265.6.C1711. [DOI] [PubMed] [Google Scholar]
- Chambers T. C., Pohl J., Raynor R. L., Kuo J. F. Identification of specific sites in human P-glycoprotein phosphorylated by protein kinase C. J Biol Chem. 1993 Mar 5;268(7):4592–4595. [PubMed] [Google Scholar]
- Dong Y., Chen G., Durán G. E., Kouyama K., Chao A. C., Sikic B. I., Gollapudi S. V., Gupta S., Gardner P. Volume-activated chloride current is not related to P-glycoprotein overexpression. Cancer Res. 1994 Oct 1;54(19):5029–5032. [PubMed] [Google Scholar]
- Díaz M., Valverde M. A., Higgins C. F., Rucăreanu C., Sepúlveda F. V. Volume-activated chloride channels in HeLa cells are blocked by verapamil and dideoxyforskolin. Pflugers Arch. 1993 Jan;422(4):347–353. doi: 10.1007/BF00374290. [DOI] [PubMed] [Google Scholar]
- Egan M., Flotte T., Afione S., Solow R., Zeitlin P. L., Carter B. J., Guggino W. B. Defective regulation of outwardly rectifying Cl- channels by protein kinase A corrected by insertion of CFTR. Nature. 1992 Aug 13;358(6387):581–584. doi: 10.1038/358581a0. [DOI] [PubMed] [Google Scholar]
- Gabriel S. E., Clarke L. L., Boucher R. C., Stutts M. J. CFTR and outward rectifying chloride channels are distinct proteins with a regulatory relationship. Nature. 1993 May 20;363(6426):263–268. doi: 10.1038/363263a0. [DOI] [PubMed] [Google Scholar]
- Gill D. R., Hyde S. C., Higgins C. F., Valverde M. A., Mintenig G. M., Sepúlveda F. V. Separation of drug transport and chloride channel functions of the human multidrug resistance P-glycoprotein. Cell. 1992 Oct 2;71(1):23–32. doi: 10.1016/0092-8674(92)90263-c. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M., Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- Gründer S., Thiemann A., Pusch M., Jentsch T. J. Regions involved in the opening of CIC-2 chloride channel by voltage and cell volume. Nature. 1992 Dec 24;360(6406):759–762. doi: 10.1038/360759a0. [DOI] [PubMed] [Google Scholar]
- Higgins C. F. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- Hoffmann E. K., Simonsen L. O. Membrane mechanisms in volume and pH regulation in vertebrate cells. Physiol Rev. 1989 Apr;69(2):315–382. doi: 10.1152/physrev.1989.69.2.315. [DOI] [PubMed] [Google Scholar]
- Hyde S. C., Emsley P., Hartshorn M. J., Mimmack M. M., Gileadi U., Pearce S. R., Gallagher M. P., Gill D. R., Hubbard R. E., Higgins C. F. Structural model of ATP-binding proteins associated with cystic fibrosis, multidrug resistance and bacterial transport. Nature. 1990 Jul 26;346(6282):362–365. doi: 10.1038/346362a0. [DOI] [PubMed] [Google Scholar]
- Jirsch J. D., Loe D. W., Cole S. P., Deeley R. G., Fedida D. ATP is not required for anion current activated by cell swelling in multidrug-resistant lung cancer cells. Am J Physiol. 1994 Sep;267(3 Pt 1):C688–C699. doi: 10.1152/ajpcell.1994.267.3.C688. [DOI] [PubMed] [Google Scholar]
- Jirsch J., Deeley R. G., Cole S. P., Stewart A. J., Fedida D. Inwardly rectifying K+ channels and volume-regulated anion channels in multidrug-resistant small cell lung cancer cells. Cancer Res. 1993 Sep 15;53(18):4156–4160. [PubMed] [Google Scholar]
- Krapivinsky G. B., Ackerman M. J., Gordon E. A., Krapivinsky L. D., Clapham D. E. Molecular characterization of a swelling-induced chloride conductance regulatory protein, pICln. Cell. 1994 Feb 11;76(3):439–448. doi: 10.1016/0092-8674(94)90109-0. [DOI] [PubMed] [Google Scholar]
- Luckie D. B., Krouse M. E., Harper K. L., Law T. C., Wine J. J. Selection for MDR1/P-glycoprotein enhances swelling-activated K+ and Cl- currents in NIH/3T3 cells. Am J Physiol. 1994 Aug;267(2 Pt 1):C650–C658. doi: 10.1152/ajpcell.1994.267.2.C650. [DOI] [PubMed] [Google Scholar]
- McEwan G. T., Hunter J., Hirst B. H., Simmons N. L. Volume-activated Cl- secretion and transepithelial vinblastine secretion mediated by P-glycoprotein are not correlated in cultured human T84 intestinal epithelial layers. FEBS Lett. 1992 Jun 15;304(2-3):233–236. doi: 10.1016/0014-5793(92)80626-r. [DOI] [PubMed] [Google Scholar]
- Mintenig G. M., Valverde M. A., Sepulveda F. V., Gill D. R., Hyde S. C., Kirk J., Higgins C. F. Specific inhibitors distinguish the chloride channel and drug transporter functions associated with the human multidrug resistance P-glycoprotein. Receptors Channels. 1993;1(4):305–313. [PubMed] [Google Scholar]
- Nilius B., Sehrer J., Droogmans G. Permeation properties and modulation of volume-activated Cl(-)-currents in human endothelial cells. Br J Pharmacol. 1994 Aug;112(4):1049–1056. doi: 10.1111/j.1476-5381.1994.tb13189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr G. A., Han E. K., Browne P. C., Nieves E., O'Connor B. M., Yang C. P., Horwitz S. B. Identification of the major phosphorylation domain of murine mdr1b P-glycoprotein. Analysis of the protein kinase A and protein kinase C phosphorylation sites. J Biol Chem. 1993 Nov 25;268(33):25054–25062. [PubMed] [Google Scholar]
- Parra-Lopez C., Lin R., Aspedon A., Groisman E. A. A Salmonella protein that is required for resistance to antimicrobial peptides and transport of potassium. EMBO J. 1994 Sep 1;13(17):3964–3972. doi: 10.1002/j.1460-2075.1994.tb06712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulmichl M., Li Y., Wickman K., Ackerman M., Peralta E., Clapham D. New mammalian chloride channel identified by expression cloning. Nature. 1992 Mar 19;356(6366):238–241. doi: 10.1038/356238a0. [DOI] [PubMed] [Google Scholar]
- Rasola A., Galietta L. J., Gruenert D. C., Romeo G. Volume-sensitive chloride currents in four epithelial cell lines are not directly correlated to the expression of the MDR-1 gene. J Biol Chem. 1994 Jan 14;269(2):1432–1436. [PubMed] [Google Scholar]
- Sarkadi B., Parker J. C. Activation of ion transport pathways by changes in cell volume. Biochim Biophys Acta. 1991 Dec 12;1071(4):407–427. doi: 10.1016/0304-4157(91)90005-h. [DOI] [PubMed] [Google Scholar]
- Solc C. K., Wine J. J. Swelling-induced and depolarization-induced C1-channels in normal and cystic fibrosis epithelial cells. Am J Physiol. 1991 Oct;261(4 Pt 1):C658–C674. doi: 10.1152/ajpcell.1991.261.4.C658. [DOI] [PubMed] [Google Scholar]
- Thiemann A., Gründer S., Pusch M., Jentsch T. J. A chloride channel widely expressed in epithelial and non-epithelial cells. Nature. 1992 Mar 5;356(6364):57–60. doi: 10.1038/356057a0. [DOI] [PubMed] [Google Scholar]
- Trezise A. E., Romano P. R., Gill D. R., Hyde S. C., Sepúlveda F. V., Buchwald M., Higgins C. F. The multidrug resistance and cystic fibrosis genes have complementary patterns of epithelial expression. EMBO J. 1992 Dec;11(12):4291–4303. doi: 10.1002/j.1460-2075.1992.tb05528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde M. A., Díaz M., Sepúlveda F. V., Gill D. R., Hyde S. C., Higgins C. F. Volume-regulated chloride channels associated with the human multidrug-resistance P-glycoprotein. Nature. 1992 Feb 27;355(6363):830–833. doi: 10.1038/355830a0. [DOI] [PubMed] [Google Scholar]
- Wang X., Wall D. M., Parkin J. D., Zalcberg J. R., Kemm R. E. P-glycoprotein expression in classical multi-drug resistant leukaemia cells does not correlate with enhanced chloride channel activity. Clin Exp Pharmacol Physiol. 1994 Feb;21(2):101–108. doi: 10.1111/j.1440-1681.1994.tb02475.x. [DOI] [PubMed] [Google Scholar]
- Zhang J. J., Jacob T. J., Valverde M. A., Hardy S. P., Mintenig G. M., Sepúlveda F. V., Gill D. R., Hyde S. C., Trezise A. E., Higgins C. F. Tamoxifen blocks chloride channels. A possible mechanism for cataract formation. J Clin Invest. 1994 Oct;94(4):1690–1697. doi: 10.1172/JCI117514. [DOI] [PMC free article] [PubMed] [Google Scholar]




