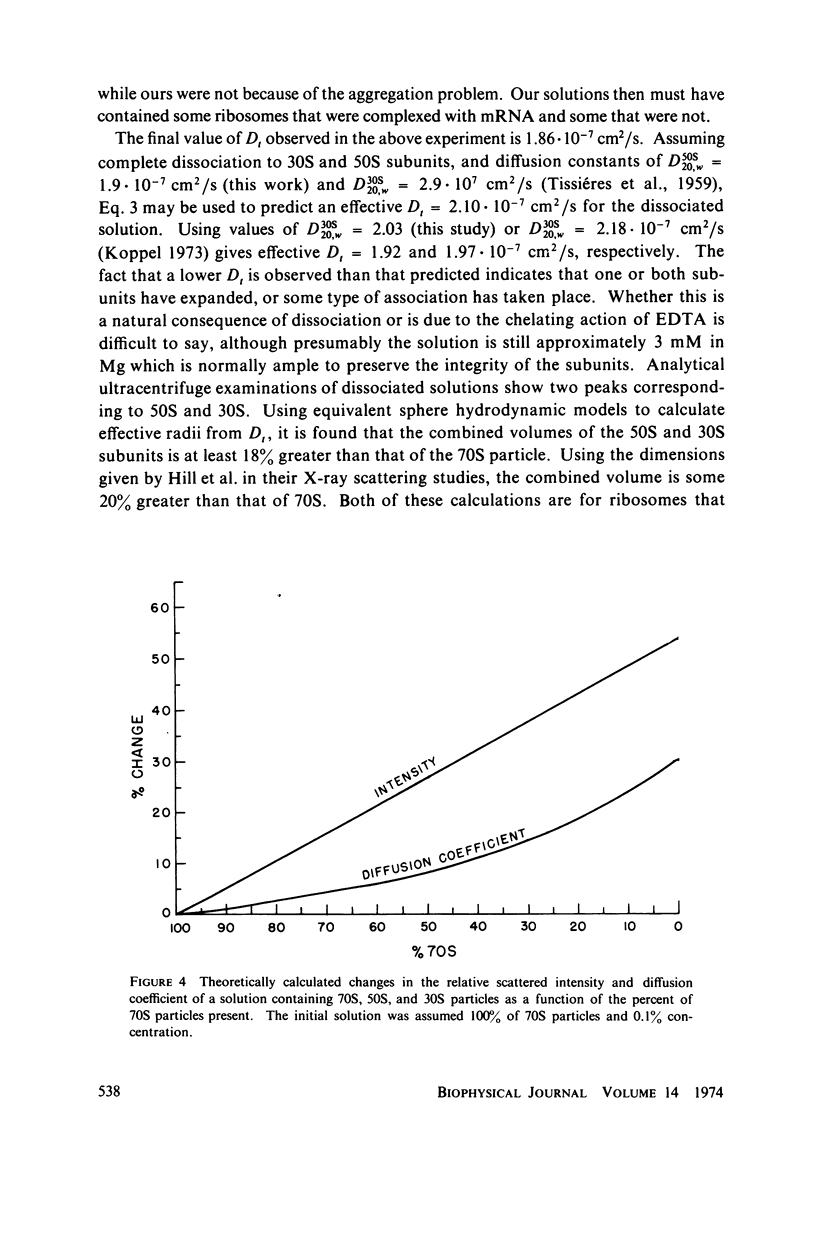
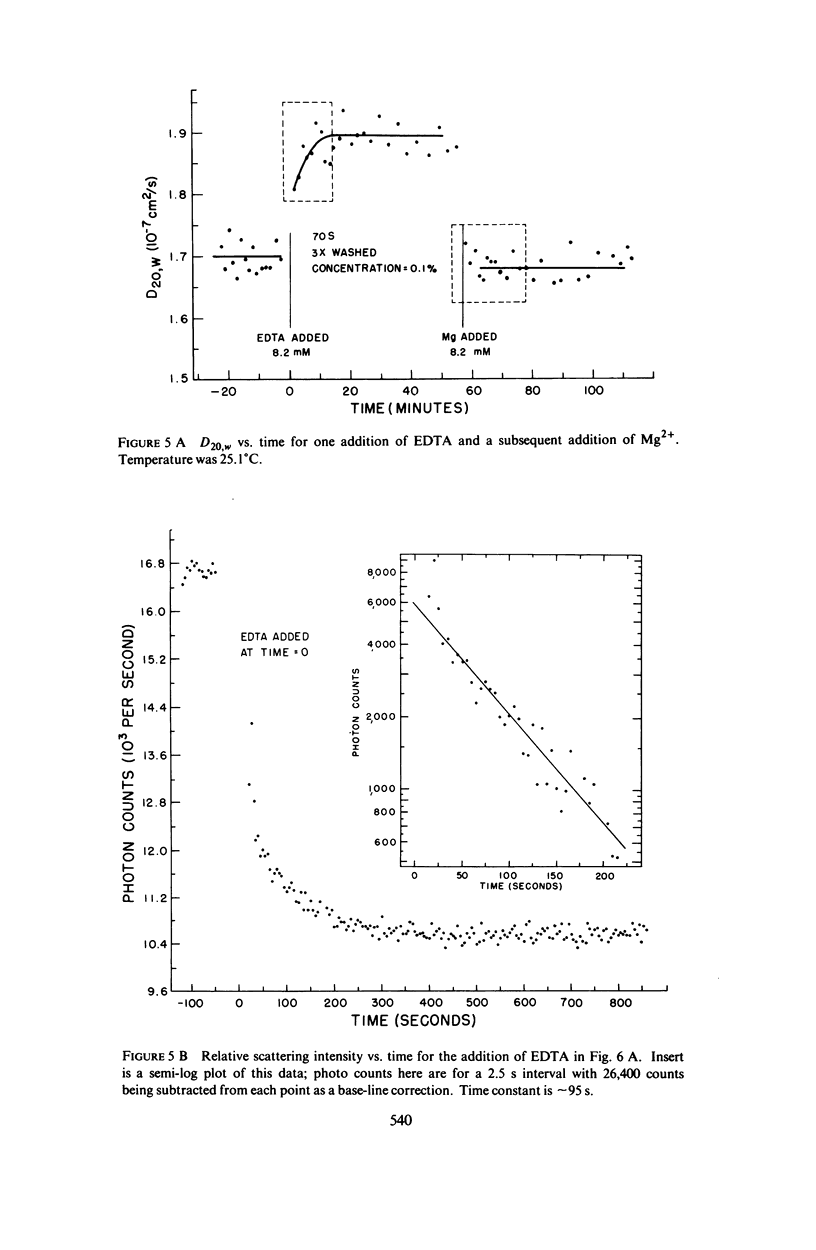
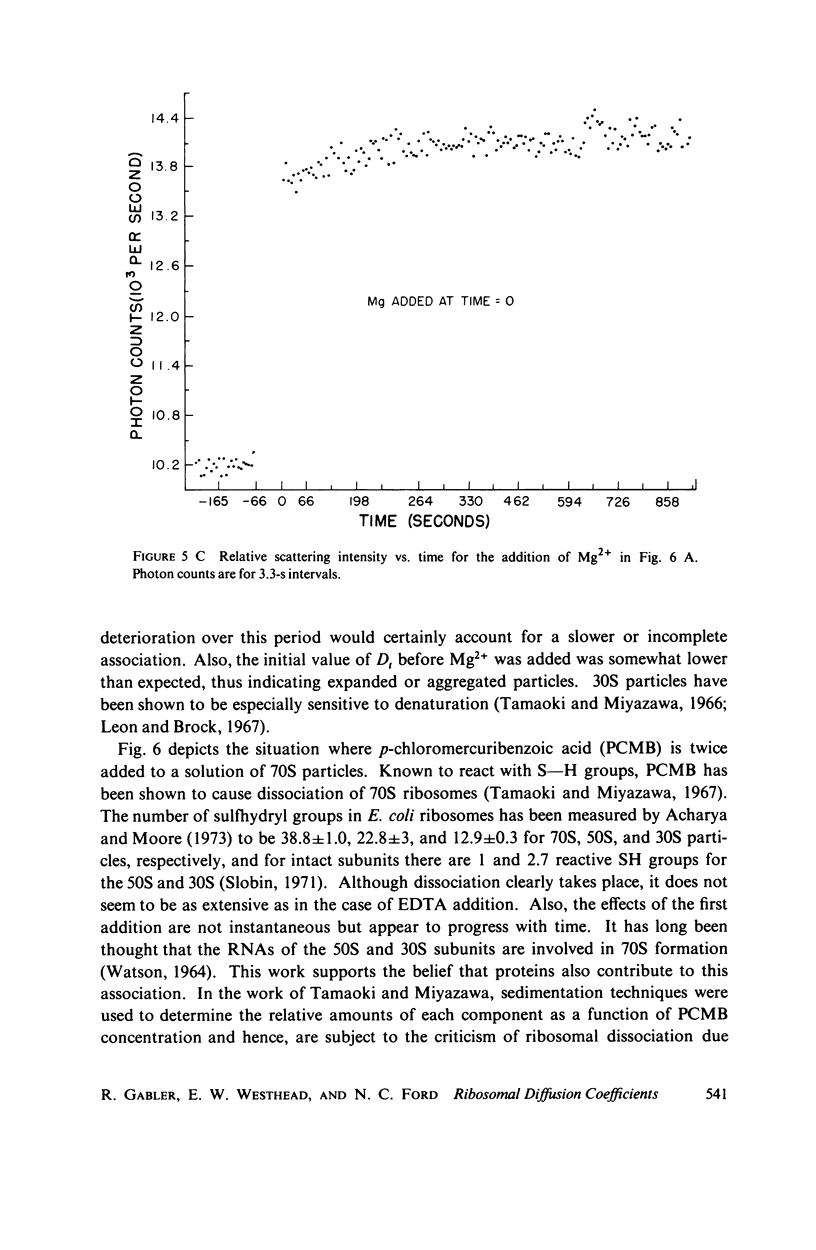
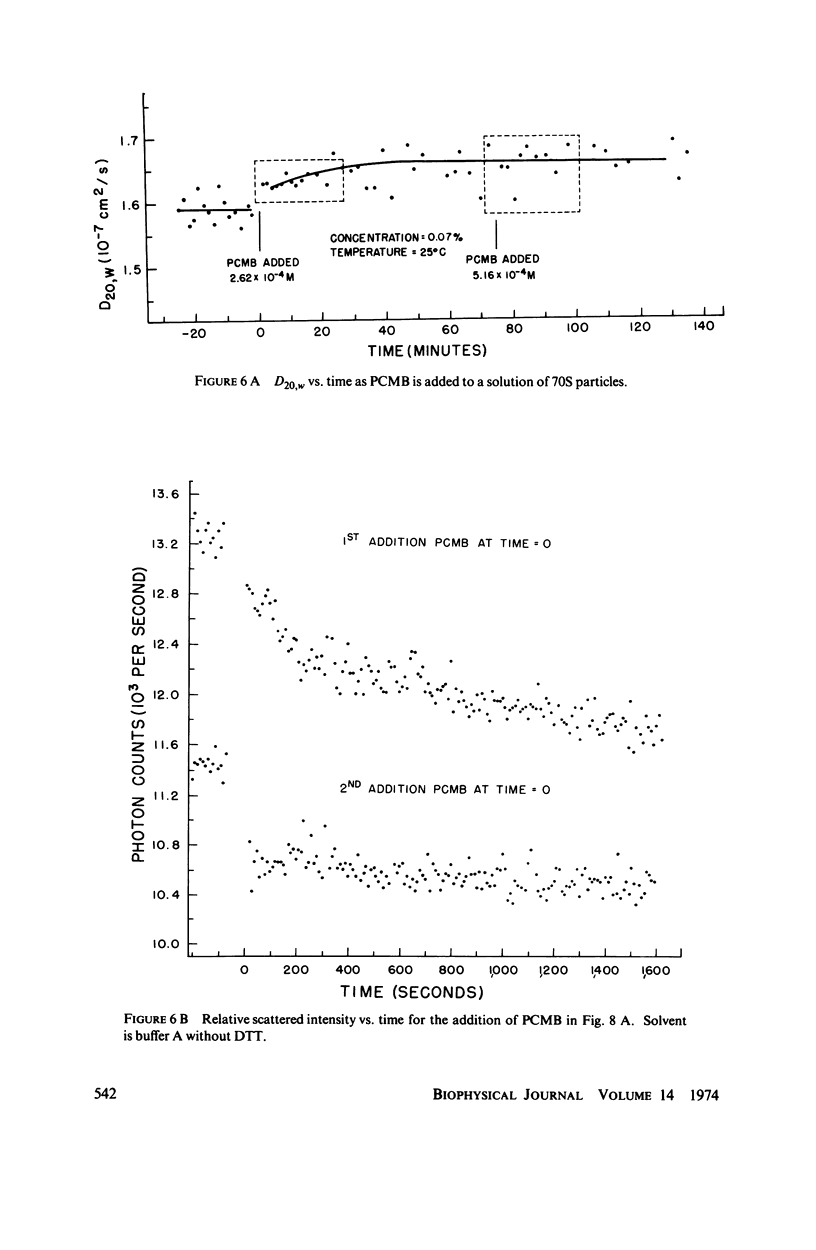
Abstract
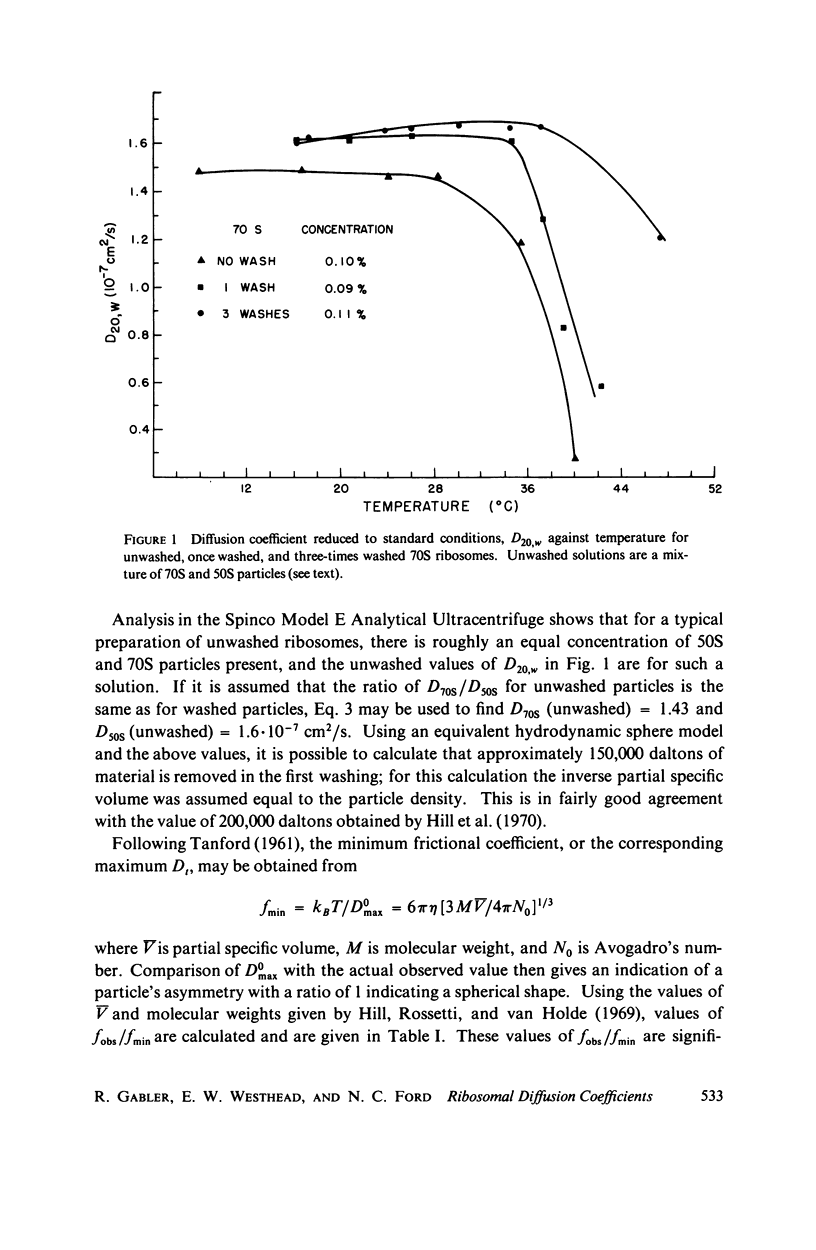
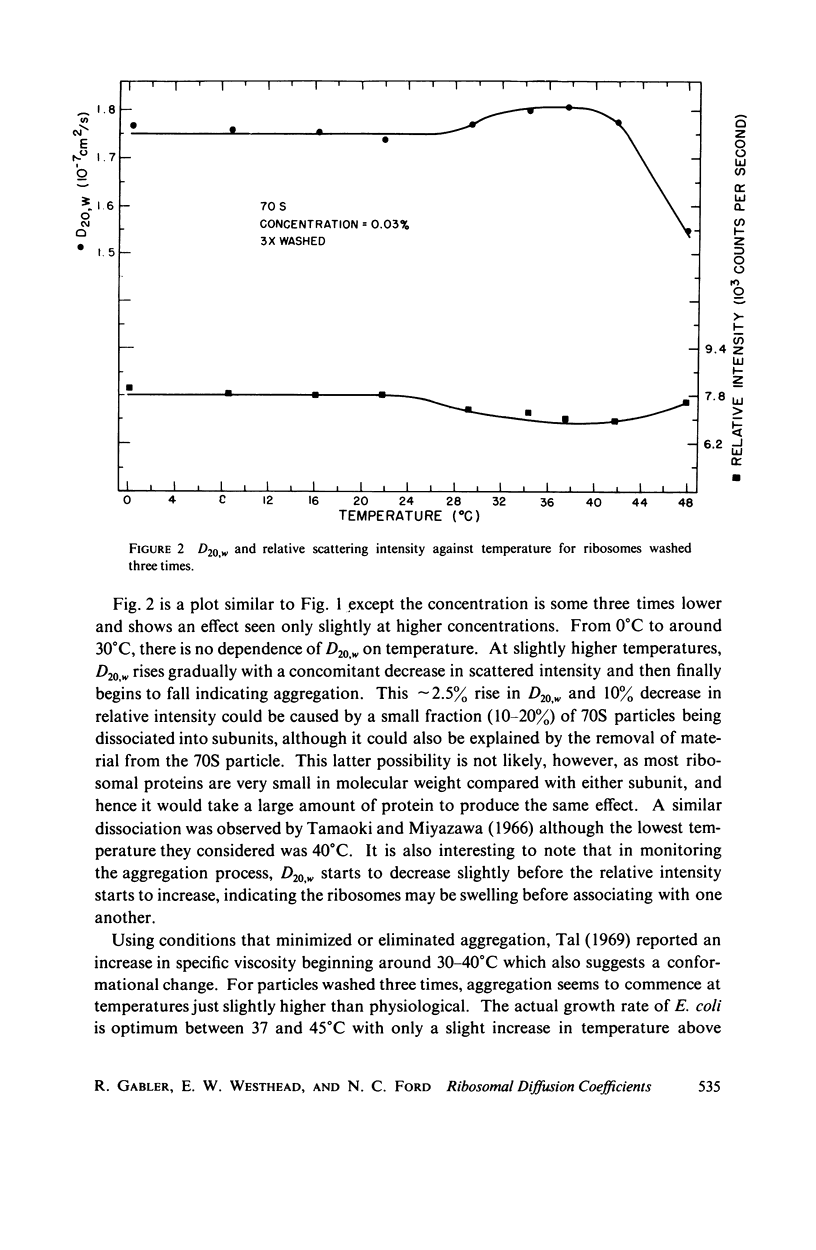
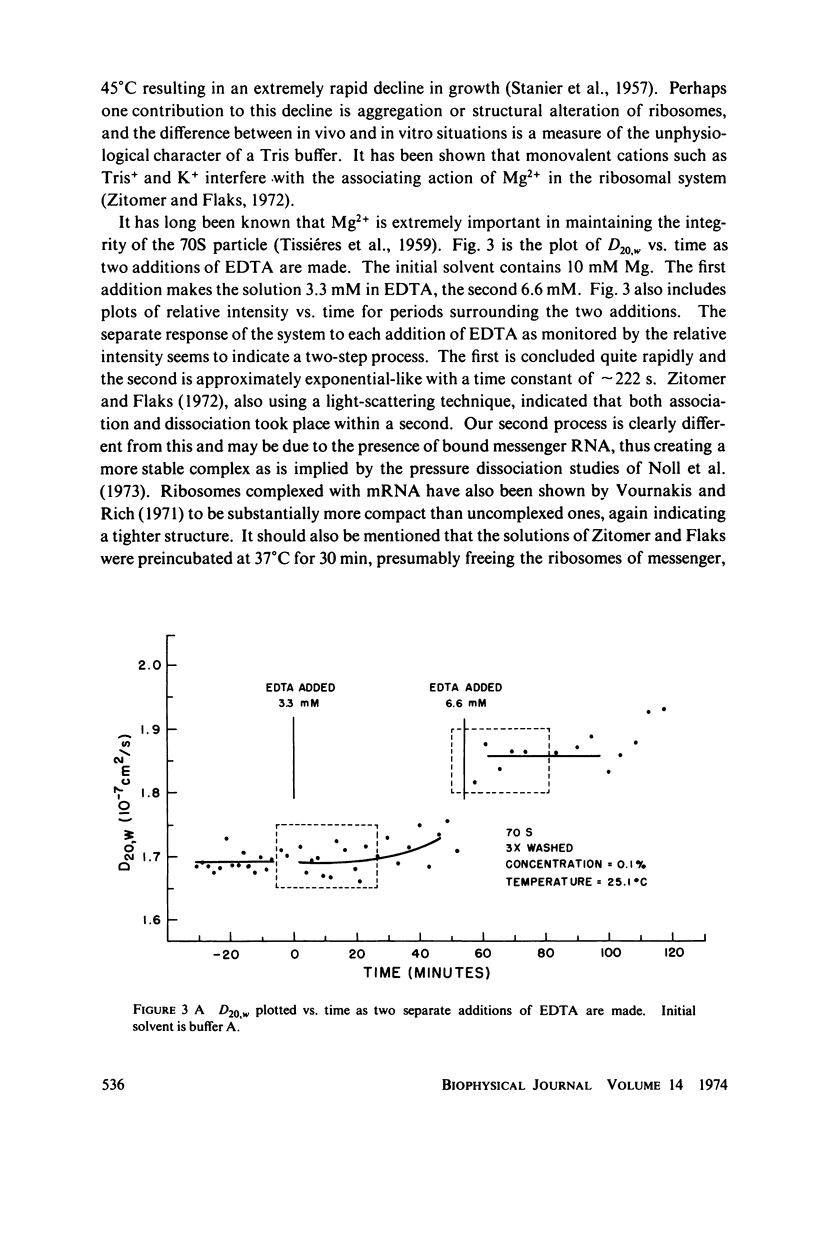
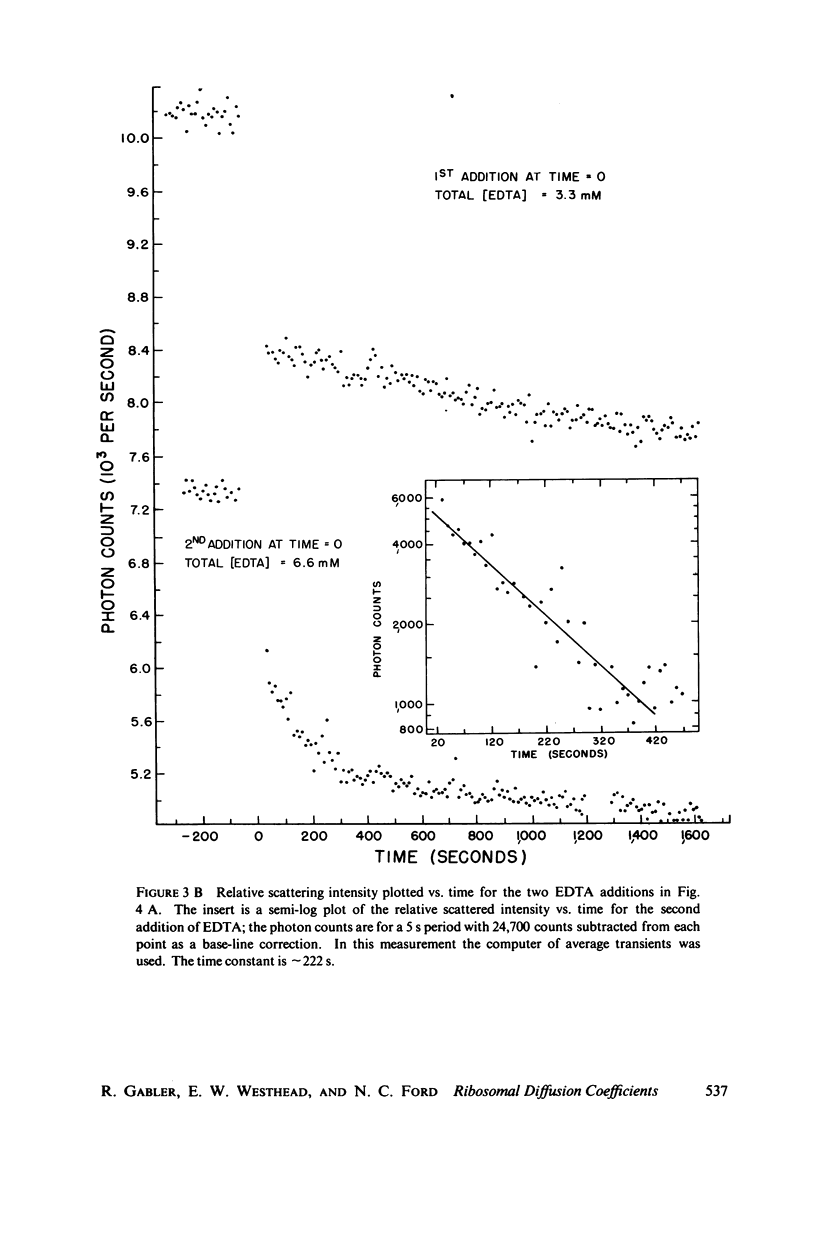
Using an optical beating technique, the diffusion coefficients and relative scattered intensity of Escherichia coli 70S, 50S, and 30S ribosomes are measured as a function of temperature and Mg2+ concentration. For solutions at 10 mM Mg2+ and between 0°C and about 40°C, the values of D20,w obtained are 1.7, 1.9, and ≈2.1 × 10-7 cm2/s, respectively. Preparative procedures drastically affect these values and equivalent hydrodynamic ellipsoids of revolution models give large axial ratios indicating extensive hydration or a deviation from the assumed shape. Calculations also indicate that the subunits expand upon dissociation. Measurements of D20,w vs. temperature indicate that 70S particles undergo a conformational change prior to dissociation and can be heat dissociated at 30-32°C at low concentrations. Treatment of 70S ribosomes with EDTA causes a biphasic dissociation reaction. Addition of Mg2+ after dissociation with EDTA shows that longer waiting times yield fewer 70S particles and that even short waiting times may yield ribosomes differing from the native conformation. Addition of p-chloromercuribenzoic acid (PCMB) is shown to dissociate 70S particles, but to a lesser extent than ethylenediaminetetraacetic acid (EDTA).
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acharya A. S., Moore P. B. Reaction of ribosomal sulfhydryl groups with 5,5'-dithiobis(2-nitrobenzoic acid). J Mol Biol. 1973 May 15;76(2):207–221. doi: 10.1016/0022-2836(73)90385-9. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesteland R. F. Unfolding of Escherichia coli ribosomes by removal of magnesium. J Mol Biol. 1966 Jul;18(2):356–371. doi: 10.1016/s0022-2836(66)80253-x. [DOI] [PubMed] [Google Scholar]
- HART R. G. Electron microscopy of the 50-S ribosomes of Escherichia coli. Biochim Biophys Acta. 1962 Jul 16;60:629–637. doi: 10.1016/0006-3002(62)90881-8. [DOI] [PubMed] [Google Scholar]
- Hill W. E., Anderegg J. W., Van Holde K. E. Effects of solvent environment and mode of preparation on the physical properties of ribosomes fron Escherichia coli. J Mol Biol. 1970 Oct 14;53(1):107–121. doi: 10.1016/0022-2836(70)90048-3. [DOI] [PubMed] [Google Scholar]
- Hill W. E., Rossetti G. P., Van Holde K. E. Physical studies of ribosomes from Escherichia coli. J Mol Biol. 1969 Sep 14;44(2):263–277. doi: 10.1016/0022-2836(69)90174-0. [DOI] [PubMed] [Google Scholar]
- Hill W. E., Thompson J. D., Anderegg J. W. X-ray scattering study of ribosomes from Escherichia coli. J Mol Biol. 1969 Aug 28;44(1):89–102. doi: 10.1016/0022-2836(69)90406-9. [DOI] [PubMed] [Google Scholar]
- Infante A. A., Baierlein R. Pressure-induced dissociation of sedimenting ribosomes: effect on sedimentation patterns. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1780–1785. doi: 10.1073/pnas.68.8.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengyel P., Söll D. Mechanism of protein biosynthesis. Bacteriol Rev. 1969 Jun;33(2):264–301. doi: 10.1128/br.33.2.264-301.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noll M., Hapke B., Schreier M. H., Noll H. Structural dynamics of bacterial ribosomes. I. Characterization of vacant couples and their relation to complexed ribosomes. J Mol Biol. 1973 Apr 5;75(2):281–294. doi: 10.1016/0022-2836(73)90021-1. [DOI] [PubMed] [Google Scholar]
- Nomura M. Bacterial ribosome. Bacteriol Rev. 1970 Sep;34(3):228–277. doi: 10.1128/br.34.3.228-277.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scafati A. R., Stornaiuolo M. R., Novaro P. Physicochemical and light scattering studies on ribosome particles. Biophys J. 1971 Apr;11(4):370–384. doi: 10.1016/S0006-3495(71)86221-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal M. Thermal denaturation of ribosomes. Biochemistry. 1969 Jan;8(1):424–435. doi: 10.1021/bi00829a058. [DOI] [PubMed] [Google Scholar]
- Tamaoki T., Miyazawa F. Dissociation of ribosomes at high temperatures. J Mol Biol. 1966 Jun;17(2):537–540. doi: 10.1016/s0022-2836(66)80164-x. [DOI] [PubMed] [Google Scholar]
- Vournakis J., Rich A. Size changes in eukaryotic ribosomes. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3021–3025. doi: 10.1073/pnas.68.12.3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON J. D. THE SYNTHESIS OF PROTEINS UPON RIBOSOMES. Bull Soc Chim Biol (Paris) 1964;46:1399–1425. [PubMed] [Google Scholar]
- Zitomer R. S., Flaks J. G. Magnesium dependence and equilibrium of the Escherichia coli ribosomal subunit association. J Mol Biol. 1972 Nov 14;71(2):263–279. doi: 10.1016/0022-2836(72)90350-6. [DOI] [PubMed] [Google Scholar]


