Abstract
Objective
To evaluate the effect of iron supplementation on the incidence of infections in children.
Design
Systematic review of randomised controlled trials.
Data sources
28 randomised controlled trials (six unpublished and 22 published) on 7892 children.
Interventions
Oral or parenteral iron supplementation or fortified formula milk or cereals.
Outcomes
Incidence of all recorded infectious illnesses, and individual illnesses, including respiratory tract infection, diarrhoea, malaria, other infections, and prevalence of positive smear results for malaria.
Results
The pooled estimate (random effects model) of the incidence rate ratio (iron v placebo) was 1.02 (95% confidence interval 0.96 to 1.08, P=0.54; P<0.0001 for heterogeneity). The incidence rate difference (iron minus placebo) for all recorded illnesses was 0.06 episodes/child year (−0.06 to 0.18, P=0.34; P<0.0001 for heterogeneity). However, there was an increase in the risk of developing diarrhoea (incidence rate ratio 1.11, 1.01 to 1.23, P=0.04), but this would not have an overall important on public health (incidence rate difference 0.05 episodes/child year, –0.03 to 0.13; P=0.21). The occurrence of other illnesses and positive results on malaria smears (adjusted for positive smears at baseline) were not significantly affected by iron administration. On meta-regression, the statistical heterogeneity could not be explained by the variables studied.
Conclusion
Iron supplementation has no apparent harmful effect on the overall incidence of infectious illnesses in children, though it slightly increases the risk of developing diarrhoea.
What is already known on this topic
Iron supplementation is recommended to prevent iron deficiency, which is a major health problem, especially in the developing countries
Conflicting data exist regarding the possibility of an increase in the incidence of infections with iron supplementation, resulting in concern about the safety of this intervention
What this study adds
Iron supplementation has no apparent harmful effect on the overall incidence of infectious illnesses in children
Iron administration increases the risk of developing diarrhoea
Fortification of foods may be the safest and most beneficial mode of supplementation in relation to infectious illnesses
Introduction
Anaemia caused by iron deficiency is a major public health problem, affecting 46% of school children globally.1 Iron deficiency has adverse effects on psychomotor development2 and on the capacity to work. The reversible consequences in childhood have prompted recommendations for early intervention. The proposed interventions rely primarily on enhancing iron intake either through supplementation or fortification of food.3,4
Because of these proposed interventions their safety needs to be unequivocally established. The role of iron in resistance to disease remains controversial. Iron deficiency may be an important defence mechanism, and the term “nutritional immunity” was coined to highlight the importance of hypoferraemia in preventing bacterial growth.5 Conversely, data suggest that iron deficiency is associated with impairment of cell mediated immunity and the bactericidal activity of neutrophils, thus increasing susceptibility to infection.6,7 Iron supplementation may also cause damage to cells mediated through free radicals.8 Objective safety data from longitudinal studies of iron supplementation are conflicting; trials have shown either beneficial effects,9 no effect,10 or an increase in infectious illnesses.11,12 Children, particularly infants and those living in developing countries, are vulnerable to infectious diseases. It is thus important to establish the safety of iron supplementation in children on a public health scale. We conducted a systematic review to determine the effect of iron supplementation on infectious illnesses.
Methods
Inclusion criteria
To be included trials had to be randomised placebo controlled trials—except for those in which iron was given parenterally, in which case trials could be non-placebo controlled because it would be difficult to administer a similar placebo; had to investigate iron supplementation through the oral or the parenteral route or as formula milk or cereals fortified with iron; and evaluate one or more infectious illnesses as an outcome measure. We also included studies in which other micronutrients and drugs were simultaneously administered if the only difference between the study and the control groups was iron supplementation.
Data collection
We searched computerised bibliographic medical databases, including Medline, Cochrane controlled trials register, Embase, IBIDS, and Healthstar. We also reviewed reference lists of identified articles and hand searched reviews, bibliographies of books, and abstracts and proceedings of international conferences or meetings. Donor agencies, “experts,” and authors of recent iron supplementation trials were contacted to identify any additional or ongoing trials. The title and abstract of the studies identified in the computerised search were scanned to exclude studies that were obviously irrelevant. We retrieved the full text of the remaining studies and identified studies that fulfilled the inclusion criteria. To avoid publication bias we included published and unpublished trials.
Quality of methods
We assessed the quality of trials using recommended criteria.13,14 Concealment of allocation was classed as adequate, unclear, inadequate, or not used. To assess completeness of follow up we classified studies by percentage of participants excluded (<3%, 3-9.9%, 10-19.9%, and ⩾20%). Blinding was classified as double blinding, single blinding, no blinding, and unclear. TG Abstracted all data.
Data abstraction
We used preformed questionnaires to abstract data. The data included in this review were derived from the published papers or were provided by the authors. Illnesses and the outcomes included were as defined by the authors. Whenever possible we contacted the authors for clarifications.
Statistical analysis
The presence of bias in the extracted data was evaluated by funnel plots.15 We used the metabias command in Stata software to perform the statistical tests for funnel plot asymmetry.16 The pooled estimates of incidence rate ratio and incidence rate difference were calculated by StatsDirect statistical software (version 1.9.5; StatsDirect, Cambridge) with fixed effects and random effects model assumptions.17 This program also computes the formal test of heterogeneity (Q statistic). We primarily report random effects estimates because most of the pooled results obtained were statistically heterogeneous. We chose incidence rate summary to account for the differences in duration of follow up in the various extracted studies. The data were recorded in the form of the total number of episodes of illness and the person time exposed (in child years). For trials in which the results were available in this format we recorded the figures directly from the publication, and this category of studies was labelled as the “actual” group. In the “computed” group of trials, the person time of follow up was not provided, and we calculated estimates from the product of the duration of follow up and the sample sizes available at the beginning and the end of the study. In some trials data were obtained by quantitative analysis of published graphs.
Some studies had reported only on the prevalence of malaria parasitaemia confirmed from smears at the beginning and the end of the supplementation period. Pooled estimates of the odds ratio of positive smears at the end of the supplementation period were computed by the “meta” command in Stata software.16 We also performed a meta-regression (restricted maximum likelihood iteration) through the “metareg” command in Stata software to determine the pooled log odds ratio of developing malaria in the group with iron supplementation compared with the placebo group. The covariate in the meta-regression equation was the log odds ratio at the beginning of the trial to adjust for the baseline differences in the prevalence of malaria.
We carried out stratified analyses for quality of methods; case detection (active field based or passive facility based); specificity of case definition; route of iron administration (parenteral, oral supplement, or fortified food); dose—this was initially planned but could not be performed as it could not be extracted for each study; duration of supplementation; type of illness (gastrointestinal, respiratory, malaria, non-diarrhoeal, or others); and baseline haemoglobin concentration in the supplemented group. The contribution of these variables to heterogeneity was also explored by meta-regression.16,17
Results
We identified 47 randomised controlled trials that were potentially eligible. Of these, 38 trials were published in medical journals or were theses9–52 and 9 were unpublished (box B1). Nineteen studies were ineligible (table 1). We therefore evaluated 28 studies (22 published10,11,31–44,47–52 two theses,45,46 and six unpublished) in this systematic review.
Unpublished studies
Papers presented at International Nutritional Anaemia Consultative Group (INACG) Symposium, Hanoi, Vietnam, 2001
Allen LH, Lopez P, Galvaz IA, Garcia DP, Isoard F, Rosado JL. Does multiple micronutrient supplementation increase haemoglobin and iron status more than iron alone?
Lonnerdal B, Domellof M, Dewey KG, Cohen R, Rivera LL, Hernell O. Effects of iron supplementation of breastfed infants in Honduras and Sweden from 4-9 or 6-9 months of age.
Ninh NX, Berger J, Tolvanen M, Trung NQ, Nhien NV, Lien DK, et al. Control of iron deficiency anaemia in Vietnamese infants by efficacy of iron and zinc supplementation to reduce anaemia and growth faltering in Vietnamese infants.
Zimmermann M, Hess S, Adou P, Torresani T, Cook J, Hurrell R. Treatment of iron deficiency in goitrous children improves the efficacy of iodized salt.
Quyen DT, Berger J, Ninh NX, Khan NC, Khoi HH. Control of iron deficiency anaemia in Vietnamese infants by weekly and daily iron supplementation: efficacy and effectiveness.
Atukorala S, de Silva A, Ahluwalia N. Evaluation of iron status of children in the presence of infections: effect of iron supplementation on iron status, infection and morbidity.*
Other unpublished papers
Rice AL, Stoltzfus RJ, Tielsch JM, Savioli L, Montresor A, Albonico M, et al. Iron supplementation and mebendazole treatment do not affect respiratory or diarrhoeal morbidity incidence rates in Tanzanian preschoolers. 1999.*
Agarwal D, Sachdev HPS, Mallika V, Singh T. Iron supplementation in breast fed, full term, low birth weight infants. 1999.*
Nagpal J, Sachdev HPS, Mallika V, Singh T. Iron supplementation with complementary feeding in predominantly breastfed infants. 2000.*
*Included in the review
Table 1.
Characteristics of excluded trials
| Reason for exclusion
|
|
|---|---|
| Andelman18 | Information not extractable |
| Burman19 | Information not extractable |
| Damodaran20 | Supplemented folic acid with iron |
| Oppenheimer21 | Same study group in included trial |
| Bates22 | Supplemented other micronutrients |
| Heresi23 | Supplemented other micronutrients |
| Chwang9 | Information not extractable |
| Heywood24 | Subset of an included trial |
| Angeles-Agdeppa25 | Supplemented other micronutrients |
| Heresi26 | Supplemented other micronutrients |
| Van Hensbroek27 | Supplemented multiple antimalarials in crossover manner |
| Beck28 | Same study group in an included trial |
| Von Stujvenberg29 | Supplemented other micronutrients |
| Picaud30 | Erythropoietin given with iron supplementation |
| Allen, Lonnerdal, Ninh, Zimmermann, Quyen | Unpublished, full text not available from authors |
Baseline characteristics of the studies
Table 2 depicts the baseline characteristics of the included trials. Thirteen trials were in children aged <1 year, 10 studies included preschool children (⩽ 5 years), and five trials included children aged >5 years. Eleven trials were from Africa, eight from Asia, five from the Americas, two from Europe, and two from Australia and New Zealand. The eligibility and exclusion criteria varied. Most of the studies used oral iron supplementation (20/28; 71%). Three trials used parenteral administration, and five studies used iron fortified foods.
Table 2.
Baseline characteristics of included trials (posted as supplied by author)
| Location
|
Age group
|
Sample size (total, iron, control)
|
Method of randomisation, allocation concealment, follow up, blinding*
|
Eligibility and exclusion criteria
|
Iron supplementation (route, dose, duration of supplementation, duration of follow up, intervention in treatment group, control group)
|
Case detection
|
Morbidities studied
|
Case definition
|
|
|---|---|---|---|---|---|---|---|---|---|
| James, 196031 | USA | 1 month | 181, 84, 97 | Unclear, B, D, C | Birth weight ⩽2000 g, survival for more than 24 hours, weight >2000 g | Parenteral, 50 mg X 5, -, 11 months; T/t: Iron dextran C: No placebo |
Clinic | URTI, LRTI, Diarrhoea | Hospital diagnosis |
| Cantwell, 197232 | New Zealand | 2 days | 238, 94, 144 | By alternate days of birth, D, A, C | Maori babies (at least 25% Maori blood) delivered at Hawkes Bay | Parenteral, 50mg X 5, -, 30 months; T/t: Iron dextran C: No placebo |
Clinic | Pneumonia, URTI, skin infections, gastroenteritis | Hospital diagnosis |
| Fuerth, 197433 | USA | 1 month | 602, 329, 273 | Alternate allocation, D, D, A | Full term Exclusion: On iron medication, vitamins, received blood transfusion, received less than 50% supplements between two visits, Hb dropped to <80 g/l during study |
Oral, 30mg/day, 18 months, 18 months; T/t: Ferrous sulphate C: Placebo containing bismuth |
Clinic | Infectious illness | Not mentioned |
| Oppenheimer, 198634 | Papua New Guinea | 2 months | 486, 236, 250 | Matched pairs randomised into treatment and control groups, B, C, B | Resident of Madang | Parenteral, 150 mg, 10 mo T/t: Iron dextran C: Saline |
Field and clinic | URTI, LRTI, TB, lung abscess, malaria, gastroenteritis, etc. | RTI- WHO classification Malaria: symptomatic Rest: not mentioned |
| Harvey, 198935 | Papua New Guinea | 8-12 years | 312, 156, 156 | Matched pairs randomised into treatment and control groups, C, C, D | Hb = 80-120 g/l | Oral, 130 mg/day, 4 months, 6 months; T/t: Ferrous sulphate C: Identical placebo (75% cellulose, 25% lactose) |
Field | Malaria prevalence | PS for malaria + |
| Smith, 198911 | Gambia | 6 months-5 years | 213, 106, 107 | Unclear, B, C, A | Hb, MCV <3rd centile of reference population Exclusion: Hb <50 g/l |
Oral, 3-6 mg/kg/day, 3 months, 3 months T/t: Ferrous sulphate in orange juice C: Orange juice |
Field | Malaria | Axillary temp >37.5°C with P. falciparum + |
| Power, 199136 | South Africa | 3-12 months | 149, 75, 74 | Stratified randomisation by purpose written computer program, B, C, A | Birth weight ⩾3000 g, Weight at 3 months = 5 kg in females and 5.5 kg in males, Hb >90 g/l at 3 months Exclusion: Blood transfusion received, serious illness before enrolment |
Fortified, 40 mg/100 mg, 9 months, 9 months; T/t: Iron enriched formula C: Standard cow's milk formula |
Clinic | RTI, GI infection, oral thrush, eye infection, others. | Not mentioned |
| Javaid, 199137 | Pakistan | 4 months | 129, 87, 42 | Unclear, B, D, D | Birth weight >2500 g | Fortified, 7.5 mg/100 mg, 8 months, 8 months; T/t: Iron fortified milk cereal C: Milk cereal |
Field | URTI, LRTI, diarrhoea | LRTI: significant complaint with +ve physical examination; Diarrhoea: >4 loose stools/day |
| Irigoyen, 199138 | USA | 6 months | 334, 228, 106 | Unclear, B, D, A | Hb ⩽115 g/l Exclusion: Prematurity, milk allergy, failure to thrive, HIV+, recent H influenzae type b meningitis, fed low iron formula, exclusively breastfed, primary physician refusal |
Oral, 3, 6 mg/kg/day, 3 months, 3 months; T/t: Ferrous sulphate C: Identical placebo† |
Clinic | Diarrhoea | Not mentioned |
| Chippaux, 199139 | Togo | 6-36 months | 190, 95, 95 | Unclear, B, D, A | Hb ⩾80 g/l | Oral, 2.5 mg/kg/day, 3 months, 9 months; T/t: Iron Betainate C: Identical placebo† |
Clinic | Malaria | Smear positive |
| Brunser, 199340 | Chile | 3 months | 400, 200, 200 | Random numbers table, A, D, A | Birth weight ⩾2500 g, W/A ⩾80% or 50th centile, Hb ⩾105 g/l | Fortified, 12 mg/l, 6 months, 6 monthsl; T/t: Iron enriched milk C: Control milk |
Field | Diarrhoea | >3 liquid stools/day or maternal report |
| Angeles, 199341 | Indonesia | 2-5 years | 80, 40, 40 | Unclear, B, B, A | W/A z score between –2 and –3, Hb = 80-110 g/l, ferritin <120μg/l | Oral, 30 mg/day, 2 months, 2 months; T/t: Ferrous sulphate, Vitamin C C: Vitamin C |
Field | Fever, RTI, Diarrhoea | Fever: Temp >37°C, Diarrhoea: >4 watery stools/d, RTI: not mentioned |
| Lawless, 199442 | Kenya | 6-11 years | 86, 44, 42 | Stratified randomisation (by gender and initial Hb value), C, A, A | Hb ⩾80 g/l Exclusion; Heavy hookworm infection, Blood in the urine indicative of S haematobium, dislike of uji, absence at the time of interval exams |
Oral, 150 mg/day, 3 months, 3 months; T/t: Ferrous sulphate C: Identical placebo† |
School | Diarrhoea, cough, malaria | PS for MP+; diarrhoea, cough—not mentioned |
| Idjradinata, 199410 | Indonesia | 12-18 months | 47, 24, 23 | Random numbers table, B, B, D | Birth weight >2.5 kg, singleton pregnancy, Hb ⩾8g/dL, wt, length and head circumference within 2 SD of NCHS standards. Exclusion: Congenital malformation, major perinatal complication, jaundice treated with phototherapy, hospital admission, supplementation with micronutreints before enrolment, chronic illness, folic acid deficiency, haemoglobinopathy or thalassaemia |
Oral, 3 mg/kg/day, 4 months, 4 months; T/t: Ferrous sulphate C: Identical placebo† |
Clinic | URTI, LRTI, gastroenteritis | Paediatrician's diagnosis |
| Hemminki, 199543 | Hungary | <45 days | 322, 164, 158 | Unclear, A, B, D | Birth weight ⩾2500 g Exclusion: Critically ill, malformations, child cared for outside home, consultation with private physician |
Fortified, 6.5 mg/l, 10.5 months, 10.5 months; T/t: Iron fortified formula C: Non-fortified formula |
Clinic | URTI, fever | Not mentioned |
| Van den Hombergh, 199644 | Tanzania | <30 months | 100, 50, 50 | Unclear, B, B, D | Hb ⩽50 g/l, PS for MP +. Exclusion: Cerebral malaria, Non-falciparum malaria, sickle cell anaemia, other significant illness |
Oral, 200 mg/day, 3 months, 3 months; T/t: Ferrous sulphate, folic acid C: Folic acid |
Clinic | Malaria, pneumonia, other infections | Malaria: smear positive Pneumonia, other infections: not mentioned |
| Adam, 199645 | Ethiopia | 6 months-7 years | 841, 431, 410 | Unclear, B, B, A | Hb = 60-110 g/l | Oral, 3mg/kg/day, 3 months, 3 months; T/t: Ferrous sulphate C: Identical placebo† |
Active | Malaria | Fever |
| Gebresellassie, 199646 | Ethiopia | 5-14 years | 500, 250, 250 | Unclear, B, C, A | Hb = 50-120 g/l, P falciparum –ve | Oral, 60mg/day, 3 months, 6 months T/t: Ferrous sulphate C: Identical placebo† |
Active | Malaria | Temp >37.5°C, P. falciparum +ve |
| Mitra, 199747 | Bangladesh | 2-48 months | 349, 172, 177 | Block randomisation of 4 homogeneous clusters, A, C, A | Exclusion: Critically ill, congenital malformations, metabolic disorders | Oral, 15mg/day, 15 months, 15 months T/t: Ferrous gluconate, vitamins‡ C: Vitamins‡ |
Field | Diarrhoea, dysentery, ARI | Diarrhoea: >2 liquid stools/d and maternal report; Dysentery: blood in stools; ARI: >50 bpm in child <1 yr, >40 bpm in child 12-15 months |
| Palupi, 199748 | Indonesia | 2-5 years | 194, 96, 98 | Unclear, B, B, A | Registered at village health centre | Oral, 15mg/week, 2 months, 2 months T/t: Ferrous sulphate C: Identical placebo† |
Clinic | Worm infestation | Stool microscopy + |
| Rosado, 199749 | Mexico | 1.5-3 years | 219, 109, 110 | Stratified randomisation (by age and sex), B, C, A | Age as stated | Oral, 20 mg/day, 12 months, 12 months Group 1 T/t: Ferrous sulphate C: Placebo† Group 2 T/t: Ferrous sulphate, zinc methionine C: Zinc methionine |
Field | RTI, diarrhoea, fever | RTI: runny nose, common cold, sore throat, cough; Diarrhoea, Fever: maternal reporting |
| Menendez, 199750 | Tanzania | 2 months | 832, 417, 415 | Block randomisation, A, D, A | Birth weight >1500 g, PCV >25% at 8 weeks. Exclusion: Congenital malformation, congenital or neonatal infection. |
Oral, 2 mg/kg/day, 4 months, 10 months Group 1 T/t: Ferrous glycine sulphate, placebo syrup‡ C: Placebo syrups‡ Group 2 T/t: Iron syrup, Deltaprim C: Deltaprim, placebo syrup‡ |
Clinic | Malaria | Axillary temp >37.5°C with P falciparum +ve |
| Rice, 1999 (unpublished) | Tanzania | 3-56 months | 614, 307, 307 | Randomisation of households of the study area into two groups, B, A, B | Age as stated | Oral, 10mg/day, 12 months, 12 months T/t: Iron sulphate C: Identical placebo† |
Field | Diarrhoea, dysentery, RTI, malaria, fever | RTI: cough with difficult breathing; Diarrhoea: >3 liquid stools/ day; Dysentery: blood in stools |
| Agarwal, 1999 (unpublished) | India | 50-80 days | 73, 37, 36 | Computer generated random numbers, A, C, A | Gestation ⩾37 weeks, birth weight <2500 g. Exclusion: Twins, congenital malformations, received blood, adverse neonatal event requiring admission in nursery, sampling before recruitment >10 ml, significant current morbidity, maternal APH |
Oral, 3 mg/kg/day, 2 months, 2 months T/t: Ferric ammonium citrate C: Identical placebo† |
Clinic | RTI | Maternal report as interpreted by paediatrician |
| Nagpal, 2000 (unpublished) | India | 4-6 months | 100, 49, 51 | Computer generated random numbers, A, D, A | Gestation ⩾37 weeks, birth weight ⩾2500 g, breast fed Exclusion: Twins, congenital malformations, received blood or iron, adverse neonatal event requiring admission in nursery, sampling before recruitment >10 ml, significant current morbidity |
Oral, 2.5mg/kg/day, 2 months, 2 months T/t: Ferric ammonium citrate C: Identical placebo† |
Clinic | RTI, diarrhoea, others | Maternal report as interpreted by paediatrician |
| Berger, 200051 | Togo | 6-36 months | 197, 100, 97 | Unclear, B, C, B | Hb ⩾80 g/l | Oral, 2-3mg/kg/day, 3 months, 9 months T/t: Iron Betainate C: Identical placebo† |
Field | URTI, LRTI, malaria, diarrhoea, cutaneous infection, fever, worms | Not mentioned |
| Singhal, 200052 | UK | 9 months | 493, 162, 331 | Separate randomisation for Asians and non-Asians, A, C, A | Birth weight >2500 g, gestation >36 weeks. Exclusion: Severe chronic disease, congenital anomalies, haematologic disorders, previously received iron or blood |
Fortified, 12mg/L, 9 months, 9 months; T/t: Iron fortified formula C: Cows' milk or standard formula |
Clinic | Chest infection, URTI, others | URTI, diarrhoea: maternal report; chest infection: treatment with antibiotics |
| Atukorala, 2001 (unpublished) | Sri Lanka | 5-10 years | 364, 262, 102 | Unclear, B, C, A | Outpatients at children's hospital | Oral, 60mg/day, 2 months, 2 months T/t: Ferrous sulphate C: Lactose |
Field | URTI, diarrhoea | URTI: clinical evidence with inflammatory parameters; diarrhoea: >2 semisolid watery stools/day |
ARI=acute respiratory illness; bpm=breaths per minute; C=intervention in the control group; GI=gastrointestinal; Hb=haemoglobin, LRTI=lower respiratory tract infection; MCV=mean corpuscular volume; MP=malarial parasite; P falciparum=Plasmodium falciparum; PS=peripheral smear; RTI=respiratory tract infection; TB=tuberculosis; URTI=upper respiratory tract infection; T/t=intervention in the treatment group;
Allocation concealment: (A) adequate; (B) unclear; (C) inadequate; (D) not used. Completeness of follow up: (A) <3% of participants excluded; (B) 3% to 9.9% of participants excluded; (C) 10% to 19.9% of participants excluded; (D) 20% or more of participants excluded. Blinding: (A) double blinding; (B) single blinding; (C) no blinding; (D) unclear.
Mentioned by the authors as being identical in appearance and/or taste; exact composition not mentioned.
Exact composition not mentioned.
Differences in the mode of administration may have implications for bioavailability of iron and its possible effect on the immune function. The supplementation dose used could influence the degree to which illness was affected. As a crude generalisation, the fortified formulas had the lowest dosage and the parenteral route had the highest. The duration of supplementation and follow up for oral intake varied from 2 months to 30 months.
The specificity of the definition used for illness was variable. Specificity of diagnosis has the potential to bias the observed effect of supplementation on illness. For example, low specificity definitions could underestimate the effect of iron supplementation on malaria due to a high rate of misclassification of non-malarial fevers as malaria. In some studies, fever was recorded as an additional infectious illness because fever in children is mostly attributed to infectious diseases.41,51 Inclusion of fever as a separate infection may lead to duplication of data because fever may accompany malaria, respiratory tract infection, and diarrhoea. However, we have included it on the assumption that an equal distribution of fever in both groups would eliminate any bias and also prevent non-inclusion of any observed infection.
The methods of surveillance varied: 15 were clinic based whereas 13 were field trials with active surveillance for cases. If iron supplementation has selective effects on mild rather than more severe episodes of illness then differences in methods of case detection may influence the observed effects of iron supplementation.
Bias detection for included studies
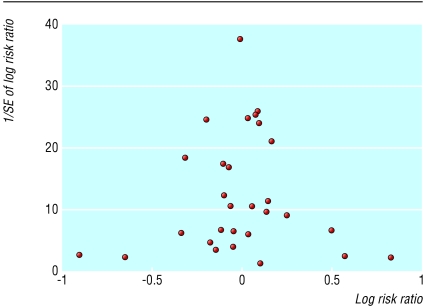
The funnel plot (fig 1) seems symmetrical, and we found no evidence of bias using the Egger (weighted regression) method (P=0.663 for bias) or the Begg (rank correlation) method (continuity corrected P=0.488).
Figure 1.
Funnel plot of extracted studies
Pooled and stratified estimates
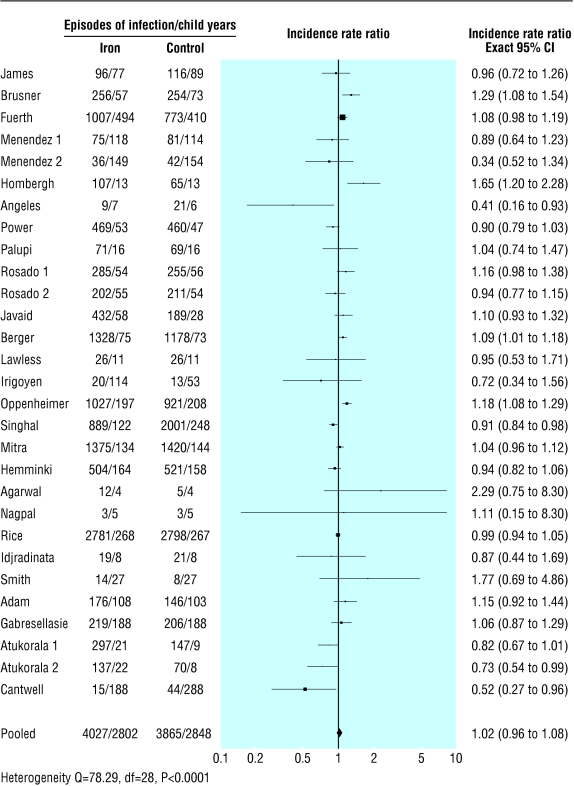
We collected data on 7892 children followed up for 5650 child years—4027 children and 2802 child years in the iron supplemented group and 3865 children and 2848 child years in the placebo group (table 3). The pooled estimate of the incidence rate ratio (iron versus placebo) for all the recorded morbidities was 1.02 (95% confidence interval 0.96 to 1.08; P=0.54; test for heterogeneity Q=78.29, P<0.0001, fig 2). Calculations of incidence rate ratio based on “actual” data (when available) and computations from sample size at the end of the study (1.03, 0.97 to 1.08, P=0.21; test for heterogeneity Q=72.19, P<0.0001) were virtually identical with computations based on sample sizes at the beginning of the study. Besides the incidence rate ratio, from the public health perspective the incidence rate difference is considered to be more informative. The incidence rate difference (iron minus placebo) for all the recorded illnesses was 0.06 episodes per child year (−0.06 to 0.18, P=0.34; test for heterogeneity Q= 80.01, P<0.0001).
Table 3.
Extracted data from included studies. Episodes of infection and exposure time (in child years) (posted as supplied by author)
| Study
|
Total infections
|
Diarrhoea
|
Respiratory tract infection
|
Malaria
|
Other infections
|
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Iron
|
Control
|
Iron
|
Control
|
Iron
|
Control
|
Iron
|
Control
|
Iron
|
Control
|
||||||||||||||||||||
| Epi
|
Obs
|
Epi
|
Obs
|
Epi
|
Obs
|
Epi
|
Obs
|
Epi
|
Obs
|
Epi
|
Obs
|
Epi
|
Obs
|
Epi
|
Obs
|
Epi
|
Obs
|
Epi
|
Obs
|
||||||||||
| James31 | 96 | 77 | 116 | 88.91 | 16 | 77 | 25 | 88.91 | 80 | 77 | 91 | 88.91 | |||||||||||||||||
| Cantwell32 | 15 | 188 | 44 | 288 | 1 | 188 | 6 | 288 | 7 | 188 | 30 | 288 | 7 | 188 | 8 | 288 | |||||||||||||
| Fuerth33 | 1007 | 493.5 | 773 | 409.5 | 1007 | 493.5 | 773 | 409.5 | |||||||||||||||||||||
| Oppenheimer34 | 1027 | 196.66 | 921 | 208.33 | 44 | 196.66 | 37 | 208.33 | 498 | 196.66 | 435 | 208.33 | 82 | 196.66 | 66 | 208.33 | 403 | 196.66 | 383 | 208.33 | |||||||||
| Smith11 | 14 | 26.5 | 8 | 26.75 | 14 | 26.5 | 8 | 26.75 | |||||||||||||||||||||
| Power36 | 469 | 52.5 | 460 | 46.5 | 84 | 52.5 | 91 | 46.5 | 105 | 52.5 | 88 | 46.5 | 280 | 52.5 | 281 | 46.5 | |||||||||||||
| Javaid37 | 432 | 58 | 189 | 28 | 250 | 58 | 105 | 28 | 182 | 58 | 84 | 28 | |||||||||||||||||
| Irigoyen38 | 20 | 114 | 13 | 53 | 20 | 114 | 13 | 53 | |||||||||||||||||||||
| Brunser40 | 256 | 56.88 | 254 | 72.57 | 256 | 56.88 | 254 | 72.57 | |||||||||||||||||||||
| Angeles41 | 9 | 6.5 | 21 | 6.16 | 2 | 6.5 | 6 | 6.16 | 4 | 6.5 | 10 | 6.16 | 3 | 6.5 | 5 | 6.16 | |||||||||||||
| Lawless42 | 26 | 11 | 26 | 10.5 | 7 | 11 | 8 | 10.5 | 19 | 11 | 18 | 10.5 | |||||||||||||||||
| Idjradinata10 | 19 | 8 | 21 | 7.66 | 19 | 8 | 21 | 7.66 | |||||||||||||||||||||
| Hemminki43 | 504 | 164 | 521 | 158 | 288 | 164 | 305 | 158 | 216 | 164 | 216 | 158 | |||||||||||||||||
| Van den Hombergh44 | 107 | 12.5 | 65 | 12.5 | 26 | 12.5 | 5 | 12.5 | 81 | 12.5 | 60 | 12.5 | |||||||||||||||||
| Adam45 | 176 | 107.75 | 146 | 102.5 | 73 | 107.75 | 67 | 102.5 | 40 | 107.75 | 32 | 102.5 | 41 | 107.75 | 32 | 102.5 | 22 | 107.75 | 15 | 102.5 | |||||||||
| Gabresellasie46 | 219 | 187.5 | 206 | 187.5 | 219 | 187.5 | 206 | 187.5 | |||||||||||||||||||||
| Mitra47 | 1375 | 134 | 1420 | 143.5 | 670 | 127 | 695 | 139 | 705 | 141 | 725 | 148 | |||||||||||||||||
| Palupi48 | 71 | 15.5 | 69 | 15.66 | 71 | 15.5 | 69 | 15.66 | |||||||||||||||||||||
| Rosado 149 | 285 | 54 | 255 | 56 | 76 | 54 | 62 | 56 | 192 | 54 | 179 | 56 | 17 | 54 | 14 | 56 | |||||||||||||
| Rosado 249 | 202 | 55 | 211 | 54 | 46 | 55 | 40 | 54 | 139 | 55 | 163 | 54 | 17 | 55 | 8 | 54 | |||||||||||||
| Menendez 150 | 75 | 118.4 | 81 | 113.8 | 75 | 118.4 | 81 | 113.8 | |||||||||||||||||||||
| Menendez 250 | 36 | 148.5 | 42 | 145.4 | 36 | 148.5 | 42 | 145.4 | |||||||||||||||||||||
| Rice (unpublished) | 2781 | 267.97 | 2798 | 267.39 | 388 | 267.98 | 376 | 267.37 | 1006 | 267.98 | 995 | 267.37 | 1387 | 267.97 | 1427 | 267.39 | |||||||||||||
| Agarwal (unpublished) | 12 | 3.75 | 5 | 3.58 | 12 | 3.75 | 5 | 3.58 | |||||||||||||||||||||
| Nagpal (unpublished) | 3 | 4.5 | 3 | 5 | 2 | 4.5 | 2 | 5 | 1 | 4.5 | 0 | 5 | 0 | 4.5 | 1 | 5 | |||||||||||||
| Berger51 | 1328 | 75 | 1178 | 72.75 | 211 | 75 | 127 | 72.75 | 623 | 75 | 627 | 72.75 | 494 | 75 | 424 | 72.75 | |||||||||||||
| Singhal52 | 889 | 121.5 | 2001 | 248.25 | 66 | 121.5 | 132 | 248.25 | 823 | 121.5 | 1869 | 248.25 | |||||||||||||||||
| Atukorala 1 (unpublished) | 297 | 21.33 | 147 | 8.66 | 23 | 21.33 | 5 | 8.66 | 274 | 21.33 | 142 | 8.66 | |||||||||||||||||
| Atukorala 2 (unpublished) | 137 | 22.33 | 70 | 8.33 | 8 | 22.33 | 2 | 8.33 | 129 | 22.33 | 68 | 8.33 | |||||||||||||||||
| Totals | 11 887 | 2802.07 | 12 064 | 2848.7 | 2243 | 1616.93 | 2053 | 1763.83 | 5153 | 1840.3 | 5871 | 1821.34 | 467 | 785.31 | 435 | 784.28 | 4024 | 1701.38 | 3705 | 1709.95 | |||||||||
Epi=no of episodes of infections observed. Obs=observation/exposure time in child years. RTI=respiratory tract infections.
Figure 2.
Forest plot for incidence rate ratio for all recorded illnesses
Stratified analysis for the effect on individual infectious illnesses showed that children in the iron supplementation group had an 11% (1% to 23%) higher risk (incidence rate ratio) of developing diarrhoea (P=0.04; test for heterogeneity Q= 30.24, P= 0.04, table 4). The effect on other individual illnesses was not significant. However, the incidence rate difference (public health impact) for diarrhoea was 0.05 episodes per child year (−0.03 to 0.13, P=0.21; test for heterogeneity Q= 42.03, P=0.001). Further stratification showed that the significantly increased risk of diarrhoea associated with iron supplementation was restricted to oral supplementation (nine studies; incidence rate ratio 1.15, 1.01 to 1.32, P=0.04; incidence rate difference 0.18 episodes per child year, −0.01 to 0.37; P=0.07). The individual studies had not determined the cause of the diarrhoea, though dysentery indicates severe infectious diarrhoea. Only two studies provided information on dysentery; they showed no difference in the incidence between the two groups. Meta-regression showed that the route of iron administration (oral versus other) was not significantly associated with incidence rate ratio for diarrhoea (risk ratio 1.06, 0.85 to 1.32, P=0.59).
Table 4.
Pooled estimates (incidence rate ratio) of effect of iron supplementation on total and individual infections
| Infection type
|
No of trials
|
Random effects model (95% CI)
|
P value
|
Tests for heterogeneity (P value)
|
|---|---|---|---|---|
| Diarrhoea | 17 | 1.11 (1.01 to 1.23) | 0.04 | 30.24 (0.04) |
| Non-diarrhoeal | 24 | 0.97 (0.95 to 1.06) | 0.99 | 63.05 (<0.0001) |
| Respiratory tract | 17 | 0.98 (0.90 to 1.06) | 0.54 | 53.18 (<0.0001) |
| Malaria | 5 | 1.07 (0.94 to 1.24) | 0.35 | 5.58 (0.35) |
| Other infections* | 13 | 1.04 (0.98 to 1.11) | 0.20 | 18.15 (0.15) |
| Lower respiratory tract† | 8 | 0.97 (0.83 to 1.23) | 0.93 | 21.91 (0.003) |
| Dysentery† | 2 | 1.00 (0.87 to 1.15) | 0.99 | 0.02 (0.90) |
Other infections included septicaemia, urinary tract infections, tuberculosis, unspecified fever, pyoderma, and infectious morbidities not classifiable under respiratory tract infections, diarrhoea, or malaria.
Included as component of respiratory tract infection or diarrhoea (as relevant). Separate stratification done to assess possible differential effects on more severe infection.
From the available data we found no increased risk of severe illness associated with iron supplementation (analysis possible only for lower respiratory tract infection and dysentery).
Malarial parasitaemia
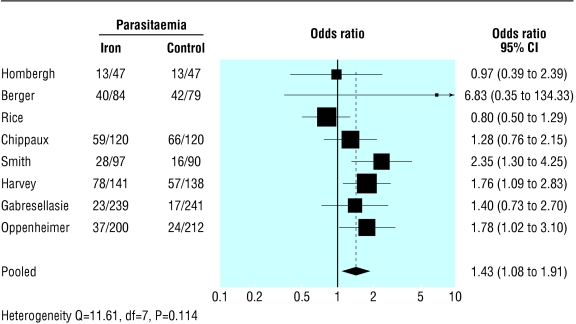
Table 5 shows the data extracted on malarial parasitaemia. The pooled odds ratio for positive smear tests for malaria at the end of the supplementation period (random effects model) was 1.43 (1.08 to 1.91, P=0.014; test for heterogeneity Q=11.611, P=0.114, fig 3. Meta-regression analysis of trials with relevant data (excluding the study by Oppenheimer et al34) indicated that this treatment effect was significantly associated with the baseline positivity of smear tests (for a unit increase in log odds ratio of baseline positivity, the treatment effect increased by 2.89; 1.37 to 6.10; P=0.005) but not iron supplementation (1.24; 0.98 to 1.57; P=0.076).
Table 5.
Extracted data from trials depicting prevalence of smears positive for malaria
| Study
|
Baseline
|
End
|
|||
|---|---|---|---|---|---|
| Iron
|
Control
|
Iron
|
Control
|
||
| Hombergh44 | 50/50 | 50/50 | 13/47 | 13/47 | |
| Berger et al51 | 59/100 | 62/97 | 40/84 | 42/79 | |
| Rice (unpublished) | 258/316 | 253/295 | 233/279 | 221/256 | |
| Chippaux39 | 72/120 | 74/120 | 59/120 | 66/120 | |
| Smith11 | 23/106 | 19/107 | 28/97 | 16/90 | |
| Harvey35 | 119/159 | 103/159 | 78/141 | 57/138 | |
| Gebresellassie46 | 30/239 | 31/241 | 23/239 | 17/241 | |
| Oppenheimer34 | — | — | 37/200 | 24/212 | |
Figure 3.
Forest plot for odds ratio of malarial parasitaemia (positive results on blood smear test) at end of supplementation period
Meta-regression analyses to explore heterogeneity
Stratified estimates indicated that iron supplementation did not significantly (P>0.05) increase the incidence of infections (incidence rate ratio and incidence rate difference), irrespective of the quality of methods, methods of surveillance, route of iron supplementation, duration of supplementation, geographic location of the study population, or the basal haemoglobin concentration of the iron supplemented group (data not presented). Meta-regression analysis showed that the treatment effect (incidence rate ratio) was not significantly associated with any of these study characteristics (table 6).
Table 6.
Meta-regression analyses for incidence rate ratio (IRR)
| Characteristic
|
Univariate analysis IRR (95% CI), P value
|
Controlled for all variables IRR (95% CI), P value
|
|---|---|---|
| Quality of study: | ||
| Allocation concealment (not adequate v adequate) | 1.02 (0.90 to 1.16), 0.716 | 0.94 (0.72 to 1.22), 0.624 |
| Completeness of follow up (⩾10% participants excluded v <10% excluded) | 1.01 (0.89 to 1.16), 0.839 | 1.17 (0.92 to 1.50), 0.202 |
| Blinding (not double blind v double blind) | 1.06 (0.95 to 1.19), 0.298 | 1.11 (0.91 to 1.36), 0.287 |
| Morbidity surveillance (passive v active) | 0.94 (0.84 to 1.05), 0.266 | 1.03 (0.83 to 1.27), 0.809 |
| Route of supplementation (oral or parenteral v fortified) | 1.04 (0.92 to 1.17), 0.555 | 1.03 (0.82 to 1.30), 0.815 |
| Geographic location (developed*v Asian or African countries) | 0.98 (0.87 to 1.11), 0.759 | 1.05 (0.81 to 1.36), 0.723 |
| Unit increase in baseline haemoglobin status of iron supplemented group (g/l) | 0.97 (0.94 to 1.01), 0.151 | 0.95 (0.90 to 1.00), 0.059 |
| Unit increase in duration of supplementation (months) | 1.00 (0.99 to 1.02), 0.864 | 0.99 (0.97 to 1.03), 0.921 |
Europe, North America, South America, and Australia and New Zealand.
Discussion
The results from our analysis of these studies show that iron supplementation does not significantly increase the risk of overall infection. However, there was an increase in the risk of developing diarrhoea, but this would not have an important overall impact on public health. The occurrence of other illnesses and malarial parasitaemia (adjusted for positive smear results at baseline) was not significantly affected by iron administration (P>0.05).
Strengths and limitations of analysis
Despite wide clinical and methodological heterogeneity in the various trials, the main inference remained stable for the various sensitivity analyses that we performed. An important caveat is the lack of uniform definitions for the individual clinical morbidities. Uniform definitions and active surveillance would have provided greater weight to the conclusions. Furthermore, not all the included trials were of high quality. We could not explain the statistical heterogeneity by various study characteristics.
There are still some questions unanswered and some new issues raised. We could not determine whether the higher risk of diarrhoea was a result of increased gastrointestinal infections or a consequence of the irritant effect of iron on the gut motility, a known effect.53 Dysentery is invariably infective in origin, and the two trials that provided information found no evidence of an increase in dysentery in children receiving iron supplements.
We could not analyse the effect of dose on the incidence of infections. However, the near absence of any important adverse effects, particularly diarrhoea, in children receiving fortified foods (compared with medicinal iron) raises the possibility of a dose related effect. Interestingly, there was also a similar significant protective effect against the development of respiratory tract infections (four studies; incidence rate ratio=0.92; 0.86 to 0.98; P=0.02). However, our meta-regression analysis showed that the route of administration was not significantly associated with incidence rate ratio. Fortification with low doses of iron is closest to the physiological situation and could theoretically be considered the safest public health intervention. There is thus a case for concomitant evaluation of the possible beneficial effects of iron fortified foods on the haematological response and infections.
Meta-regression analysis suggested that the risk of acquiring infectious illnesses is inversely associated with the baseline haemoglobin concentration. Stratified analysis also suggested increased risk of infections in children who had a mean baseline concentration below 100 g/l. Iron supplementation promotes production of free radicals, and this may have a deleterious effect on the immunity of a child. Ironically, defences against free radicals are compromised the most in iron deficiency and malnutrition,54,55 which are conditions likely to benefit the most from iron supplementation. Interestingly, all the studies included in this stratified subset were from regions of the African continent where malaria is endemic. Some data suggest indirectly that iron deficiency in such regions decreases the susceptibility to disease related to malaria, HIV, and tuberculosis.56 The safety of iron supplementation in people with anaemia, particularly in regions where malaria is endemic, may be difficult to determine because of the ethical problem of withholding treatment in a control group.
Acknowledgments
We thank Sunil Sinha for facilitating access to Embase and two PhD dissertations. We also thank L Satyanaryana for help in conducting the meta-regression analysis. Part of this paper was presented as a poster at the International Nutritional Anaemia Consultative Group symposium 2001, held at Hanoi, Vietnam.
Footnotes
Editorial by Tomkins
Funding: None.
Competing interests: International Life Science Institute (ILSI) sponsored TG for travel to Hanoi, Vietnam for the purpose of attending the International Nutritional Anaemia Consultative Group symposium and presenting part of the analysis as a poster.
References
- 1.United Nations Administrative Committee on Coordination Sub-Committee on Nutrition (ACC/SCN) Fourth report on the world nutrition situation. Geneva: ACC/SCN in collaboration with International Food Policy Research Institute; 2000. [Google Scholar]
- 2.Pollitt E. Iron deficiency anaemia and cognitive function. Ann Rev Nutr. 1993;13:521–537. doi: 10.1146/annurev.nu.13.070193.002513. [DOI] [PubMed] [Google Scholar]
- 3.American Academy of Pediatrics-Committee on Nutrition. Nutritional needs of low-birth-weight infants. Pediatrics. 1985;75:976–986. [PubMed] [Google Scholar]
- 4.Hurrell RF. Preventing iron deficiency through food fortification. Nutr Rev. 1997;55:210–222. doi: 10.1111/j.1753-4887.1997.tb01608.x. [DOI] [PubMed] [Google Scholar]
- 5.Kochan I. The role of iron in bacterial infections with special consideration of host-tubercle bacillus interaction. Curr Top Microbiol Immunol. 1973;60:1–30. doi: 10.1007/978-3-642-65502-9_1. [DOI] [PubMed] [Google Scholar]
- 6.Chandra RK. Reduced bactericidal capacity of polymorphs in iron deficiency. Arch Dis Child. 1973;48:864–867. doi: 10.1136/adc.48.11.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhaskaram P, Reddy V. Cell mediated immunity in iron and vitamin deficient children. BMJ. 1975;iii:522–524. doi: 10.1136/bmj.3.5982.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaduska MB, Burkitt MJ, Xiang DH, Mason RP. Iron supplementation generates hydroxyl radical in vivo. An ESR spin trapping investigation. J Clin Invest. 1995;96:1653–1657. doi: 10.1172/JCI118205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chwang L, Soemantri AG, Polloitt E. Iron supplementation and physical growth of rural Indonesian children. Am J Clin Nutr. 1988;47:496–501. doi: 10.1093/ajcn/47.3.496. [DOI] [PubMed] [Google Scholar]
- 10.Idjradinata P, Watkins WE, Pollitt E. Adverse effect of iron supplementation on weight gain of iron replete young children. Lancet. 1994;343:1252–1254. doi: 10.1016/s0140-6736(94)92151-2. [DOI] [PubMed] [Google Scholar]
- 11.Smith AW, Hendrickse RG, Harrison C, Hayes RJ, Greenwood BM. The effects on malaria of treatment of iron deficiency anaemia with oral iron in Gambian children. Ann Trop Paediatr. 1989;9:17–23. doi: 10.1080/02724936.1989.11748589. [DOI] [PubMed] [Google Scholar]
- 12.Murray MJ, Murray AB, Murray MB, Murray CJ. The adverse effect of iron repletion on the course of certain infections. BMJ. 1978;ii:1113–1115. doi: 10.1136/bmj.2.6145.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Juni P, Altman DG, Egger M. Assessing the quality of randomised controlled trials. In: Egger M, Smith GD, Altman DG, editors. Systematic reviews in health care: meta-analysis in context. London: BMJ Publishing; 2001. pp. 87–108. [Google Scholar]
- 14.Clarke M, Oxman AD, editors. Cochrane Library. Issue 1. Oxford: Update Software; 2001. Assessment of study quality. Cochrane reviewers handbook 4.1.1 [updated December 2000] [Google Scholar]
- 15.Sterne JAC, Egger M, Smith GD. Investigating and dealing with publication and other biases. In: Egger M, Smith GD, Altman DG, editors. Systematic reviews in health care: meta-analysis in context. London: BMJ Publishing; 2001. pp. 189–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sterne JAC, Bradburn MJ, Egger M. Meta-analysis in STATA TM. In: Egger M, Smith GD, Altman DG, editors. Systematic reviews in health care: meta-analysis in context. London: BMJ Books; 2001. pp. 347–369. [Google Scholar]
- 17.Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Smith GD, Altman DG, editors. Systematic reviews in health care: meta-analysis in context. London: BMJ Books; 2001. pp. 285–312. [Google Scholar]
- 18.Andelman MB, Bernard RS. Utilization of dietary iron by term infants. Am J Dis Child. 1966;111:45–55. doi: 10.1001/archpedi.1966.02090040081007. [DOI] [PubMed] [Google Scholar]
- 19.Burman D. Haemoglobin levels in normal infants aged 3 to 24 months, and the effect of iron. Arch Dis Child. 1972;47:261–271. doi: 10.1136/adc.47.252.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Damodaran M, Naidu AN, Sarma KVR. Anaemia and morbidity in preschool children. Indian J Med Res. 1979;69:448–456. [PubMed] [Google Scholar]
- 21.Oppenheimer SJ, Gibson FD, Macfarlane SB, Moody JB, Harrison C, Spencer A, et al. Iron supplementation increases prevalence and effects of malaria: report on clinical studies in Papua New Guinea. Trans R Soc Trop Med Hyg. 1986;80:603–612. doi: 10.1016/0035-9203(86)90154-9. [DOI] [PubMed] [Google Scholar]
- 22.Bates CJ, Powers HJ, Lamb WH, Gelman W, Webb E. Effect of supplementary vitamins and iron on malaria indices in rural Gambian children. Trans R Soc Trop Med Hyg. 1987;81:286–291. doi: 10.1016/0035-9203(87)90244-6. [DOI] [PubMed] [Google Scholar]
- 23.Heresi G, Olivares M, Pizarro F, Cayazzo M, Stekel A. Effect of an iron fortified milk on morbidity in infancy: a field trial. Nutr Res. 1987;7:915–922. [Google Scholar]
- 24.Heywood A, Oppenheimer S, Heywood P, Jolley D. Behavioural effects of iron supplementation in infants in Madang, Papua New Guinea. Am J Clin Nutr. 1989;50:630–640. doi: 10.1093/ajcn/50.3.630. [DOI] [PubMed] [Google Scholar]
- 25.Angeles-Agdeppa I, Schultnik W, Sastroamidjojo S, Gross R, Karyadi D. Weekly micronutrient supplementation to build iron stores in female Indonesian adolescents. Am J Clin Nutr. 1997;66:177–183. doi: 10.1093/ajcn/66.1.177. [DOI] [PubMed] [Google Scholar]
- 26.Heresi G, Pizarro F, Olivares M, Cayazzo M, Hertrampf E, Walter T, et al. Effect of supplementation with an iron fortified milk on incidence of diarrhoea and respiratory infection in urban-resident infants. Scand J Infect Dis. 1995;27:385–389. doi: 10.3109/00365549509032736. [DOI] [PubMed] [Google Scholar]
- 27.Van Hensbroek MB, Morris-Jones S, Meisner S, Jaffar S, Bayo L, Dackour R, et al. Iron, but not folic acid, combined with effective antimalarial therapy promotes haematological recovery in African children after acute falciparum malaria. Trans R Soc Trop Med Hyg. 1995;89:672–676. doi: 10.1016/0035-9203(95)90438-7. [DOI] [PubMed] [Google Scholar]
- 28.Beck HP, Felger I, Vounatsou P, Hirt R, Tanner M, Alonso P, et al. Effect of iron supplementation and malaria prophylaxis in infants on Plasmodium falciparum genotypes and multiplicity of infection. Trans R Soc Trop Med Hyg. 1999;93:S1/41–S1/45. doi: 10.1016/s0035-9203(99)90326-7. [DOI] [PubMed] [Google Scholar]
- 29.Van Stuijvenberg ME, Kvalsvig JD, Faber M, Kruger M, Kenoyer DG, Benalde AJS. Effect of iron-, iodine-, and β-carotene-fortified biscuits on the micronutrient status of primary school children: a randomised controlled trial. Am J Clin Nutr. 1999;69:497–503. doi: 10.1093/ajcn/69.3.497. [DOI] [PubMed] [Google Scholar]
- 30.Picaud JC, Rivet C, Salle BL, Basson E, Lasne Y, Chapuis-Cellier C, et al. Early iron supplementation in preterm infants with erythropoietin treatment: Safety and efficacy. Prenat Neonat Med. 1999;4:472–478. [Google Scholar]
- 31.James JA, Combes M. Iron deficiency in the premature infant: Significance and prevention by intramuscular administration of iron-dextran. Pediatrics. 1960;26:368–374. [PubMed] [Google Scholar]
- 32.Cantwell RJ. Iron deficiency anaemia of infancy: some clinical principles illustrated by the response of Maori infants to neonatal parenteral iron administration. Clin Pediatr. 1972;11:443–449. doi: 10.1177/000992287201100807. [DOI] [PubMed] [Google Scholar]
- 33.Fuerth JH. Iron supplementation of the diet in full-term infants: a controlled study. J Pediatr. 1974;80:974–979. doi: 10.1016/s0022-3476(72)80010-6. [DOI] [PubMed] [Google Scholar]
- 34.Oppenheimer SJ, Macfarlane SBJ, Moody JB, Bunari O, Hendrickse RG. Effect of iron prophylaxis on morbidity due to infectious disease: report on clinical studies in Papua New Guinea. Trans R Soc Trop Med Hyg. 1986;80:596–602. doi: 10.1016/0035-9203(86)90153-7. [DOI] [PubMed] [Google Scholar]
- 35.Harvey PWJ, Heywood PF, Nesheim MC, Galme K, Zegans M, Habicht JP, et al. The effect of iron therapy on malarial infection in Papua New Guinean schoolchildren. Am J Trop Med Hyg. 1989;40:12–18. doi: 10.4269/ajtmh.1989.40.12. [DOI] [PubMed] [Google Scholar]
- 36.Power HM, Heese HDV, Beatty DW, Hughes J, Dempster WS. Iron fortification of infant milk formula: the effect on iron status and immune function. Ann Trop Paediatr. 1991;11:57–66. doi: 10.1080/02724936.1991.11747479. [DOI] [PubMed] [Google Scholar]
- 37.Javaid N, Haschke F, Pietschnig B, Schuster E, Huemer C, Shebaz A, et al. Interactions between infections, malnutrition and iron nutritional status in Pakistani infants. Acta Paediatr Scand. 1991;374:141–150. doi: 10.1111/j.1651-2227.1991.tb12017.x. [DOI] [PubMed] [Google Scholar]
- 38.Irigoyen M, Davidson LL, Carriero D, Seaman C. Randomised, placebo-controlled trial of iron supplementation in infants with low haemoglobin levels fed iron-fortified formula. Pediatrics. 1991;88:320–326. [PubMed] [Google Scholar]
- 39.Chippaux JP, Schneider D, Aplogan A, Dyck JL, Berger J. Effets de la supplementation en fer sur l'infection palustre. Bull Soc Path Ex. 1991;84:54–62. [PubMed] [Google Scholar]
- 40.Brunser O, Espinoza J, Araya M, Pacheco I, Cruchet S. Chronic iron intake and diarrhoeal disease in infants: a field study in a less-developed country. Eur J Clin Nutr. 1993;47:317–326. [PubMed] [Google Scholar]
- 41.Angeles IT, Schultnik WJ, Matulessi P, Gross R, Sastroamidjojo S. Decreased rate of stunting among anaemic Indonesian preschool children through iron supplementation. Am J Clin Nutr. 1993;58:339–342. doi: 10.1093/ajcn/58.3.339. [DOI] [PubMed] [Google Scholar]
- 42.Lawless JW, Latham MC, Stephenson LS, Kinoti SN, Pertet AM. Iron supplementation improves appetite and growth in anaemic Kenyan primary school children. J Nutr. 1994;124:645–654. doi: 10.1093/jn/124.5.645. [DOI] [PubMed] [Google Scholar]
- 43.Hemminki E, Nemet K, Horvath M, Malin M, Schuler D, Hollan S. Impact of iron fortification of milk formulas on infants growth and health. Nutr Res. 1995;4:491–503. [Google Scholar]
- 44.Van den Hombergh J, Dalderop E, Smit Y. Does iron therapy benefit children with severe malaria-associated anaemia? A clinical trial with 12 weeks supplementation of oral iron in young children from the Turiani division, Tanzania. J Trop Pediatr. 1996;42:220–227. doi: 10.1093/tropej/42.4.220. [DOI] [PubMed] [Google Scholar]
- 45.Adam Z. Iron supplementation and malaria: a randomised, placebo-controlled field trial in rural Ethiopia. London, University of London. 1996. (PhD thesis). [Google Scholar]
- 46.Gebreselassie H. Quebec: McGill University; 1996. Iron supplementation and malaria infection: Results of a randomised controlled field trial. (PhD thesis). [Google Scholar]
- 47.Mitra AK, Akramuzzaman SM, Fuchs GJ, Rahman MM, Mahalanabis D. Long-term oral supplementation with iron is not harmful for young children in a poor community of Bangladesh. J Nutr. 1997;127:1451–1455. doi: 10.1093/jn/127.8.1451. [DOI] [PubMed] [Google Scholar]
- 48.Palupi L, Schultnik W, Achadi E, Gross R. Effective community intervention to improve haemoglobin status in preschoolers receiving once-weekly iron supplementation. Am J Clin Nutr. 1997;65:1057–1061. doi: 10.1093/ajcn/65.4.1057. [DOI] [PubMed] [Google Scholar]
- 49.Rosado JL, Lopez P, Munoz E, Martinez H, Allen LH. Zinc supplementation reduced morbidity, but neither zinc nor iron supplementation affected growth or body composition of Mexican preschoolers. Am J Clin Nutr. 1997;65:13–19. doi: 10.1093/ajcn/65.1.13. [DOI] [PubMed] [Google Scholar]
- 50.Menendez C, Kahigwa E, Hirt R, Vounatsou P, Aponte JJ, Font F, et al. Randomised placebo-controlled trial of iron supplementation and malaria chemoprophylaxis for prevention of severe anaemia and malaria in Tanzanian infants. Lancet. 1997;350:844–850. doi: 10.1016/S0140-6736(97)04229-3. [DOI] [PubMed] [Google Scholar]
- 51.Berger J, Dyck JC, Galan P, Aplogan A, Scnheider D, Traissac P, et al. Effect of daily iron supplementation on iron status, cell-mediated immunity, and incidence of infections in 6-36 month old Togolese children. Eur J Clin Nutr. 2000;54:29–35. doi: 10.1038/sj.ejcn.1600888. [DOI] [PubMed] [Google Scholar]
- 52.Singhal A, Morley R, Abbott R, Fairweather-Tait S, Stephenson T, Lucas A. Clinical safety of iron fortified formulas. Pediatrics. 2000;105:e38. doi: 10.1542/peds.105.3.e38. [DOI] [PubMed] [Google Scholar]
- 53.Hallberg L, Ryttinger L, Solvell L. Side effects of oral iron therapy. A double blind study of different iron compounds in tablet form. Acta Med Scand Suppl. 1966;459:3–10. doi: 10.1111/j.0954-6820.1966.tb19403.x. [DOI] [PubMed] [Google Scholar]
- 54.Srigiridhar K, Nair KM. Iron deficient intestine is more susceptible to peroxidative damage during iron supplementation in rats. Free Radic Biol Med. 1998;25:660–665. doi: 10.1016/s0891-5849(98)00086-0. [DOI] [PubMed] [Google Scholar]
- 55.Tatli MM, Vural H, Koc A, Kosecik M, Atas A. Altered anti-oxidant status and increased lipid peroxidation in marasmic children. Pediatr Int. 2000;42:289–292. doi: 10.1046/j.1442-200x.2000.01217.x. [DOI] [PubMed] [Google Scholar]
- 56.Oppenheimer SJ. Iron and its relation to immunity and infectious disease. J Nutr. 2001;131(suppl):616–635S. doi: 10.1093/jn/131.2.616S. [DOI] [PubMed] [Google Scholar]