Abstract
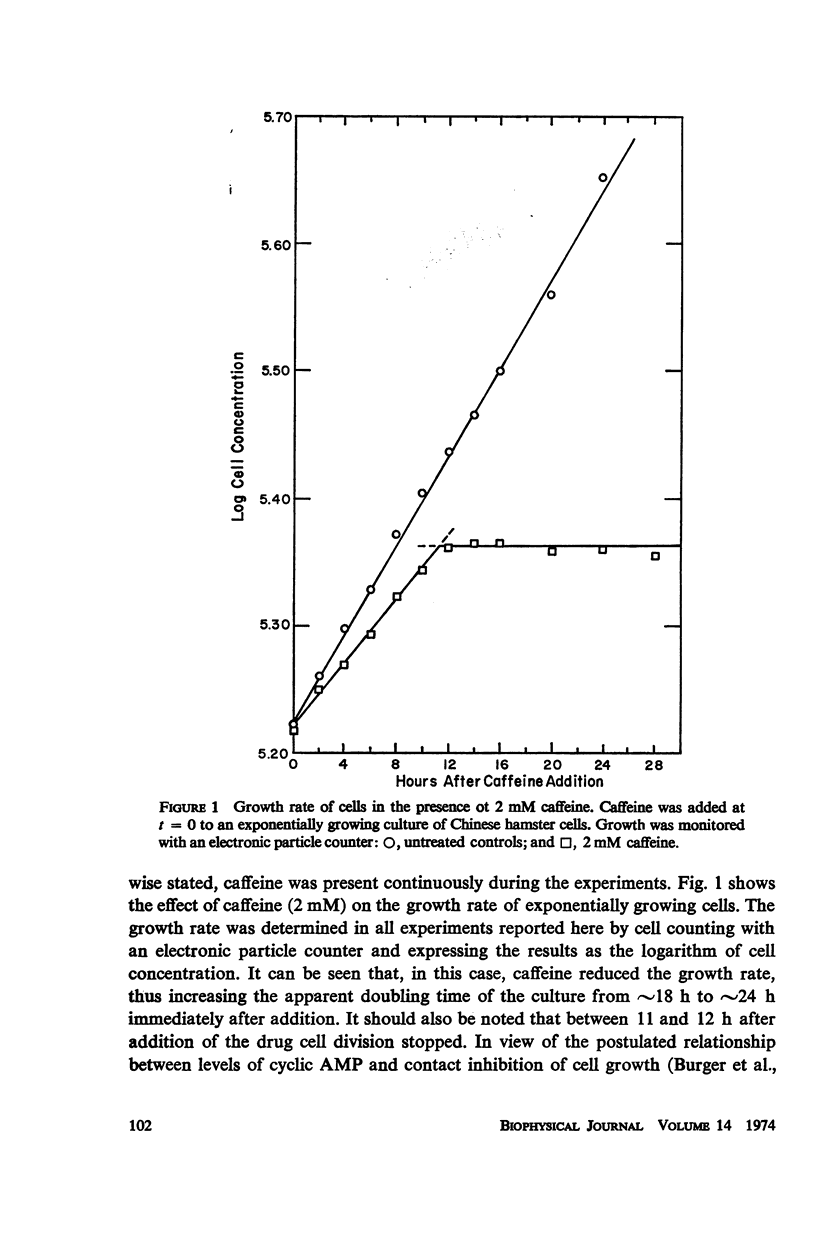
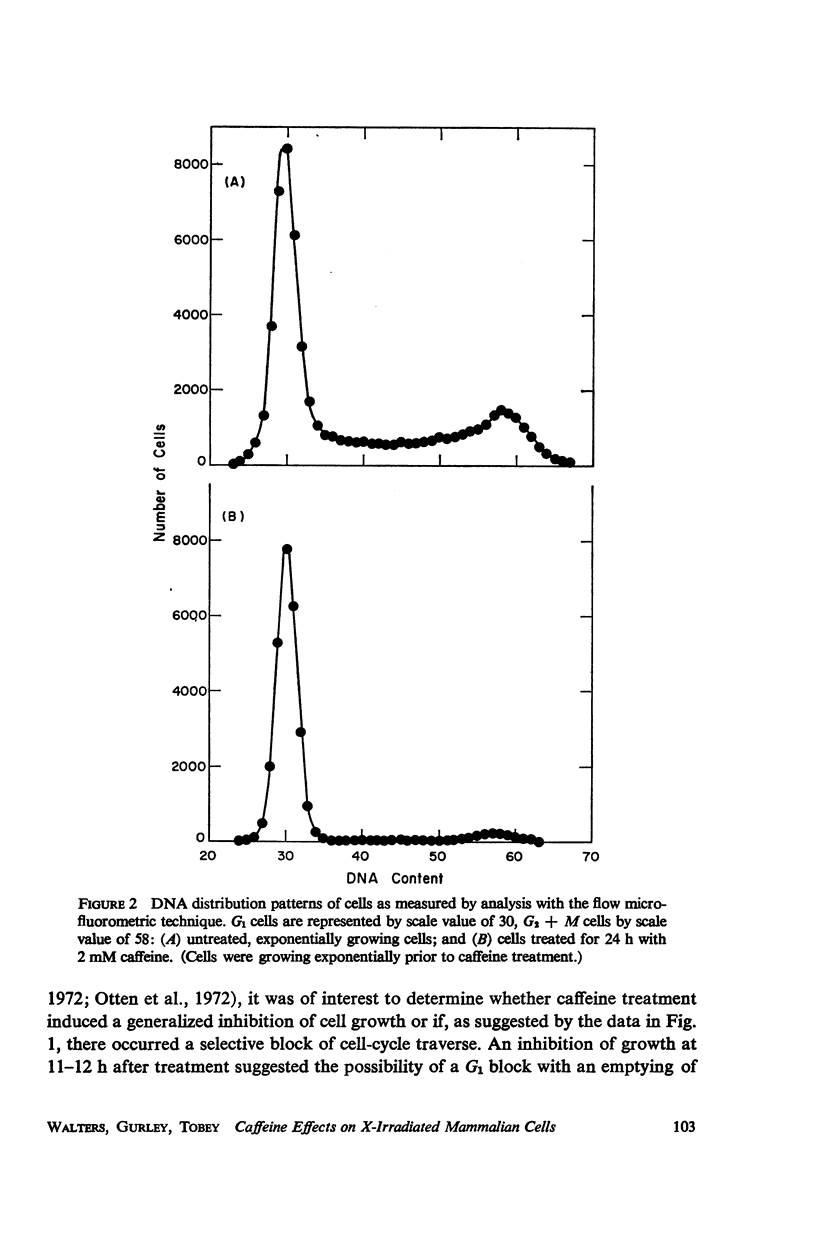
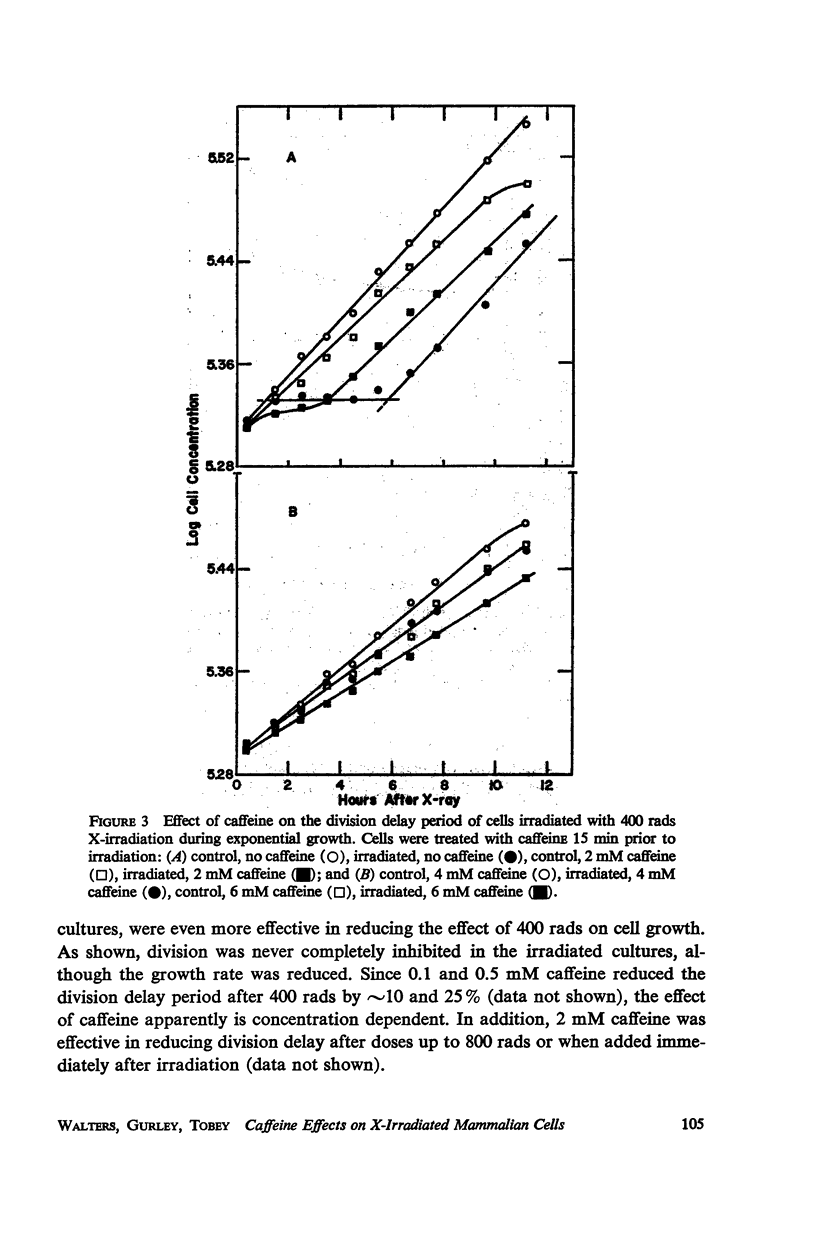
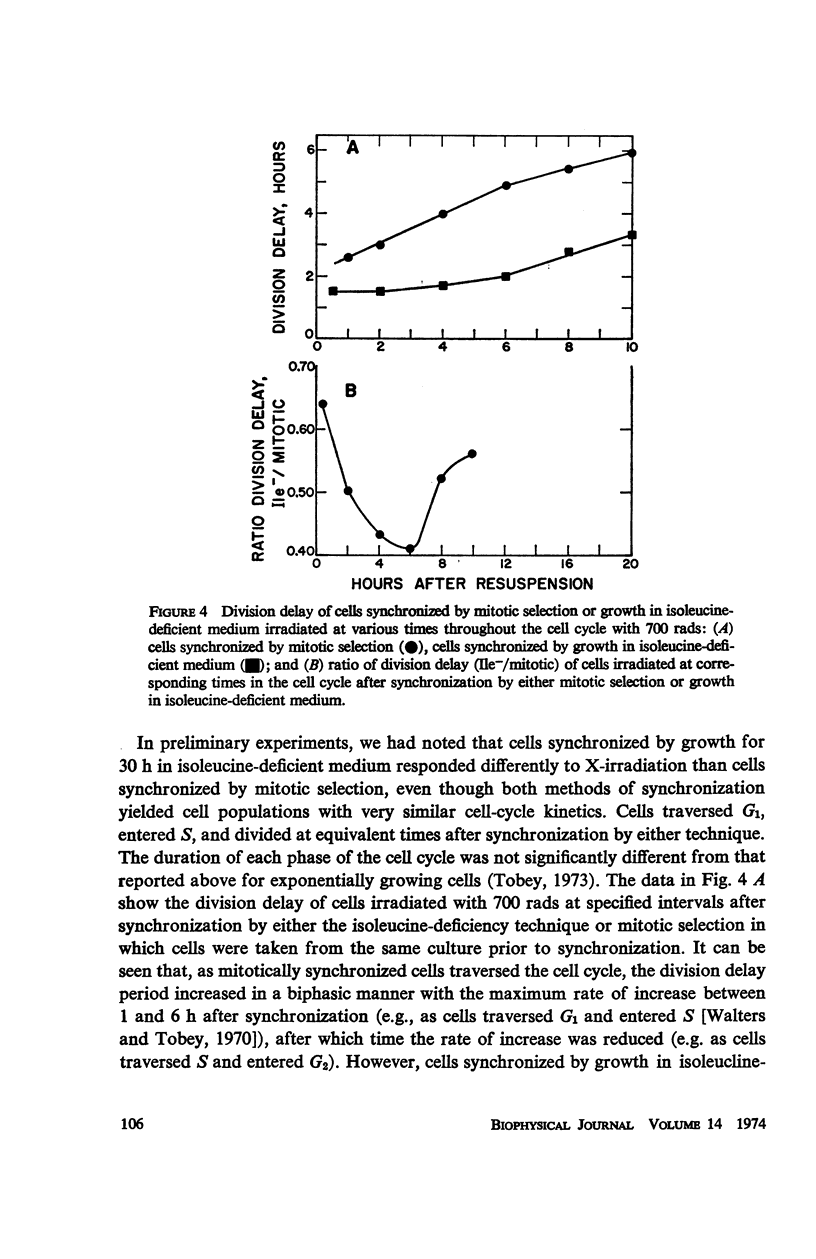
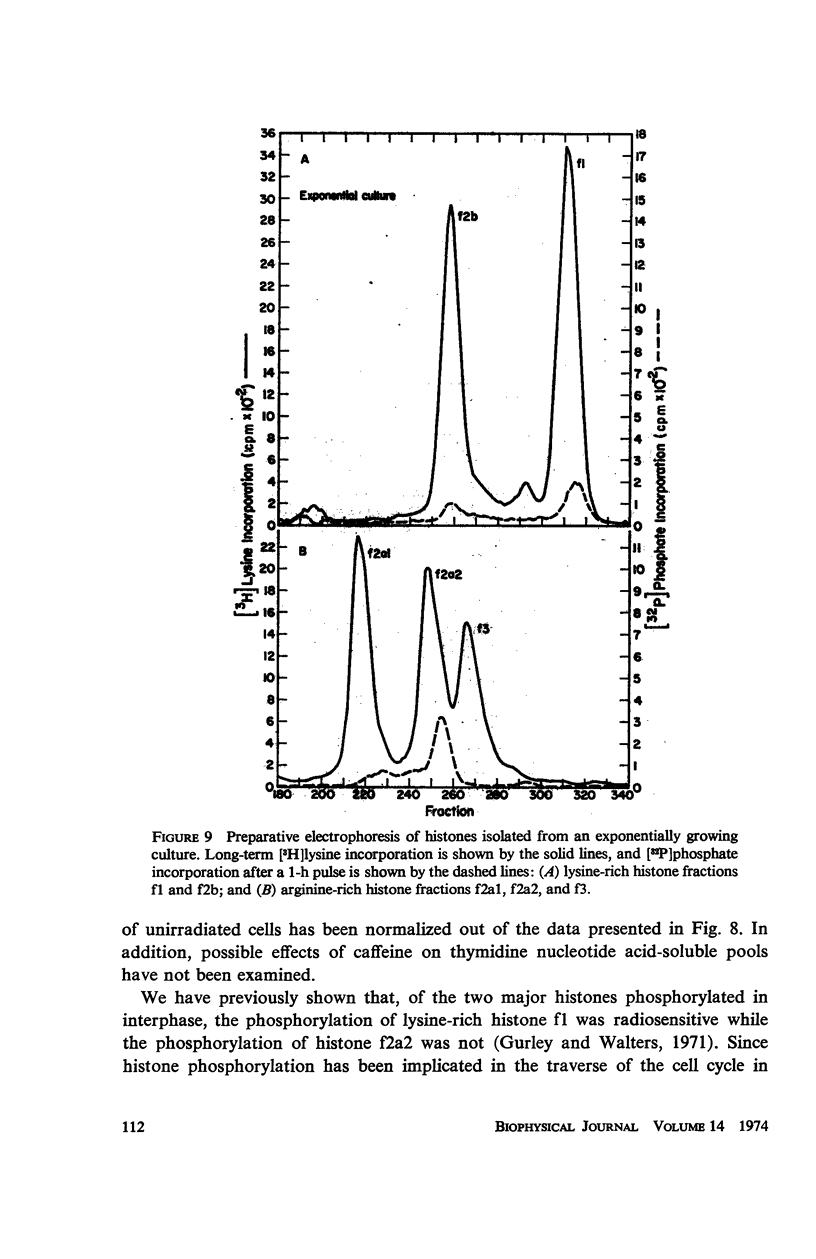
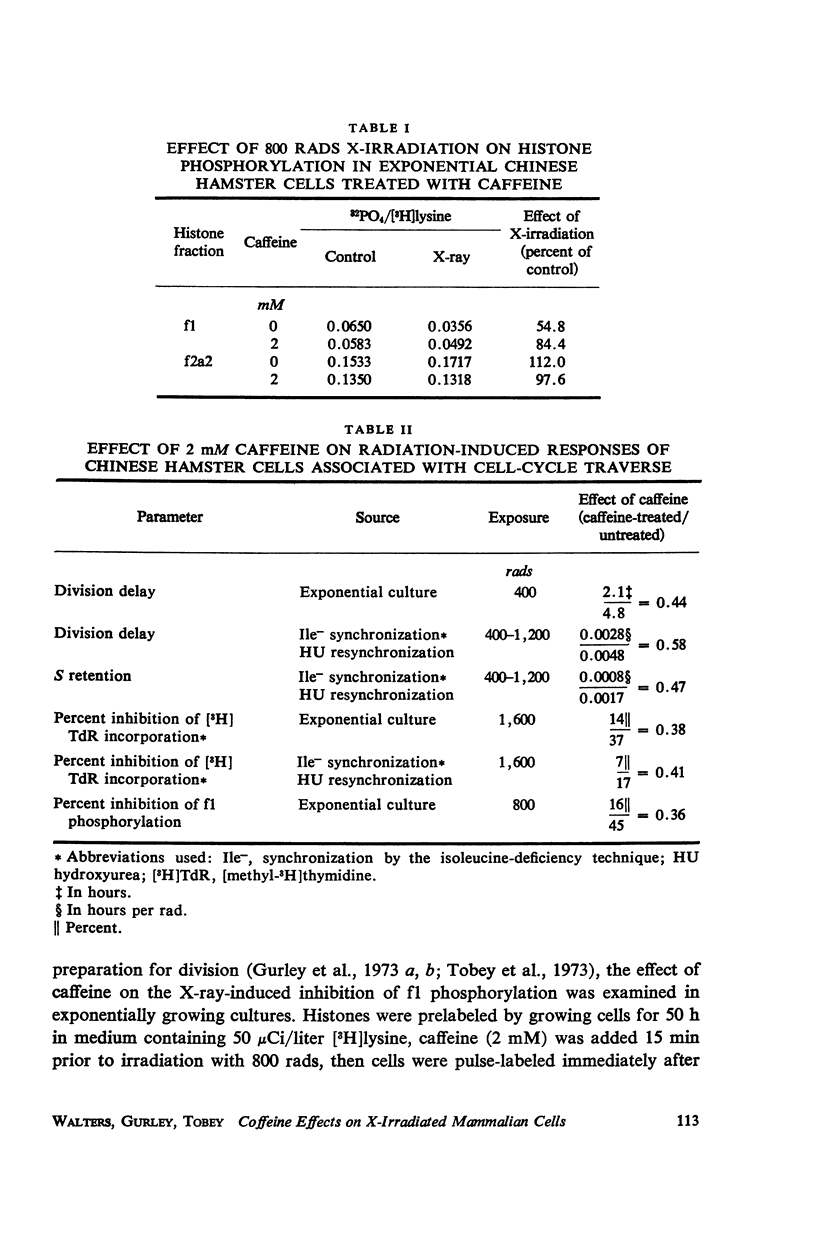
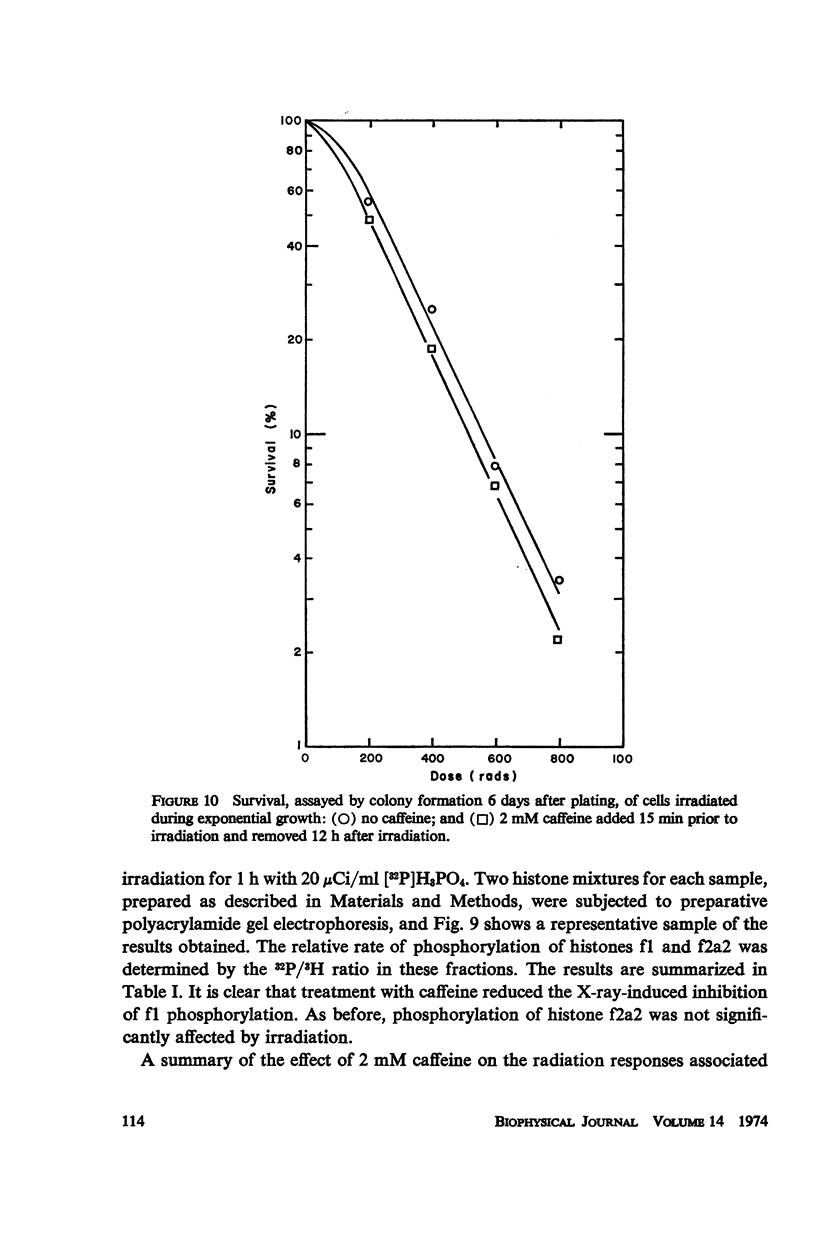
Caffeine induced a state of G1 arrest when added to an exponentially growing culture of Chinese hamster cells (line CHO). In addition to its effect on cell-cycle traverse, caffeine ameliorated a number of the responses of cells to ionizing radiation. The duration of the division delay period following X-irradiation of caffeine-treated cells was reduced, and the magnitude of reduction was dependent on caffeine concentration. Cells irradiated during the DNA synthetic phase in the presence of caffeine were delayed less in their exit from S, measured autoradiographically, and the radiation-induced reduction of radioactive thymidine incorporation into DNA was lessened. Cells synchronized by isoleucine deprivation, while being generally less sensitive to the effects of ionizing radiation than mitotically synchronized cells, were equally responsive to the effects of caffeine. The X-ray-induced reduction of phosphorylation of lysine-rich histone F1 was less in caffeine-treated cells than in untreated cells. Finally, survival after irradiation was only slightly reduced in caffeine-treated cells. A possible role of cyclic AMP in cell-cycle traverse of irradiated cells is discussed.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burger M. M., Bombik B. M., Breckenridge B. M., Sheppard J. R. Growth control and cyclic alterations of cyclic AMP in the cell cycle. Nat New Biol. 1972 Oct 11;239(93):161–163. doi: 10.1038/newbio239161a0. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E. Repair replication of mammalian cell DNA: effects of compounds that inhibit DNA synthesis or dark repair. Radiat Res. 1969 Feb;37(2):334–348. [PubMed] [Google Scholar]
- Cleaver J. E., Thomas G. H. Single strand interruptions in DNA and the effects of caffeine in Chinese hamster cells irradiated with ultraviolet light. Biochem Biophys Res Commun. 1969 Jul 23;36(2):203–208. doi: 10.1016/0006-291x(69)90315-5. [DOI] [PubMed] [Google Scholar]
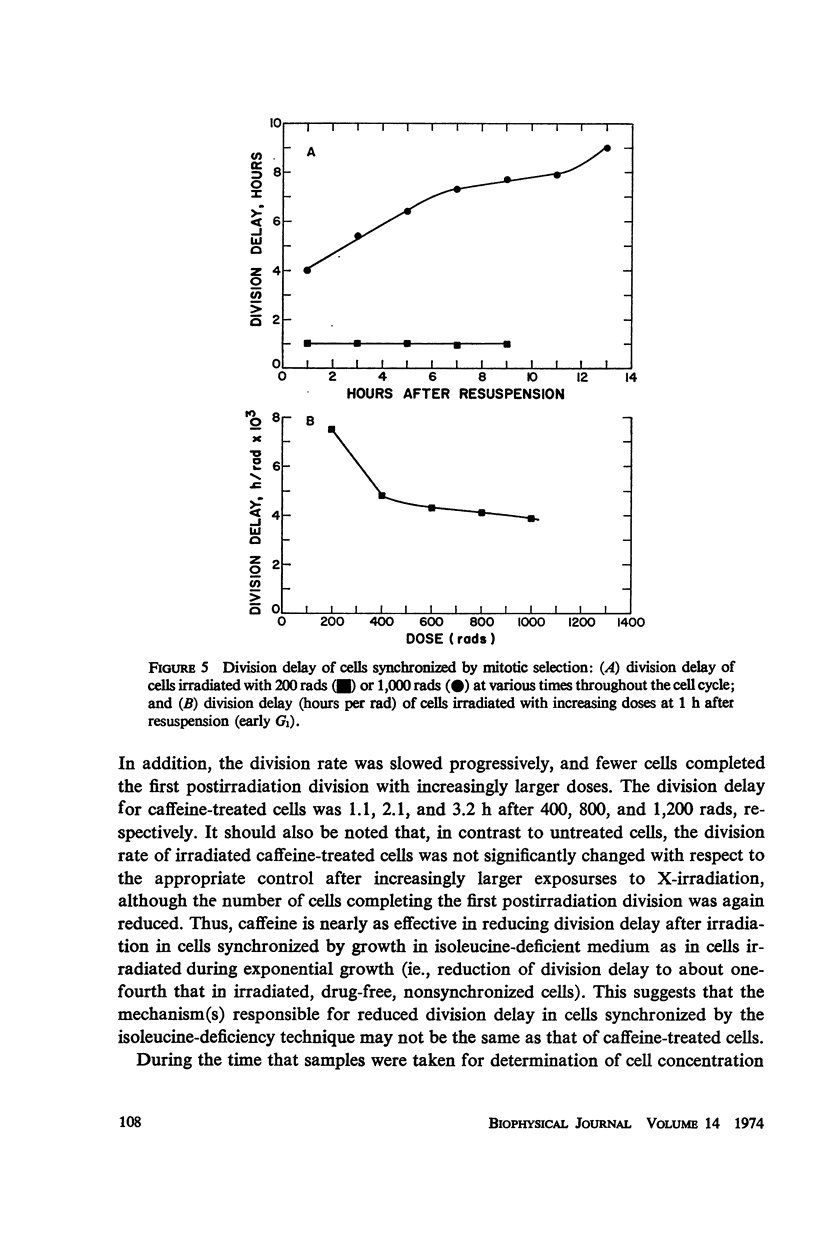
- DEWEY W. C., HUMPHREY R. M., JONES B. A. RELATIONSHIP BETWEEN RADIATION-INDUCED MITOTIC DELAY AND DOUBLING-TIME OF CELLS. Int J Radiat Biol Relat Stud Phys Chem Med. 1964;8:605–607. doi: 10.1080/09553006414550731. [DOI] [PubMed] [Google Scholar]
- Domon M., Rauth A. M. Effects of caffeine on ultraviolet-irradiated mouse L cells. Radiat Res. 1969 Jul;39(1):207–221. [PubMed] [Google Scholar]
- Enger M. D., Tobey R. A. Effects of isoleucine deficiency on nucleic acid and protein metabolism in cultured Chinese hamster cells. Continued ribonucleic acid and protein synthesis in the absence of deoxyribonucleic acid synthesis. Biochemistry. 1972 Jan 18;11(2):269–277. doi: 10.1021/bi00752a019. [DOI] [PubMed] [Google Scholar]
- Froehlich J. E., Rachmeler M. Effect of adenosine 3'-5'-cyclic monophosphate on cell proliferation. J Cell Biol. 1972 Oct;55(1):19–31. doi: 10.1083/jcb.55.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurley L. R., Hardin J. M. The metabolism of histone fractions. I. Synthesis of histone fractions during the life cycle of mammalian cells. Arch Biochem Biophys. 1968 Nov;128(2):285–292. doi: 10.1016/0003-9861(68)90034-9. [DOI] [PubMed] [Google Scholar]
- Gurley L. R., Walters R. A. Response of histone turnover and phosphorylation to X irradiation. Biochemistry. 1971 Apr 27;10(9):1588–1593. doi: 10.1021/bi00785a013. [DOI] [PubMed] [Google Scholar]
- Gurley L. R., Walters R. A., Tobey R. A. The metabolism of histone fractions. VI. Differences in the phosphorylation of histone fractions during the cell cycle. Arch Biochem Biophys. 1973 Jan;154(1):212–218. doi: 10.1016/0003-9861(73)90051-9. [DOI] [PubMed] [Google Scholar]
- House W., Waddell A. Detection of mycoplasma in cell cultures. J Pathol Bacteriol. 1967 Jan;93(1):125–132. doi: 10.1002/path.1700930112. [DOI] [PubMed] [Google Scholar]
- Langan T. A. Action of adenosine 3',5'-monophosphate-dependent histone kinase in vivo. J Biol Chem. 1969 Oct 25;244(20):5763–5765. [PubMed] [Google Scholar]
- Lehmann A. R. Effect of caffeine on DNA synthesis in mammalian cells. Biophys J. 1972 Oct;12(10):1316–1325. doi: 10.1016/S0006-3495(72)86165-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacManus J. P., Whitfield J. F., Youdale T. Stimulation by epinephrine of adenyl cyclase activity, cyclic AMP formation, DNA synthesis and cell proliferation in populations of rat thymic lymphocytes. J Cell Physiol. 1971 Feb;77(1):103–116. doi: 10.1002/jcp.1040770112. [DOI] [PubMed] [Google Scholar]
- Moore E. C., Hurlbert R. B. Regulation of mammalian deoxyribonucleotide biosynthesis by nucleotides as activators and inhibitors. J Biol Chem. 1966 Oct 25;241(20):4802–4809. [PubMed] [Google Scholar]
- Otten J., Johnson G. S., Pastan I. Regulation of cell growth by cyclic adenosine 3',5'-monophosphate. Effect of cell density and agents which alter cell growth on cyclic adenosine 3',5'-monophosphate levels in fibroblasts. J Biol Chem. 1972 Nov 10;247(21):7082–7087. [PubMed] [Google Scholar]
- Perris A. D., Whitfield J. F., Rixon R. H. Stimulation of mitosis in bone marrow and thymus of normal and irradiated rats by divalent cations and parathyroid extract. Radiat Res. 1967 Nov;32(3):550–563. [PubMed] [Google Scholar]
- Rasmussen H. Cell communication, calcium ion, and cyclic adenosine monophosphate. Science. 1970 Oct 23;170(3956):404–412. doi: 10.1126/science.170.3956.404. [DOI] [PubMed] [Google Scholar]
- Rauth A. M. Evidence for dark-reactivation of ultraviolet light damage in mouse L cells. Radiat Res. 1967 May;31(1):121–138. [PubMed] [Google Scholar]
- Reimann E. M., Brostrom C. O., Corbin J. D., King C. A., Krebs E. G. Separation of regulatory and catalytic subunits of the cyclic 3',5'-adenosine monophosphate-dependent protein kinase(s) of rabbit skeletal muscle. Biochem Biophys Res Commun. 1971 Jan 22;42(2):187–194. doi: 10.1016/0006-291x(71)90086-6. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Pardee A. B. Opposite effects of dibutyryl adenosine 3':5' cyclic monophosphate and serum on growth of Chinese hamster cells. J Cell Physiol. 1972 Oct;80(2):273–279. doi: 10.1002/jcp.1040800215. [DOI] [PubMed] [Google Scholar]
- Scaife J. F. Cyclic 3'-5'-adenosine monophosphate: its possible role in mammalian cell mitosis and radiation-induced mitotic G2-delay. Int J Radiat Biol Relat Stud Phys Chem Med. 1971;19(2):191–195. doi: 10.1080/09553007114550251. [DOI] [PubMed] [Google Scholar]
- Shepherd G. R., Walters R. A., Hardin J. M., Noland B. J. The effects of x-irradiation on histone acetylation and methylation in cultured mammalian cells. Arch Biochem Biophys. 1972 Mar;149(1):175–182. doi: 10.1016/0003-9861(72)90312-8. [DOI] [PubMed] [Google Scholar]
- Smith E. L., DeLange R. J., Bonner J. Chemistry and biology of the histones. Physiol Rev. 1970 Apr;50(2):159–170. doi: 10.1152/physrev.1970.50.2.159. [DOI] [PubMed] [Google Scholar]
- Tobey R. A., Anderson E. C., Petersen D. F. Properties of mitotic cells prepared by mechanically shaking monolayer cultures of Chinese hamster cells. J Cell Physiol. 1967 Aug;70(1):63–68. doi: 10.1002/jcp.1040700109. [DOI] [PubMed] [Google Scholar]
- Tobey R. A., Crissman H. A. Preparation of large quantities of synchronized mammalian cells in late G1 in the pre-DNA replicative phase of the cell cycle. Exp Cell Res. 1972 Dec;75(2):460–464. doi: 10.1016/0014-4827(72)90453-3. [DOI] [PubMed] [Google Scholar]
- WHITFIELD J. F., BROHEE H., YOUDALE T. REDUCTION OF MITOTIC DELAY IN IRRADIATED SUSPENSION CULTURES OF RAT THYMOCYTES BY AN ELEVATED SALT CONCENTRATION. Exp Cell Res. 1964 Jun;35:207–210. doi: 10.1016/0014-4827(64)90085-0. [DOI] [PubMed] [Google Scholar]
- WHITFIELD J. F., RIXON R. H. Prevention of postirradiation mitotic delay in cultures of L mouse cells by calcium salts. Exp Cell Res. 1962 Jun;27:154–157. doi: 10.1016/0014-4827(62)90055-1. [DOI] [PubMed] [Google Scholar]
- Walters R. A., Petersen D. F. Radiosensitivity of mammalian cells. II. Radiation effects on macromolecular synthesis. Biophys J. 1968 Dec;8(12):1487–1504. doi: 10.1016/S0006-3495(68)86568-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters R. A., Tobey R. A. Radiosensitivity of mammalian cells. IV. Effects of x-irradiation on the DNA synthetic period in synchronized cells. Biophys J. 1970 Jun;10(6):556–562. doi: 10.1016/S0006-3495(70)86319-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters R. A., Tobey R. A., Ratliff R. L. Cell-cycle-dependent variations of deoxyribonucleoside triphosphate pools in Chinese hamster cells. Biochim Biophys Acta. 1973 Sep 7;319(3):336–347. doi: 10.1016/0005-2787(73)90173-1. [DOI] [PubMed] [Google Scholar]
- Whitfield J. F., MacManus J. P., Rixon R. H. Cyclic AMP-mediated stimulation of thymocyte proliferation by low concentrations of cortisol. Proc Soc Exp Biol Med. 1970 Sep;134(4):1170–1174. doi: 10.3181/00379727-134-34967. [DOI] [PubMed] [Google Scholar]
- Whitfield J. F., MacManus J. P., Rixon R. H. The possible mediation by cyclic AMP of parathyroid hormone-induced stimulation of mitotic activity and deoxyribonucleic acid synthesis in rat thymic lymphocytes. J Cell Physiol. 1970 Apr;75(2):213–224. doi: 10.1002/jcp.1040750210. [DOI] [PubMed] [Google Scholar]
- Whitfield J. F., Rixon R. H., MacManus J. P., Balk S. D. Calcium, cyclic adenosine 3',5'-monophosphate, and the control of cell proliferation: a review. In Vitro. 1973 Jan-Feb;8(4):257–278. doi: 10.1007/BF02615905. [DOI] [PubMed] [Google Scholar]
- Whitfield J. F., Rixon R. H., Youdale T. The calcium-dependent stimulation of mitotic activity in normal and irradiated rat thymocyte populations by somatotrophic hormone. Int J Radiat Biol Relat Stud Phys Chem Med. 1969;15(4):385–388. doi: 10.1080/09553006914550601. [DOI] [PubMed] [Google Scholar]


