Abstract
Objectives
To determine the prevalence of left ventricular systolic dysfunction, and of heart failure due to different causes, in patients with risk factors for these conditions.
Design
Epidemiological study, including detailed clinical assessment, electrocardiography, and echocardiography.
Setting
16 English general practices, representative for socioeconomic status and practice type.
Participants
1062 patients (66% response rate) with previous myocardial infarction, angina, hypertension, or diabetes.
Main outcome measures
Prevalence of systolic dysfunction, both with and without symptoms, and of heart failure, in groups of patients with each of the risk factors.
Results
Definite systolic dysfunction (ejection fraction <40%) was found in 54/244 (22.1%, 95% confidence interval 17.1% to 27.9%) patients with previous myocardial infarction, 26/321 (8.1%, 5.4% to 11.6%) with angina, 7/388 (1.8%, 0.7% to 3.7%) with hypertension, and 12/208 (5.8%, 3.0% to 9.9%) with diabetes. In each group, approximately half of these patients had symptoms of dyspnoea, and therefore had heart failure. Overall rates of heart failure, defined as symptoms of dyspnoea plus objective evidence of cardiac dysfunction (systolic dysfunction, atrial fibrillation, or clinically significant valve disease) were 16.0% (11.6% to 21.2%) in patients with previous myocardial infarction, 8.4% (5.6% to 12.0%) in those with angina, 2.8% (1.4% to 5.0%) in those with hypertension, and 7.7% (4.5% to 12.2%) in those with diabetes.
Conclusion
Many people with ischaemic heart disease or diabetes have systolic dysfunction or heart failure. The data support the need for trials of targeted echocardiographic screening, in view of the major benefits of modern treatment. In contrast, patients with uncomplicated hypertension have similar rates to the general population.
What is already known on this topic
The prognosis and symptoms of patients with left ventricular systolic dysfunction and heart failure can be greatly improved by modern treatments
Many patients with heart failure do not have an assessment of left ventricular function, resulting in undertreatment of the condition
What this study adds
Patients with a history of ischaemic heart disease (especially those with previous myocardial infarction) or diabetes commonly have left ventricular systolic dysfunction
These patients would be candidates for a targeted echocardiographic screening programme
In contrast, the yield from screening patients with uncomplicated hypertension would be low
Introduction
Heart failure, the most common and important precursor of which is left ventricular systolic dysfunction, causes high mortality and major impairment of quality of life.1–3 It is also a major cause of healthcare expenditure through frequent hospital admissions.4 The symptoms and prognosis of patients with overt heart failure due to systolic dysfunction are greatly improved by angiotensin converting enzyme inhibitors,5 and the use of these drugs in patients with asymptomatic systolic dysfunction can also delay or prevent progression to symptomatic heart failure.6,7 In addition, β blockers further improve survival,8,9 as does spironolactone in more severe cases.10 Modern management, especially when combined with a dedicated nurse led service, can significantly reduce hospital admission rates.11
Unfortunately, heart failure is difficult to diagnose, as relevant symptoms are non-specific. Many patients with heart failure do not have their left ventricular function assessed,12,13 and undertreatment of heart failure is a major consequence. Patients with asymptomatic left ventricular dysfunction are even less likely to have been assessed. Many patients are therefore denied the benefit of strongly evidence based treatments. Systematically screening for heart failure and systolic dysfunction is one method that could improve case identification and thereby potentially improve treatment rates. Such a policy might tackle some of the deficiencies identified in the national service framework for coronary heart disease in England.14
A screening programme for a disease must fulfil certain well established criteria before being widely adopted.15,16 The condition should be the precursor of an important health problem; the clinical course of the condition should be understood, and there should be a recognisable latent or early symptomatic phase; an accepted treatment that reduces disability, death, or both should be available; a valid and acceptable test for the condition should be available; and screening should be cost effective.
Left ventricular systolic dysfunction, the most important cause of heart failure, would seem to meet most of these criteria, although no trial evidence for screening currently exists. The most common risk factors for systolic dysfunction and heart failure, in major trials in heart failure, are myocardial infarction, angina, hypertension, and diabetes mellitus.17 A screening programme is most cost effective if it is targeted at the patients at highest risk, so identification of how powerfully each of these risk factors predicts systolic dysfunction is important. We investigated this in a prospective substudy of the community based echocardiographic heart of England screening (ECHOES) study.
Methods
Full details of the separate, random population sample section of the echocardiographic heart of England screening study were recently published.18 Briefly, 16 general practices in the West Midlands region of England were randomly selected, after practices had been stratified geographically and socioeconomically. In this section of the study, we identified all patients with an electronic practice record of myocardial infarction, angina, hypertension, or diabetes. We excluded registered patients who had died or moved and those with severe psychiatric disorders, immobility, or terminal illness.
We sent invitations to 1617 patients selected randomly from the lists obtained. We based eligibility for inclusion on disease registers and did not validate diagnoses before the study. Patients with more than one risk factor were eligible for inclusion in more than one category but were included in the analysis in more than one category only if randomly selected from lists of patients with each risk factor. All patients who agreed to participate were assessed in their own general practice by clinical history (including prescribed drugs), determination of New York Heart Association functional class, clinical examination, resting 12 lead electrocardiography, and echocardiography including Doppler studies.18
We defined heart failure according to European Society of Cardiology criteria—namely, appropriate symptoms (New York Heart Association functional class II or worse) plus objective evidence of cardiac dysfunction.19 We classed left ventricular systolic function as “definitely impaired” (ejection fraction <40%), “borderline” (40-50%), or “normal” (>50%). We considered valve disease to be clinically significant in this analysis only if aortic stenosis with a gradient >20 mm Hg, mitral stenosis with a valve area <1.5 cm2, or regurgitant lesions of at least moderate severity were present. Owing to a lack of agreed diagnostic criteria, we did not attempt to diagnose diastolic dysfunction.
We stratified patients by age and sex and calculated rates for all observations. We used SPSS 9.0 for Windows and Minitab to analyse the data. We calculated 95% confidence intervals for prevalence figures by using the exact binomial method. We did univariate analysis and logistic regression analysis to identify the predictive factors for systolic dysfunction. The study had full ethical approval, and all patients gave written informed consent.
Results
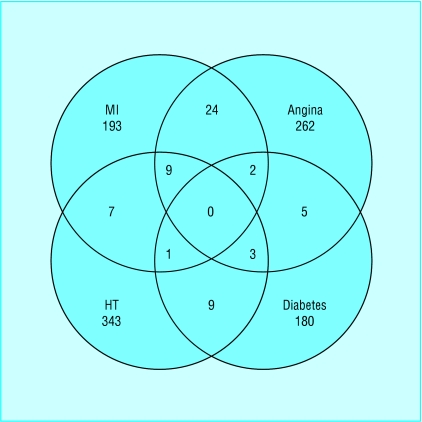
A total of 1062 patients (66%) attended for screening. Table 1 lists demographic and clinical data relating to the participants, and the figure shows details of the relations between the subgroups. Table 2 shows the results for left ventricular systolic dysfunction in patients with the defined risk factors, and table 3 gives the results for symptomatic heart failure, subdivided into differing aetiology.
Table 1.
Demographic details and clinical data of patients with medical records of risk factors (obtained during clinical history taking, examination, and assessment). Values are numbers (percentages) unless stated otherwise
| Characteristic
|
Previous myocardial infarction (n=244)
|
Angina (n=321)
|
Hypertension (n=388)
|
Diabetes (n=208)
|
|---|---|---|---|---|
| Mean (SD) age (years) | 67.3 (9.5) | 68.0 (9.7) | 65.7 (9.5) | 66.5 (9.9) |
| Sex (male:female) | 153:91 | 173:148 | 197:191 | 111:97 |
| Ever smoked | 181 (74.2) | 209 (65.1) | 236 (60.8) | 122 (58.7) |
| Current smoker | 36 (14.8) | 35 (10.9) | 52 (13.4) | 24 (11.5) |
| Non-white | 4 (1.6) | 7 (2.2) | 21 (5.4) | 23 (11.1) |
| Patient reported history of myocardial infarction | 223 (91.4) | 112 (34.9) | 42 (10.8) | 35 (16.8) |
| Patient reported history of angina | 128 (52.5) | 258 (80.4) | 62 (16.0) | 49 (23.6) |
| Patient reported history of hypertension | 96 (39.3) | 147 (45.8) | 365 (94.1) | 100 (48.1) |
| Patient reported history of diabetes | 31 (12.7) | 29 (9.0) | 43 (11.1) | 204 (98.1) |
| Family history of myocardial infarction (age <65) | 74 (30.3) | 99 (30.8) | 103 (26.5) | 47 (22.6) |
| Mean (SD) height (m) | 1.67 (0.10) | 1.66 (0.09) | 1.67 (0.09) | 1.66 (0.10) |
| Mean (SD) weight (kg) | 76.4 (13.3) | 75.2 (13.9) | 76.6 (14.6) | 77.3 (14.9) |
| Mean (SD) heart rate | 68 (15) | 68 (15) | 71 (15) | 77 (13) |
| Mean (SD) forced expiratory volume in 1 second (l) | 2.09 (0.76) | 2.03 (0.69) | 2.11 (0.70) | 2.10 (0.74) |
| Mean (SD) forced vital capacity (l) | 2.53 (0.89) | 2.43 (0.78) | 2.49 (0.78) | 2.46 (0.87) |
| Drugs prescribed: | ||||
| Angiotensin converting enzyme inhibitors | 61 (25.0) | 47 (14.6) | 93 (24.0) | 57 (27.4) |
| Diuretics | 89 (36.5) | 88 (27.4) | 148 (38.1) | 64 (30.8) |
| β blockers | 80 (32.8) | 103 (32.1) | 126 (32.5) | 20 (9.6) |
| Calcium antagonists | 70 (28.7) | 143 (44.5) | 130 (33.5) | 49 (23.6) |
| Aspirin | 170 (69.7) | 173 (53.9) | 75 (19.3) | 48 (23.1) |
| Digoxin | 18 (7.4) | 16 (5.0) | 9 (2.3) | 14 (6.7) |
| Mean (SD) systolic blood pressure (mm Hg) | 149 (24) | 153 (22) | 164 (21) | 156 (23) |
| Mean (SD) diastolic blood pressure (mm Hg) | 81 (10) | 83 (10) | 91 (12) | 84 (11) |
| New York Heart Association functional class: | ||||
| Class I | 129 (52.9) | 149 (46.4) | 278 (71.6) | 127 (61.1) |
| Class II | 79 (32.4) | 132 (41.1) | 92 (23.7) | 65 (31.3) |
| Class III | 25 (10.2) | 27 (8.4) | 10 (2.6) | 8 (3.8) |
| Class IV | 11 (4.5) | 13 (4.0) | 8 (2.1) | 8 (3.8) |
| Any electrocardiographic abnormality | 216 (88.5) | 220 (68.5) | 205 (52.8) | 126 (60.6) |
Table 2.
Prevalence of ejection fraction <40% and 40-50% by sex in the diagnostic groups. Values are numbers (percentages)
| Men
|
Women
|
Total
|
|
|---|---|---|---|
| Ejection fraction <40% | |||
| Myocardial infarction | 36/153 (23.5) | 18/91 (19.8) | 54/244 (22.1) |
| Angina | 18/173 (10.4) | 8/148 (5.4) | 26/321 (8.1) |
| Hypertension | 3/197 (1.5) | 4/191 (2.1) | 7/388 (1.8) |
| Diabetes | 9/111 (8.1) | 3/97 (3.1) | 12/208 (5.8) |
| Ejection fraction 40-50% | |||
| Myocardial infarction | 33/153 (21.6) | 15/91 (16.5) | 48/244 (19.7) |
| Angina | 28/173 (16.2) | 11/148 (7.4) | 39/321 (12.1) |
| Hypertension | 23/197 (11.7) | 8/191 (4.2) | 31/388 (8.0) |
| Diabetes | 9/111 (8.1) | 10/97 (10.3) | 19/208 (9.1) |
Table 3.
Heart failure on defined criteria (exertional dyspnoea with objective evidence of cardiac dysfunction) in diagnostic groups. Values are numbers (percentages)
| EF <40% alone*
|
EF <40% + AF
|
EF <40% + valve disease†
|
EF <40% + AF + valve disease†
|
EF 40-50% + AF
|
EF 40-50% + valve disease†
|
EF 40-50% + AF + valve disease†
|
AF (EF >50%)
|
Valve disease† (EF >50%)
|
AF + valve disease† (EF >50%)
|
Total definite HF
|
EF 40-50% alone
|
Total HF (definite + probable)
|
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Previous myocardial infarction (n=244) | 28 (11.5) | 0 | 1 (0.4) | 2 (0.8) | 2 (0.8) | 1 (0.4) | 0 | 5 (2.0) | 0 | 0 | 39 (16) | 22 (9.0) | 67 (27.5) |
| Angina (n=321) | 13 (4.0) | 0 | 4 (1.2) | 1 (0.3) | 0 | 0 | 0 | 5 (1.6) | 3 (0.9) | 1 (0.3) | 27 (8.4) | 22 (6.9) | 51 (15.9) |
| Hypertension (n=388) | 3 (0.8) | 1 (0.3) | 0 | 0 | 1 (0.3) | 0 | 0 | 2 (0.5) | 4 (1.0) | 0 | 11 (2.8) | 12 (3.1) | 25 (6.4) |
| Diabetes (n=208) | 6 (2.9) | 2 (1.0) | 0 | 2 (1.0) | 0 | 0 | 0 | 3 (1.4) | 2 (1.0) | 1 (0.5) | 16 (7.7) | 9 (4.3) | 25 (12.0) |
AF=atrial fibrillation; EF=ejection fraction; HF=heart failure.
In addition, six patients with previous myocardial with EF <40% had no symptoms of dyspnoea at time of assessment but were taking diuretics or angiotensin converting enzyme inhibitors; this applied also to two of hypertension group, two of angina group, and none of diabetes group.
Of the four patients with previous myocardial infarction and valve disease, one had mitral stenosis (MS) and three had mitral regurgitation (MR). Of the four with hypertension and valve disease, three had MR and one had aortic stenosis (AS). Of the nine with angina and valve disease, seven had MR and two had AS. Of the five with diabetes and valve disease, three had MR and two had AS.
History of myocardial infarction
—We found definite systolic dysfunction in 54 (22.1%, 95% confidence interval 17.1% to 27.9%) of the 244 patients with a history of myocardial infarction (table 2). Of these patients, 31 (57.4%) had symptoms of dyspnoea, 17 were asymptomatic and on no treatment, and six were asymptomatic but taking anti-heart failure treatment. An additional 48 (19.7%) patients had borderline systolic function. In addition to the 31 patients with ejection fraction <40% and symptoms of dyspnoea, seven patients had atrial fibrillation alone, and one had pronounced heart valve disease as well as symptoms of dyspnoea (table 3). A total of 39 patients (16.0%, 11.6% to 21.2%) in this risk group therefore had symptoms of dyspnoea with objective evidence of some form of cardiac dysfunction (ejection fraction <40%, atrial fibrillation, valve disease) and thus had definite heart failure. If patients with ejection fraction 40-50% and symptoms of dyspnoea are included, along with those with ejection fraction <40% and taking drugs for heart failure, but with no current symptoms, the overall prevalence of heart failure (both definite and probable) was 27.5% (22.0% to 33.5%).
History of angina
—We found definite systolic dysfunction in 26 (8.1%, 5.4% to 11.6%) of the 321 patients with a history of angina (table 2), of whom 18 (69.2%) had symptoms of dyspnoea. A further 39 (12.1%) patients had borderline left ventricular dysfunction. In addition to the 18 patients with ejection fraction <40% and symptoms of dyspnoea, five patients had atrial fibrillation, three had pronounced heart valve disease, and one had both, as well as symptoms of dyspnoea (table 3). Therefore, a total of 27 patients (8.4%, 5.6% to 12.0%) with a record of symptoms of angina also had symptoms of dyspnoea and objective evidence of some form of cardiac dysfunction and thus had definite heart failure. Including patients with borderline systolic dysfunction and symptoms of dyspnoea, and those with definite systolic dysfunction rendered asymptomatic on treatment, the overall prevalence of heart failure (definite and probable) was 15.9% (12.1% to 20.4%).
History of hypertension
—As detailed in tables 2 and 3, we found definite systolic dysfunction in only seven (1.8%, 0.7% to 3.7%) of the 388 patients with a history of hypertension, and four (57.1%) of these had symptoms of dyspnoea. Another 31 (8.0%) patients had an ejection fraction of 40-50%. As well as the four patients with ejection fraction <40% and symptoms of dyspnoea, three patients had atrial fibrillation and four had pronounced heart valve disease, in association with symptoms of dyspnoea. A total of 11 patients (2.8%, 1.4% to 5.0%) with a record of hypertension therefore had definite heart failure. If the 12 patients with borderline systolic function and symptoms of dyspnoea and the two with definitely impaired systolic function rendered asymptomatic on treatment are included as having heart failure, the overall prevalence was 6.4% (4.2% to 9.4%).
History of diabetes
—We found an ejection fraction of <40% in 12 (5.8%, 3.0% to 9.9%) of the 208 patients identified as having a history of diabetes (table 2). Ten (83.3%) of these patients had symptoms of shortness of breath. A further 19 (9.1%) patients had an ejection fraction of 40-50%. In addition to the 10 patients with ejection fraction <40% and symptoms, three patients had atrial fibrillation, two had heart valve disease, and one had both, as well as symptoms of dyspnoea (table 3). Therefore, a total of 16 patients (7.7%, 4.5% to 12.2%) with diabetes had symptoms of dyspnoea with objective evidence of cardiac dysfunction and thus had definite heart failure. Including patients with borderline systolic function and symptoms, the overall prevalence of heart failure was 12.0% (7.9% to 17.2%).
Statistical analysis
—Univariate analysis showed that patients with definite systolic dysfunction differed significantly from the rest of the population studied with respect individually to age, sex, history of myocardial infarction, angina, or hypertension, smoking status, and current systolic blood pressure (table 4). Multiple logistic regression analysis identified only age, previous myocardial infarction, and current systolic blood pressure below 150 mm Hg as independent predictors (table 5).
Table 4.
Univariate analysis of possible predictors of ejection fraction <40%. Values are percentages unless stated otherwise
| Variable
|
LVEF <40%
|
LVEF ⩾40%
|
P value
|
|---|---|---|---|
| Mean (SD) age (years) | 69.6 (9.0) | 66.6 (9.7) | 0.0049 |
| Mean (SD) height (m) | 1.68 (0.09) | 1.66 (0.1) | 0.15 |
| Mean (SD) weight (kg) | 77.9 (14.4) | 76.0 (14.2) | 0.26 |
| Body mass index >25 | 70.1 | 70.2 | 0.99 |
| Sex (male) | 67.8 | 52.7 | 0.007 |
| Angina | 68.3 | 46.5 | <0.001 |
| Previous myocardial infarction | 85.9 | 30.9 | <0.001 |
| Hypertension | 45.7 | 67.5 | <0.001 |
| Diabetes | 22.4 | 26.7 | 0.34 |
| Caucasian | 95.4 | 95.2 | 0.92 |
| Ever smoked | 31.0 | 37.0 | 0.27 |
| Current smoker | 89.7 | 78.7 | 0.047 |
| Current systolic blood pressure >150 mm Hg | 40.2 | 60.4 | <0.001 |
| Current diastolic blood pressure >90 mm Hg | 20.7 | 27.3 | 0.18 |
| Family history myocardial infarction | 25.6 | 29.6 | 0.45 |
| Family history hypertension | 26.7 | 32.1 | 0.33 |
| Family history diabetes | 23.5 | 23.3 | 0.96 |
| Taking antihypertensive drugs | 82.8 | 79.2 | 0.43 |
LVEF=left ventricular ejection fraction.
Table 5.
Logistic regression analysis of predictors of ejection fraction <40%
| Variable
|
χ2
|
P value
|
Odds ratio (95% CI)
|
|---|---|---|---|
| Previous myocardial infarction | 60.3 | <0.001 | 12.21 (6.49 to 22.97) |
| Age | 7.2 | 0.007 | 1.04 (1.01 to 1.06) |
| Current systolic blood pressure >150 mm Hg | 5.3 | 0.02 | 0.56 (0.34 to 0.92) |
Discussion
Heart failure is common, and we have shown, in the general population arm of the echocardiographic heart of England screening study,18 that most patients with left ventricular systolic dysfunction and heart failure in the community have one or more of the risk factors investigated here. In the population aged 45 and older, we found systolic dysfunction in 1.8% of patients, and 81% of these had a history of one or more of the selected risk factors. We found definite heart failure in 2.3% of patients, and 71% of these patients had one or more of the risk factors. Screening only patients with these risk factors would therefore identify most people with systolic dysfunction and symptomatic heart failure.
The most powerful predictor of systolic dysfunction was previous myocardial infarction. A high prevalence of systolic dysfunction also occurred in the groups with angina and diabetes, but regression analysis did not show these to be independent risk factors. Confining screening to patients with a history of ischaemic heart disease would give a high yield of candidates for evidence based treatments, which alter prognosis as well as symptoms.
In the Framingham heart study hypertension, alone or associated with ischaemic or rheumatic heart disease, was the most common condition predisposing to heart failure, diagnosed with a clinical and radiological scoring system.2 In contrast, our data and those from Glasgow suggest that uncomplicated hypertension is less important in predicting left ventricular dysfunction.20 Even including all causes of heart failure, using objective criteria for diagnosis, the prevalence was only slightly higher in patients with hypertension than in the general population (2.8% v 2.3%). The reasons for the differences are not clear. Patients with hypertension in our study had all been identified and treated, which may have reduced development of heart failure. Indeed, recent Framingham data show greatly improved treatment of hypertension.21 Alternatively, some people diagnosed with heart failure by the Framingham scoring system may not have met the diagnostic criteria used here; hypertension is a common cause of radiological cardiomegaly, a major Framingham criterion. Our finding on regression analysis that systolic blood pressure below 150 mm Hg is a predictor of systolic dysfunction is counterintuitive and is likely to reflect the use of angiotensin converting enzyme inhibitors and other antihypertensive drugs in patients with previously identified systolic dysfunction.
Diastolic dysfunction may be responsible for some cases of heart failure, but we did not assess this in our study because of controversy over diagnostic criteria.22 We report only on heart failure due to systolic dysfunction, atrial fibrillation, and valve disease, as the diagnosis of these is well defined and established treatments are available. We report on ejection fractions of <40% and 40-50% separately, as patients with an ejection fraction <40% were all qualitatively impaired and the figure of <40% has been used as an entry criterion for trials of treatment in systolic dysfunction,9,23 whereas uncertainty exists about the 40-50% group, hence the classification as “borderline.”
We obtained the data in table 1 clinically and through investigation, whereas details of which patients had the risk factors were obtained from computerised records. Apparent differences (for example, only 91% of patients with a computerised record of history of myocardial infarction gave this diagnosis on questioning) may be due to patients' uncertainty about the diagnosis, incorrect practice record data, or both. A 98% agreement in the case of diabetes suggests that practice records are on the whole accurate. All but 28 of the patients with a computerised record of myocardial infarction had electrocardiographic abnormalities. In any event, our method represents “real life,” replicating a standard method of selecting patients for screening in routine practice.
We believe that these are the first data on the potential diagnostic yield from a targeted, systematic screening programme for heart failure and systolic dysfunction. Such practice based electronic record searching for the at risk population and subsequent echocardiography are feasible. This method could be used in any healthcare system in which population diagnostic registers are maintained and echocardiography facilities are available. Formal trials are now justified to explore the cost effectiveness of such a strategy, including estimates of the screening interval needed to detect incident cases.
The national service framework for coronary heart disease states that “primary care teams and hospitals should put in place models of care so that they use a systematic approach to identify people at high risk of heart failure (e.g. people who have had an acute myocardial infarction).”14 Our data support this approach.
Figure.
Venn diagram showing relations between subgroups with different risk factors (HT=hypertension; MI=myocardial infarction)
Acknowledgments
S McLeod assisted in practice recruitment and coordination, and T Marshall assisted with the initial sampling frame of practices and patients. D Wosornu contributed to study design and carried out some clinical assessments and reports. The following practices kindly agreed to take part in the study: Albrighton Surgery, Albrighton; Baker St Surgery, Stoke on Trent; Castle Practice, Castle Bromwich; Church St Surgery, Kidderminster; Droitwich Health Centre, Droitwich; Elgar House Surgery, Redditch; Great Barr Group Practice, Birmingham; Hollies Medical Practice, Tamworth; Holmcroft Surgery, Stafford; Laurie Pike Health Centre, Handsworth, Birmingham; Lee Bank Health Centre, Birmingham; Ley Hill Surgery, Sutton Coldfield; Moss Grove Surgery, Kingswinford; Red Roofs Surgery, Nuneaton; Riverbrook Medical Centre, Stirchley, Birmingham; and Shirley Medical Centre, Shirley, Solihull.
Footnotes
Funding: NHS research and development cardiovascular disease and stroke programme.
Competing interests: FDRH is a member of the European Society of Cardiology working party on heart failure, chair of the British Primary Care Cardiovascular Society, and Treasurer of the British Society for Heart Failure. MKD is chairman of the British Society for Heart Failure. FDRH and MKD have received travel sponsorship and honorariums from several biotechnology and pharmaceutical companies with cardiovascular products for plenary talks and attendance at major cardiology scientific congresses and conferences.
References
- 1.The CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. N Engl J Med. 1987;316:1429–1435. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- 2.McKee PA, Castelli WP, McNamara P, Kannell WB. The natural history of heart failure: the Framingham study. N Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 3.Davis RC, Hobbs FDR, Kenkre JE, Roalfe AK, McLeod S, Hare R, et al. Quality of life in heart failure, as measured by the SF-36 health status questionnaire. Eur Heart J. 1998;19(abstr suppl):639. [Google Scholar]
- 4.Sutton GC. Epidemiological aspects of heart failure. Am Heart J. 1990;120:1538–1540. doi: 10.1016/0002-8703(90)90055-3. [DOI] [PubMed] [Google Scholar]
- 5.Garg R, Yusuf S.for the Collaborative Group on ACE Inhibitor Trials. Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure JAMA 19952731450–1456. [PubMed] [Google Scholar]
- 6.The SOLVD Investigators. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med. 1992;327:685–691. doi: 10.1056/NEJM199209033271003. [DOI] [PubMed] [Google Scholar]
- 7.Pfeffer MA, Braunwald E, Moy LA, Basta L, Brown EJ, Cuddy TE.for the SAVE Investigators. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction: results of the survival and ventricular enlargement trial N Engl J Med 1992327669–677. [DOI] [PubMed] [Google Scholar]
- 8.CIBIS-II Investigators and Committees. The cardiac insufficiency bisoprolol study II. Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- 9.MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: metoprolol CR/XL randomised intervention trial in congestive heart failure (MERIT-HF) Lancet. 1999;353:2001–2007. [PubMed] [Google Scholar]
- 10.Pitt B, Zannad F, Remm WJ, Cody R, Castaigne A, Perez A, et al. for the Randomised Aldactone Evaluation Study Investigators. The effect of spironolactone on morbidity and mortality in patients with severe heart failure N Engl J Med 1999341709–717. [DOI] [PubMed] [Google Scholar]
- 11.Blue L, Lang E, McMurray JJV, Davie AP, McDonagh TA, Murdoch DR, et al. Randomised controlled trial of specialist nurse intervention in heart failure. BMJ. 2001;323:715–718. doi: 10.1136/bmj.323.7315.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clarke KW, Gray D, Hampton JR. Evidence of inadequate investigation and treatment of patients with heart failure. Br Heart J. 1994;71:584–587. doi: 10.1136/hrt.71.6.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hobbs FDR, Jones MI, Allan TS, Wilson S, Jones MI, Tobias R. European survey of primary care physician perceptions and practice on heart failure diagnosis and management (Euro-HF) Eur Heart J. 2000;21:1877–1887. doi: 10.1053/euhj.2000.2170. [DOI] [PubMed] [Google Scholar]
- 14.Department of Health. National service framework for coronary heart disease. London: Stationery Office; 2000. [Google Scholar]
- 15.Wilson JMG, Junger G. Principles and practice of screening for disease. World Health Organisation Public Health Paper. 1968;34:14–38. [Google Scholar]
- 16.McMurray JV, McDonagh TA, Davie AP, Cleland JGF, Francis CM, Morrison C. Should we screen for asymptomatic left ventricular dysfunction to prevent heart failure? Eur Heart J. 1998;19:842–846. doi: 10.1093/eurheartj/19.6.842. [DOI] [PubMed] [Google Scholar]
- 17.The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 18.Davies MK, Hobbs FDR, Davis RC, Kenkre JE, Roalfe AK, Hare R, et al. Prevalence of left-ventricular systolic dysfunction and heart failure in the echocardiographic heart of England screening study: a population based study. Lancet. 2001;358:439–444. doi: 10.1016/s0140-6736(01)05620-3. [DOI] [PubMed] [Google Scholar]
- 19.Task Force on Heart Failure of the European Society of Cardiology. Guidelines for the diagnosis of heart failure. Eur Heart J. 1995;16:741–751. [PubMed] [Google Scholar]
- 20.McDonagh TA, Morrison CE, Lawrence A, Ford I, Tunstall-Pedoe H, McMurray JJV, et al. Symptomatic and asymptomatic left ventricular systolic dysfunction in an urban population. Lancet. 1997;350:829–833. doi: 10.1016/S0140-6736(97)03033-X. [DOI] [PubMed] [Google Scholar]
- 21.Mosterd A, D'Agostino RB, Silbershatz H, Sytkowski PA, Kannel WB, Grobee DE, et al. Trends in the prevalence of hypertension, antihypertensive therapy and left ventricular hypertrophy. N Engl J Med. 1999;340:1221–1227. doi: 10.1056/NEJM199904223401601. [DOI] [PubMed] [Google Scholar]
- 22.Davie AP, Francis CM, Caruana L, Sutherland GR, McMurray JJV. The prevalence of left ventricular diastolic filling abnormalities in patients with suspected heart failure. Eur Heart J. 1997;18:981–984. doi: 10.1093/oxfordjournals.eurheartj.a015387. [DOI] [PubMed] [Google Scholar]
- 23.Pitt B, Segal R, Martinez FA, Meurers G, Cowley AJ, Thomas I, et al. on behalf of the ELITE Study Investigators. Randomised trial of losartan versus captopril in patients over 65 with heart failure (evaluation of losartan in the elderly study, ELITE) Lancet 1997349747–752. [DOI] [PubMed] [Google Scholar]