Abstract
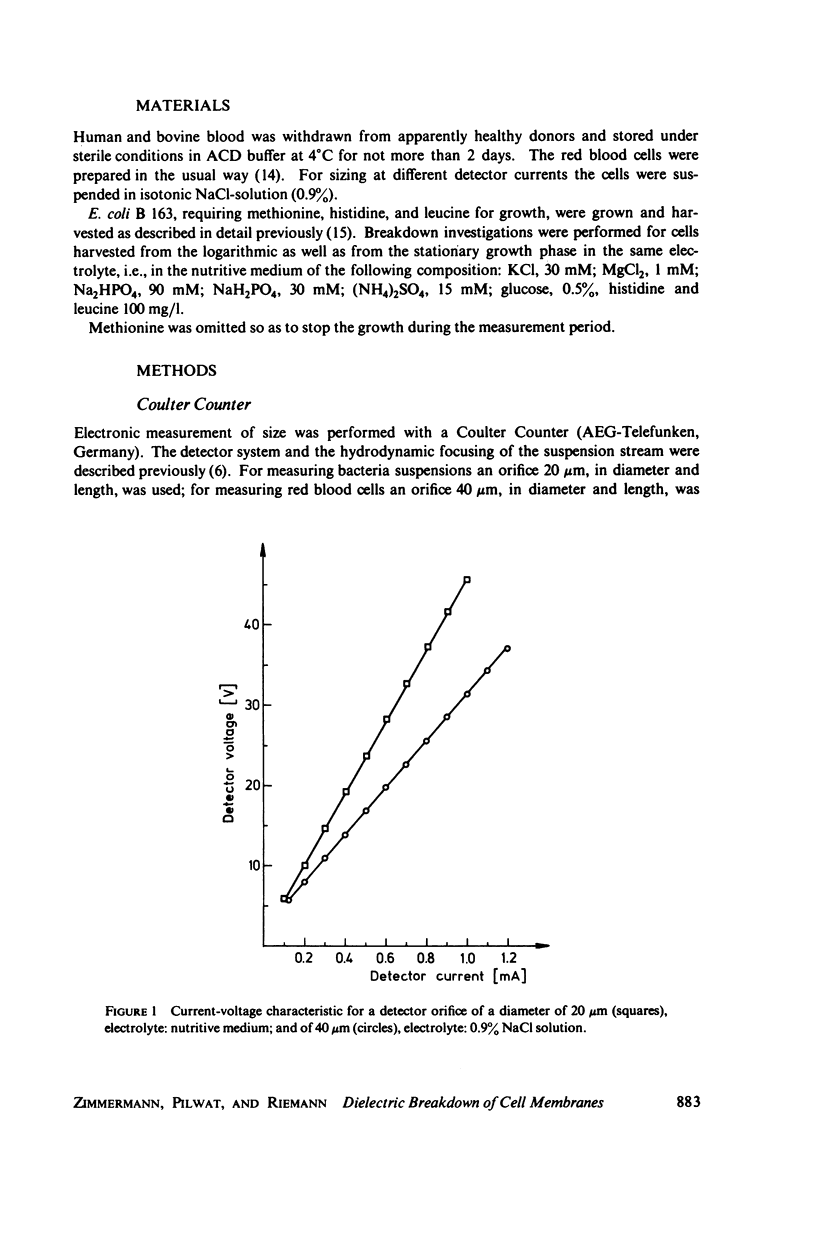
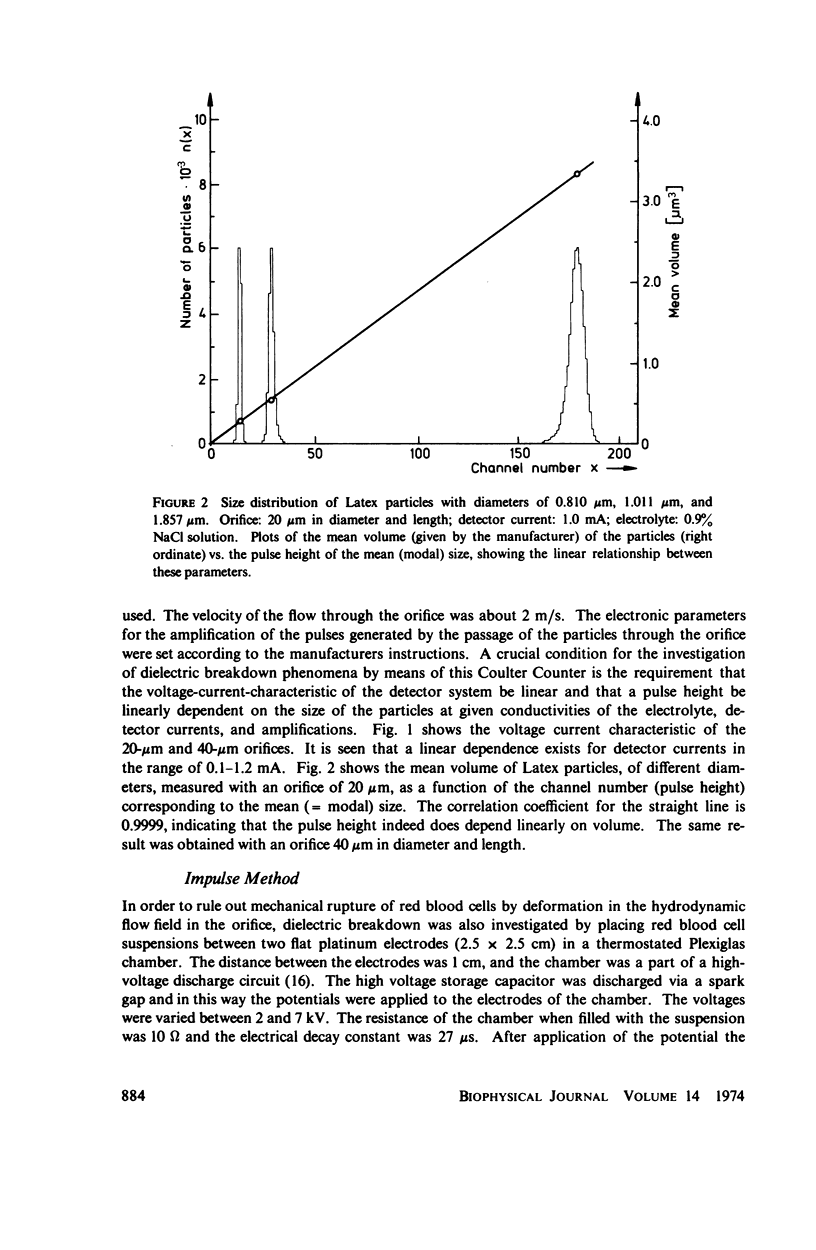
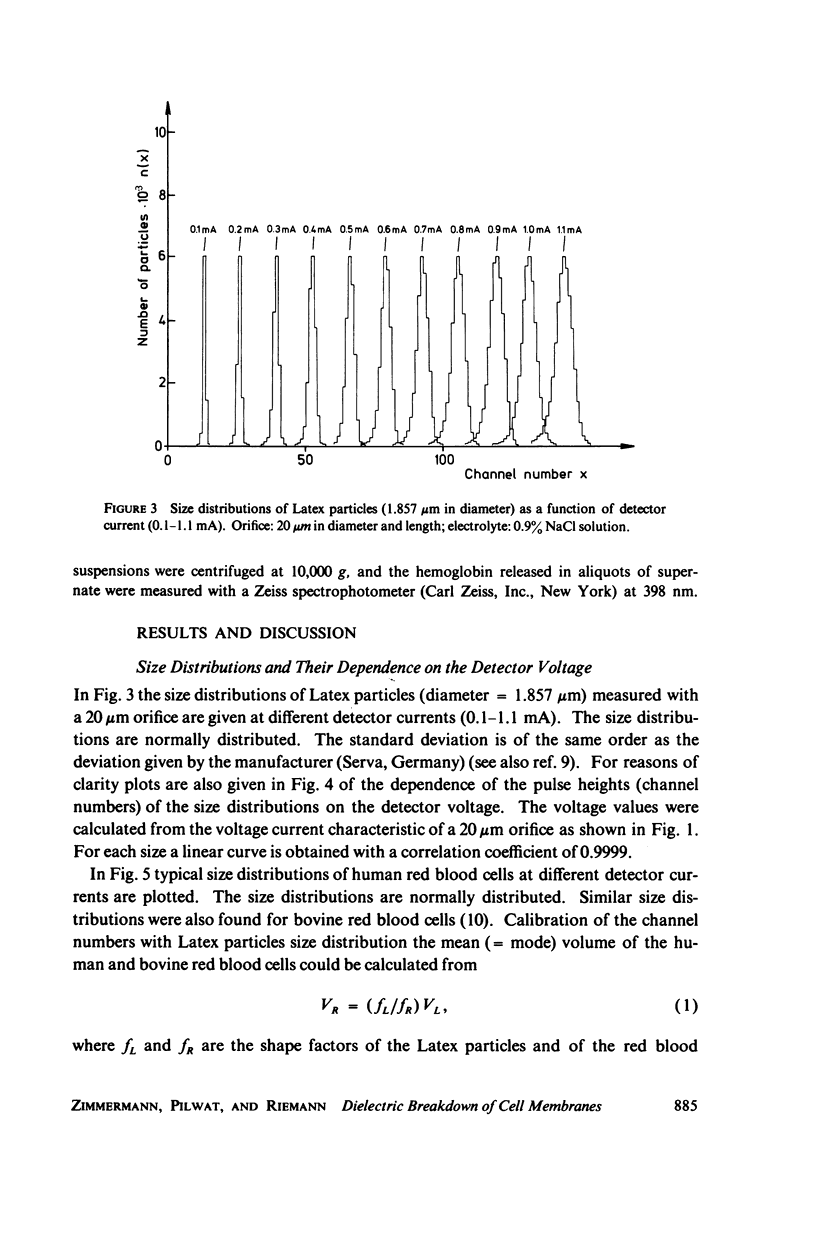
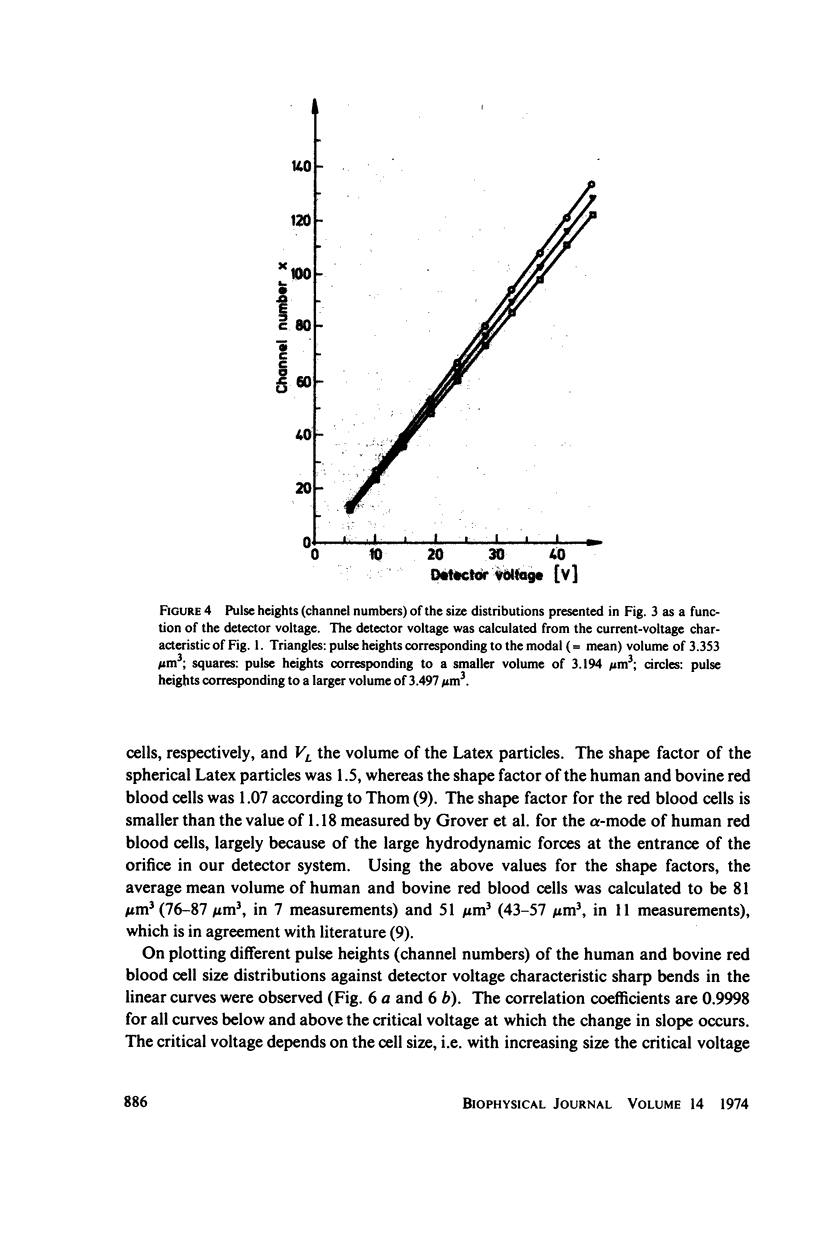
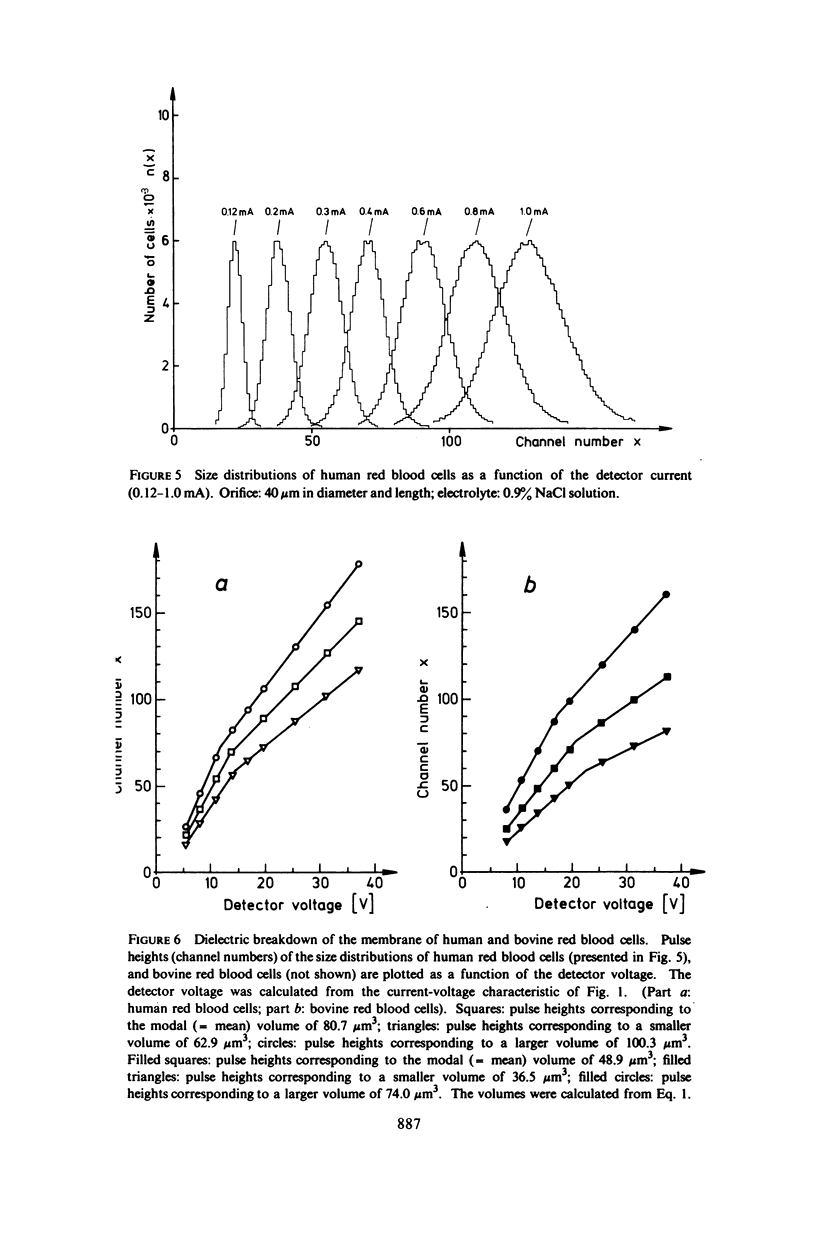
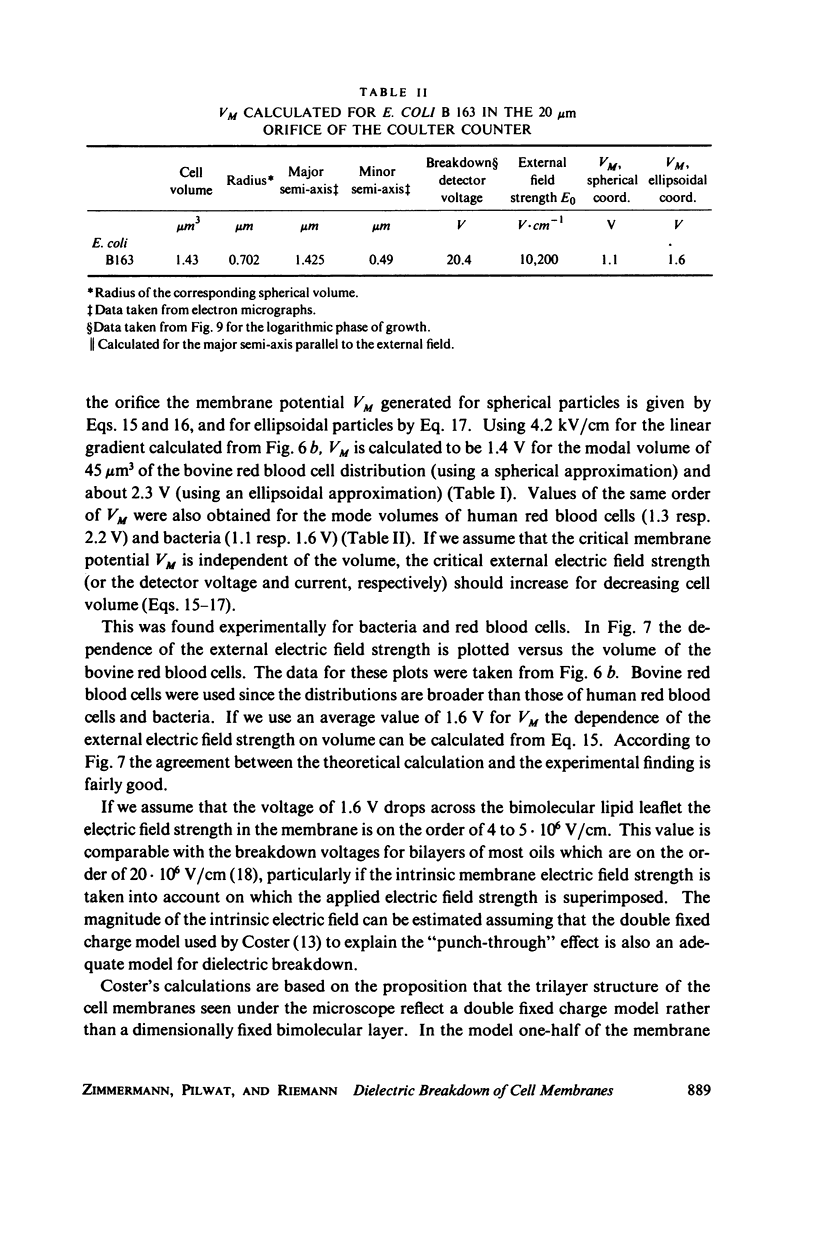
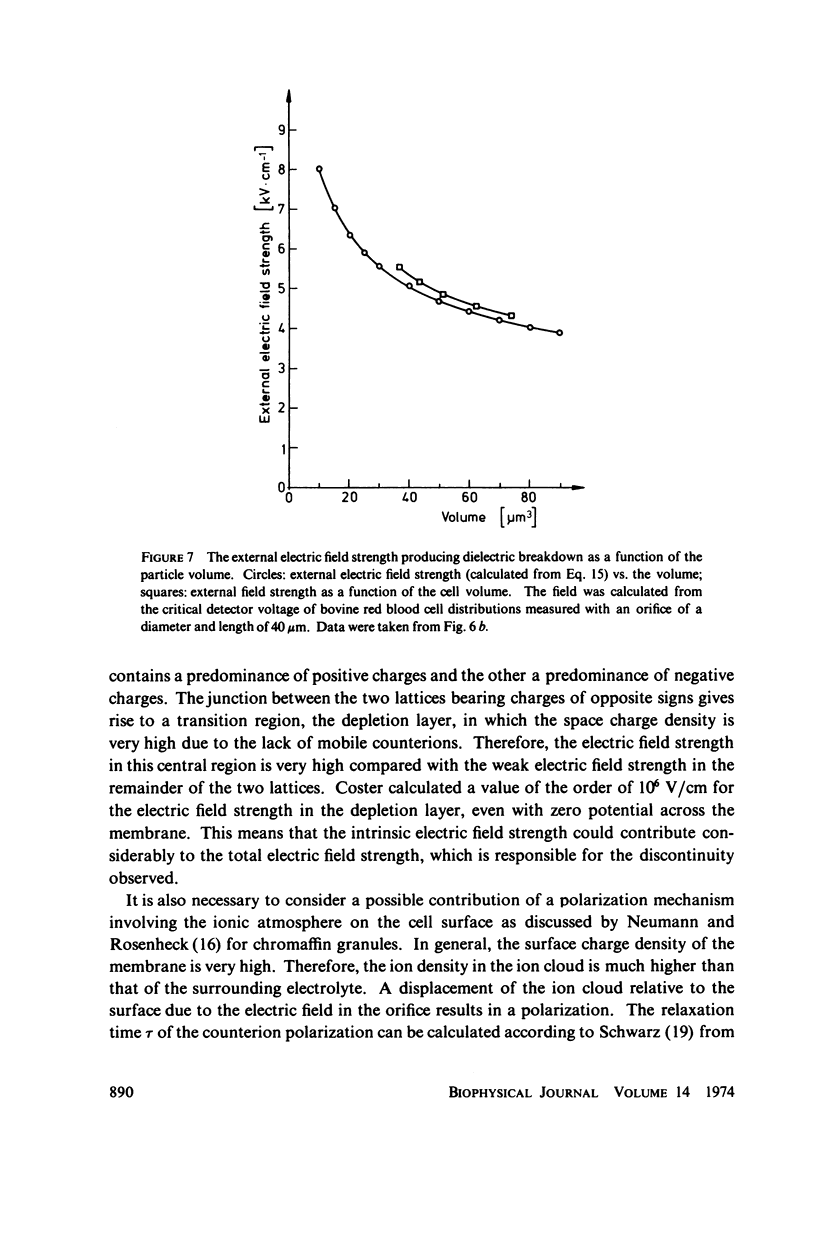
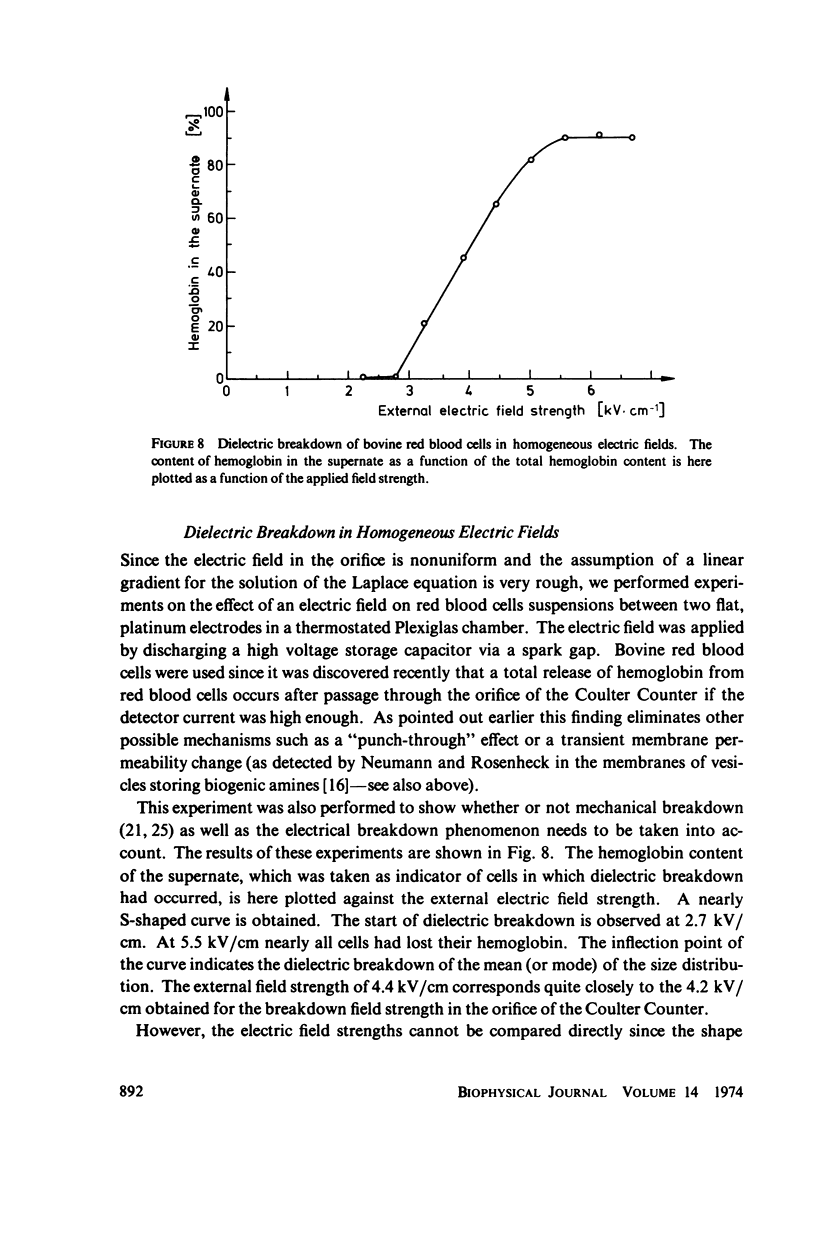
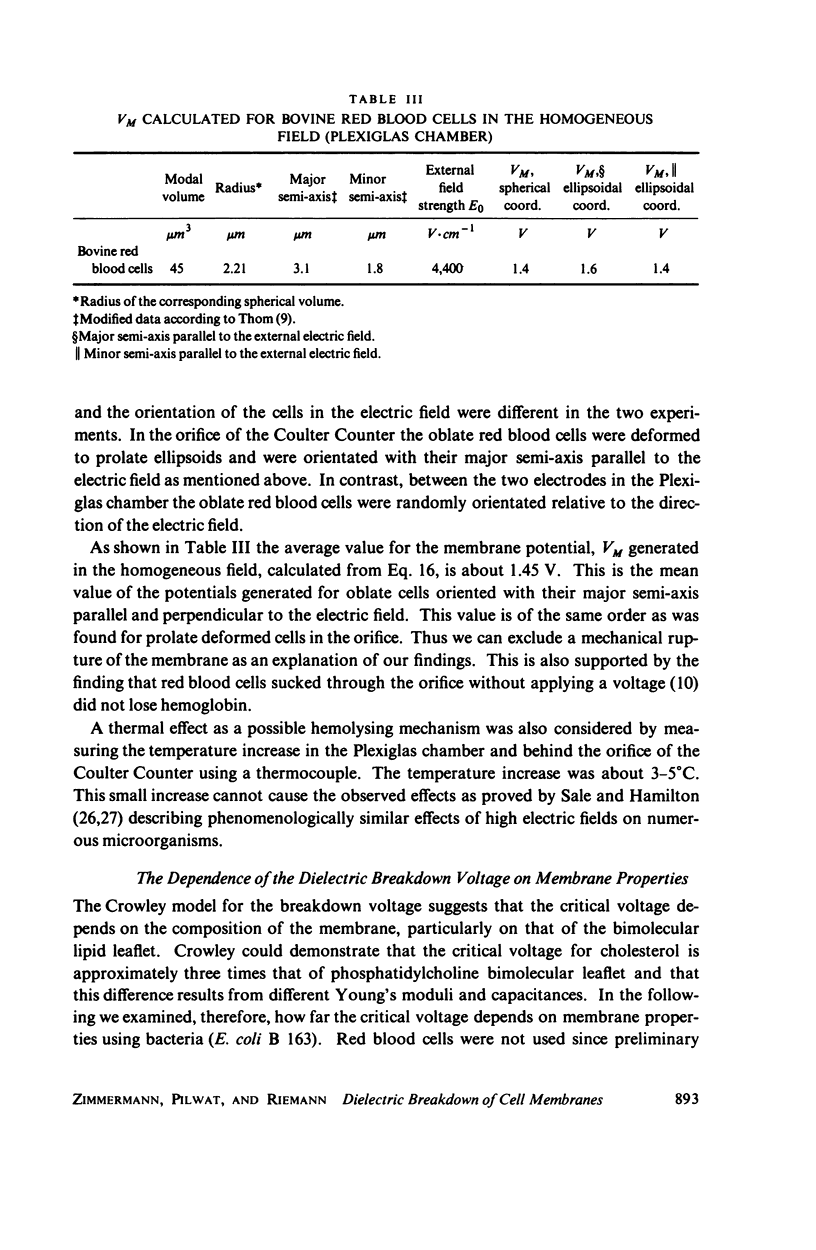
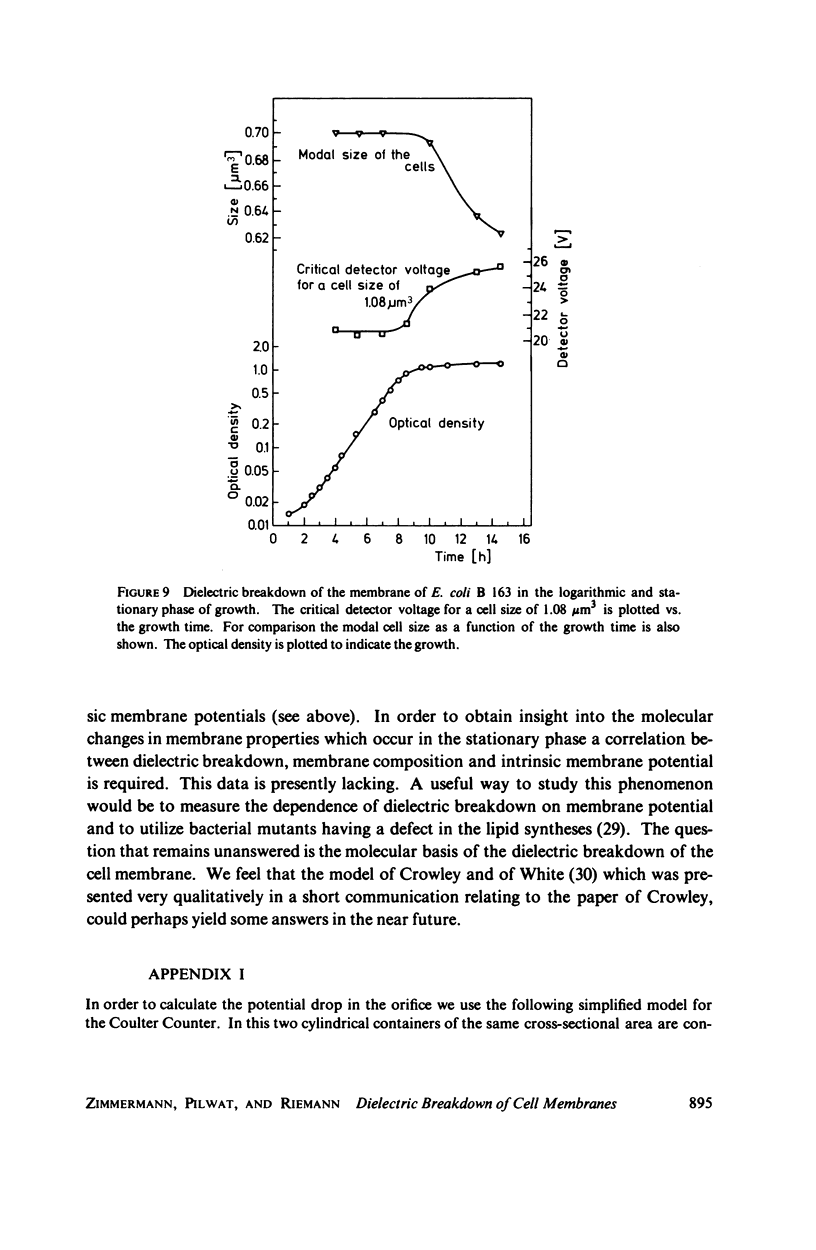
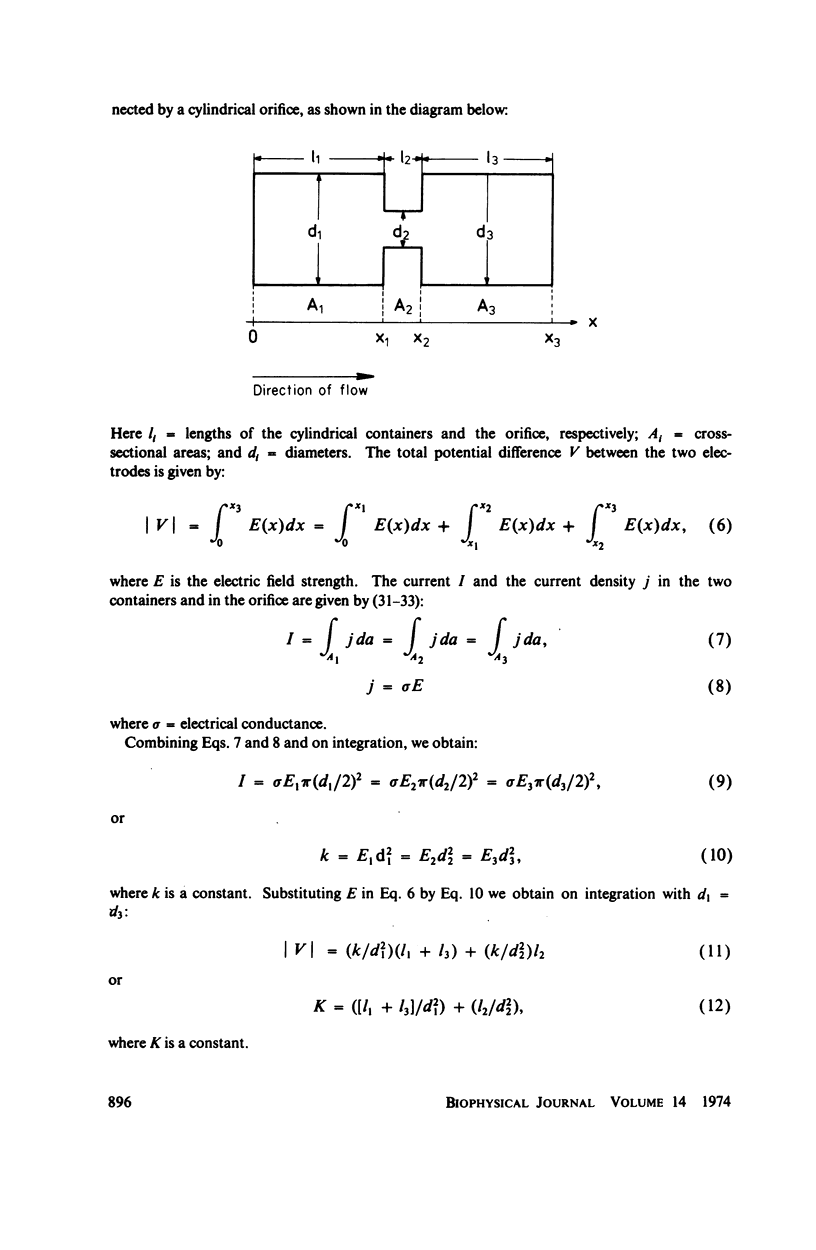
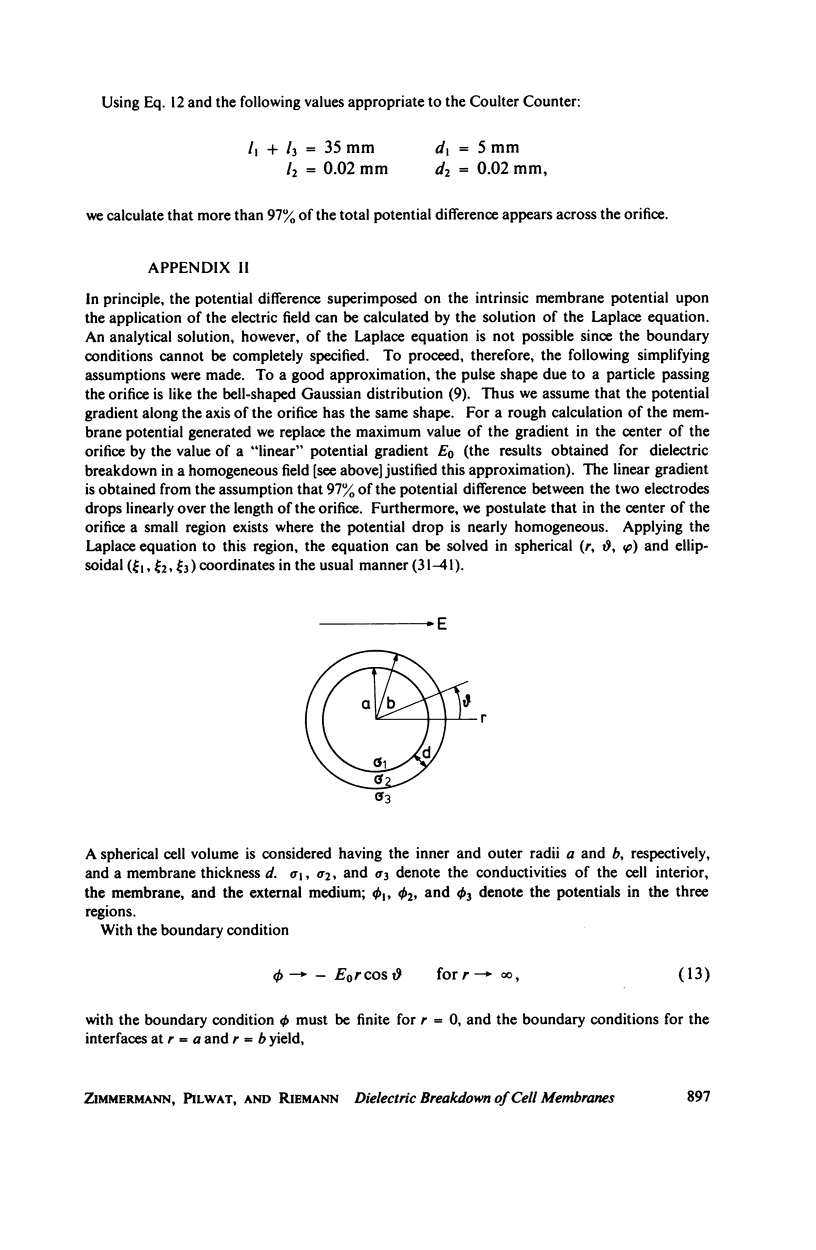
With human and bovine red blood cells and Escherichia coli B, dielectric breakdown of cell membranes could be demonstrated using a Coulter Counter (AEG-Telefunken, Ulm, West Germany) with a hydrodynamic focusing orifice. In making measurements of the size distributions of red blood cells and bacteria versus increasing electric field strength and plotting the pulse heights versus the electric field strength, a sharp bend in the otherwise linear curve is observed due to the dielectric breakdown of the membranes. Solution of Laplace's equation for the electric field generated yields a value of about 1.6 V for the membrane potential at which dielectric breakdown occurs with modal volumes of red blood cells and bacteria. The same value is also calculated for red blood cells by applying the capacitor spring model of Crowley (1973. Biophys. J. 13:711). The corresponding electric field strength generated in the membrane at breakdown is of the order of 4 · 106 V/cm and, therefore, comparable with the breakdown voltages for bilayers of most oils. The critical detector voltage for breakdown depends on the volume of the cells. The volume-dependence predicted by Laplace theory with the assumption that the potential generated across the membrane is independent of volume, could be verified experimentally. Due to dielectric breakdown the red blood cells lose hemoglobin completely. This phenomenon was used to study dielectric breakdown of red blood cells in a homogeneous electric field between two flat platinum electrodes. The electric field was applied by discharging a high voltage storage capacitor via a spark gap. The calculated value of the membrane potential generated to produce dielectric breakdown in the homogeneous field is of the same order as found by means of the Coulter Counter. This indicates that mechanical rupture of the red blood cells by the hydrodynamic forces in the orifice of the Coulter Counter could also be excluded as a hemolysing mechanism. The detector voltage (or the electric field strength in the orifice) depends on the membrane composition (or the intrinsic membrane potential) as revealed by measuring the critical voltage in E. coli B harvested from the logarithmic and stationary growth phases. The critical detector voltage increased by about 30% for a given volume on reaching the stationary growth phase.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernhardt J., Pauly H. On the generation of potential differences across the membranes of ellipsoidal cells in an alternating electrical field. Biophysik. 1973;10(3):89–98. doi: 10.1007/BF01189915. [DOI] [PubMed] [Google Scholar]
- Bodemann H., Passow H. Factors controlling the resealing of the membrane of human erythrocyte ghosts after hypotonic hemolysis. J Membr Biol. 1972;8(1):1–26. doi: 10.1007/BF01868092. [DOI] [PubMed] [Google Scholar]
- Coster H. G. A quantitative analysis of the voltage-current relationships of fixed charge membranes and the associated property of "punch-through". Biophys J. 1965 Sep;5(5):669–686. doi: 10.1016/S0006-3495(65)86745-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coster H. G., George E. P., Simons R. The electrical characteristics of fixed charge membranes: solution of the field equations. Biophys J. 1969 May;9(5):666–684. doi: 10.1016/S0006-3495(69)86411-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coster H. G. Letter: The double fixed charge membrane model: an hypothesis concerning the structure and morphogenesis of cell membranes. Biophys J. 1973 Oct;13(10):1119–1123. doi: 10.1016/S0006-3495(73)86049-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley J. M. Electrical breakdown of bimolecular lipid membranes as an electromechanical instability. Biophys J. 1973 Jul;13(7):711–724. doi: 10.1016/S0006-3495(73)86017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover N. B., Naaman J., Ben-Sasson S., Doljanski F. Electrical sizing of particles in suspensions. 3. Rigid spheroids and red blood cells. Biophys J. 1972 Sep;12(9):1099–1117. doi: 10.1016/s0006-3495(72)86147-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover N. B., Naaman J., Ben-Sasson S., Doljanski F. Electrical sizing of particles in suspensions. I. Theory. Biophys J. 1969 Nov;9(11):1398–1414. doi: 10.1016/S0006-3495(69)86461-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover N. B., Naaman J., Ben-Sasson S., Doljanski F., Nadav E. Electrical sizing of particles in suspensions. II. Experiments with rigid spheres. Biophys J. 1969 Nov;9(11):1415–1425. doi: 10.1016/S0006-3495(69)86462-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochmuth R. M., Mohandas N., Blackshear P. L., Jr Measurement of the elastic modulus for red cell membrane using a fluid mechanical technique. Biophys J. 1973 Aug;13(8):747–762. doi: 10.1016/S0006-3495(73)86021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON S. L., WOODBURY J. W. MEMBRANE RESISTANCE OF HUMAN RED CELLS. J Gen Physiol. 1964 May;47:827–837. doi: 10.1085/jgp.47.5.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann E., Rosenheck K. Permeability changes induced by electric impulses in vesicular membranes. J Membr Biol. 1972 Dec 29;10(3):279–290. doi: 10.1007/BF01867861. [DOI] [PubMed] [Google Scholar]
- RAND R. P., BURTON A. C. MECHANICAL PROPERTIES OF THE RED CELL MEMBRANE. I. MEMBRANE STIFFNESS AND INTRACELLULAR PRESSURE. Biophys J. 1964 Mar;4:115–135. doi: 10.1016/s0006-3495(64)86773-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAND R. P. MECHANICAL PROPERTIES OF THE RED CELL MEMBRANE. II. VISCOELASTIC BREAKDOWN OF THE MEMBRANE. Biophys J. 1964 Jul;4:303–316. doi: 10.1016/s0006-3495(64)86784-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull R. J. Letter: An alternate explanation for the permeability changes induced by electrical impulses in vesicular membranes. J Membr Biol. 1973 Dec 31;14(2):193–196. doi: 10.1007/BF01868077. [DOI] [PubMed] [Google Scholar]
- Ubitschek H. E. Linear cell growth in Escherichia coli. Biophys J. 1968 Jul;8(7):792–804. doi: 10.1016/s0006-3495(68)86521-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White S. H., Thompson T. E. Capacitance, area, and thickness variations in thin lipid films. Biochim Biophys Acta. 1973 Sep 27;323(1):7–22. doi: 10.1016/0005-2736(73)90428-8. [DOI] [PubMed] [Google Scholar]
- Zimmermann U., Pilwat G., Günther T. Regulation of the intracellular potassium concentration in Escherichia coli B 525. Biochim Biophys Acta. 1973 Jul 6;311(3):442–451. doi: 10.1016/0005-2736(73)90324-6. [DOI] [PubMed] [Google Scholar]
- Zimmermann U., Pilwat G., Riemann F. Reversibler dielektrischer Durchbruch von Zellmembranen in elektrostatischen Feldern. Z Naturforsch C. 1974 May-Jun;29(5):304–305. [PubMed] [Google Scholar]
- Zimmermann U., Schulz J., Pilwat G. Transcellular ion flow in Escherichia coli B and electrical sizing of bacterias. Biophys J. 1973 Oct;13(10):1005–1013. doi: 10.1016/S0006-3495(73)86041-2. [DOI] [PMC free article] [PubMed] [Google Scholar]



