Abstract
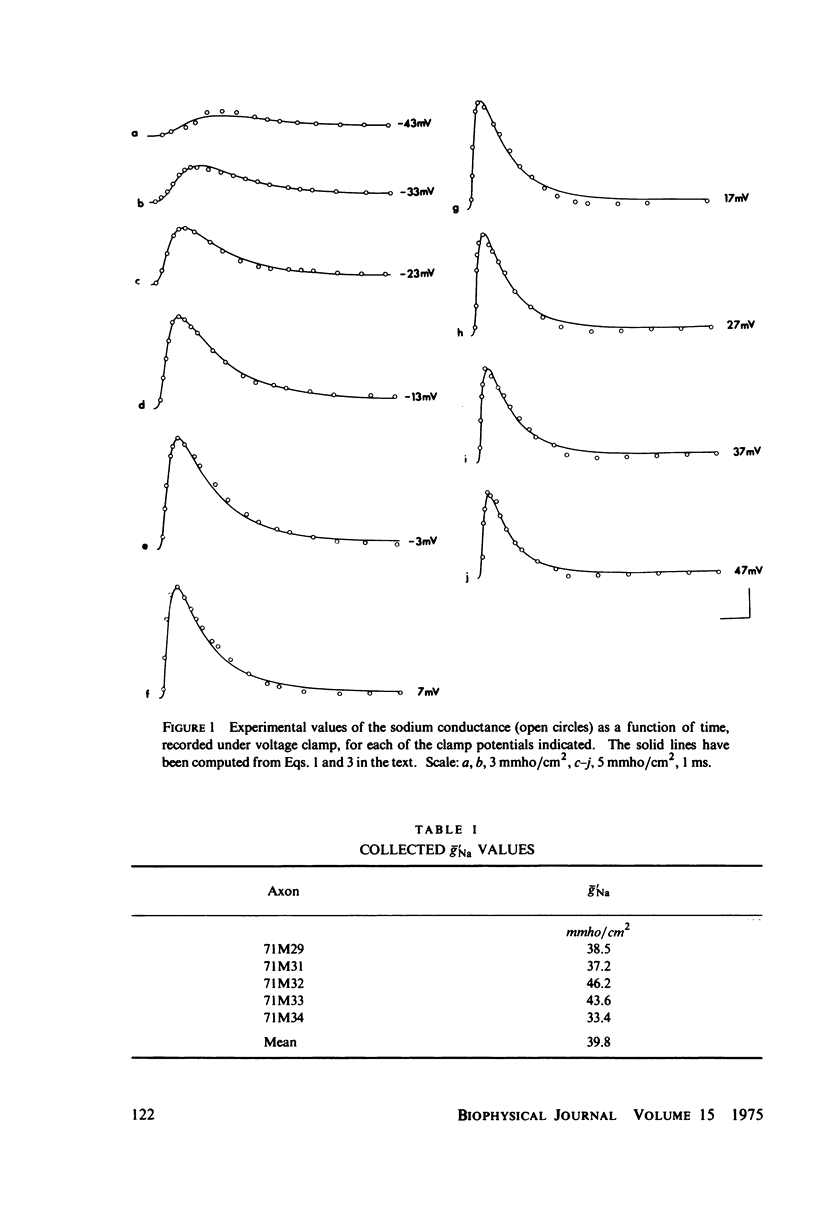
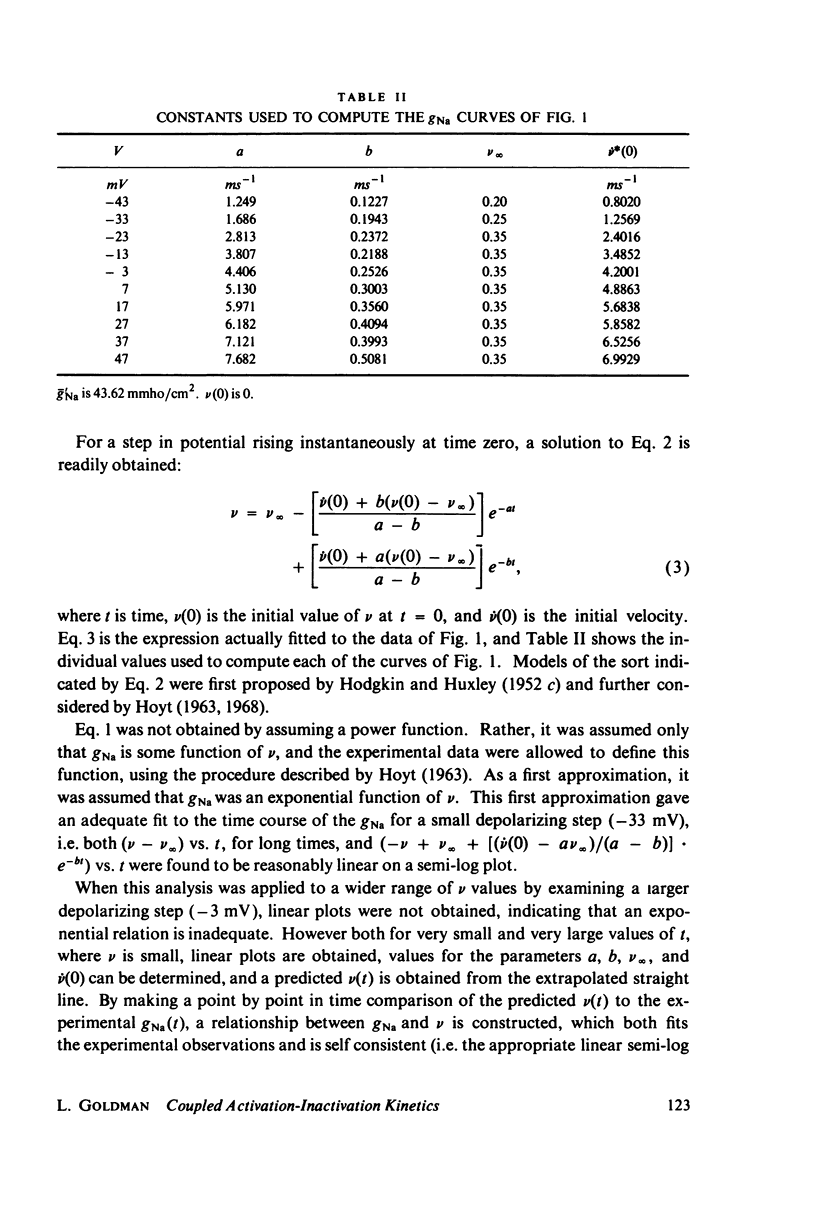
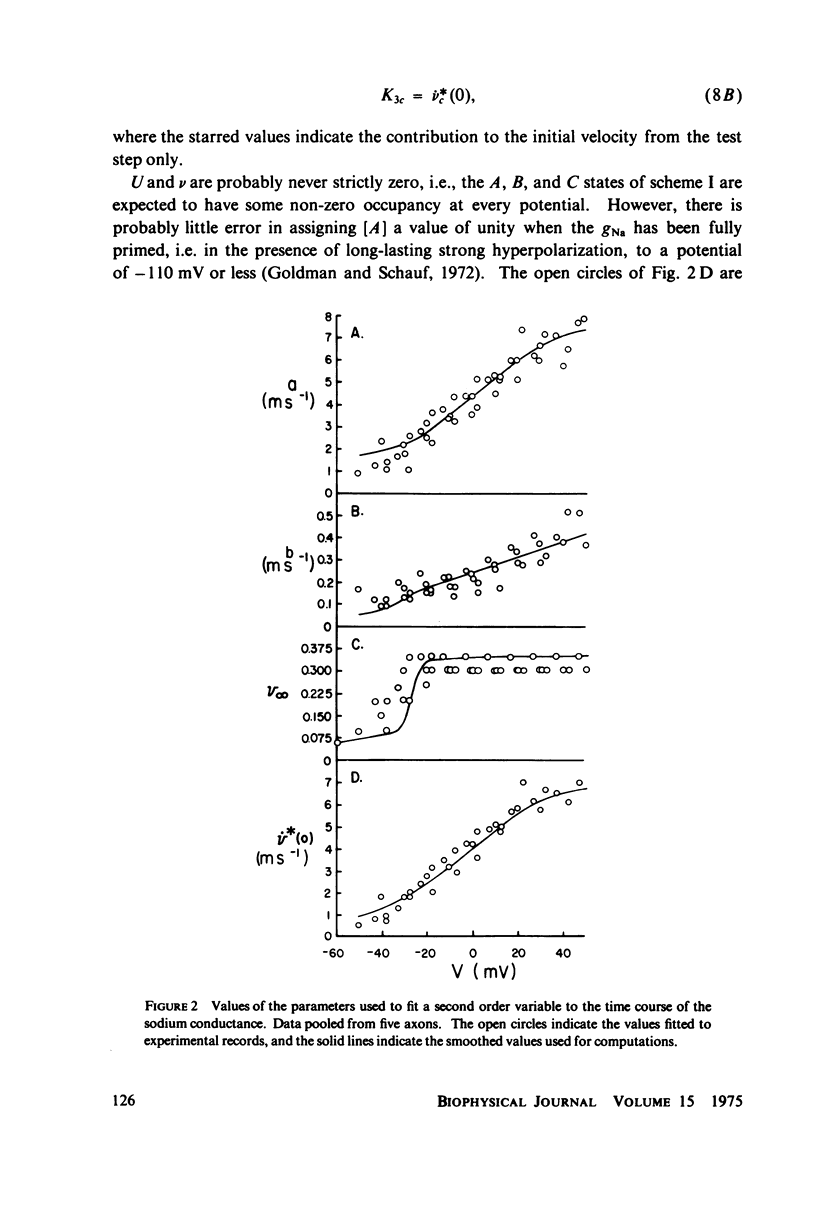
A variety of experimental observations in Myxicola and other preparations indicate that the sodium conductance, gNa, has properties quite different from those described by the m and h variables of Hodgkin and Huxley. A new quantitative description of the gNa is presented in which the gNa is assumed to be proportional to the fifth power of a generalized second-order variable, i.e., gNa = g'Na times v to the fifth, v = -Kav + K2U = K3, U = K4U + K5v + K6. This model is shown to be able to quantitatively simulate all of the experimentally observed behavior of the gNa. A view of the sodium gate consistent with these kinetics is to imagine it to be composed of five independent subunits, each of the type A eq. B eq. C eq. A where A represents the resting state, B the conducting state, and C the inactivated state. A model in which the subunit is of the type A eq. B eq. C could not simulate the experimental observations. It was concluded that two processes are sufficient to account for all of the behavior of the gNa.
Full text
PDF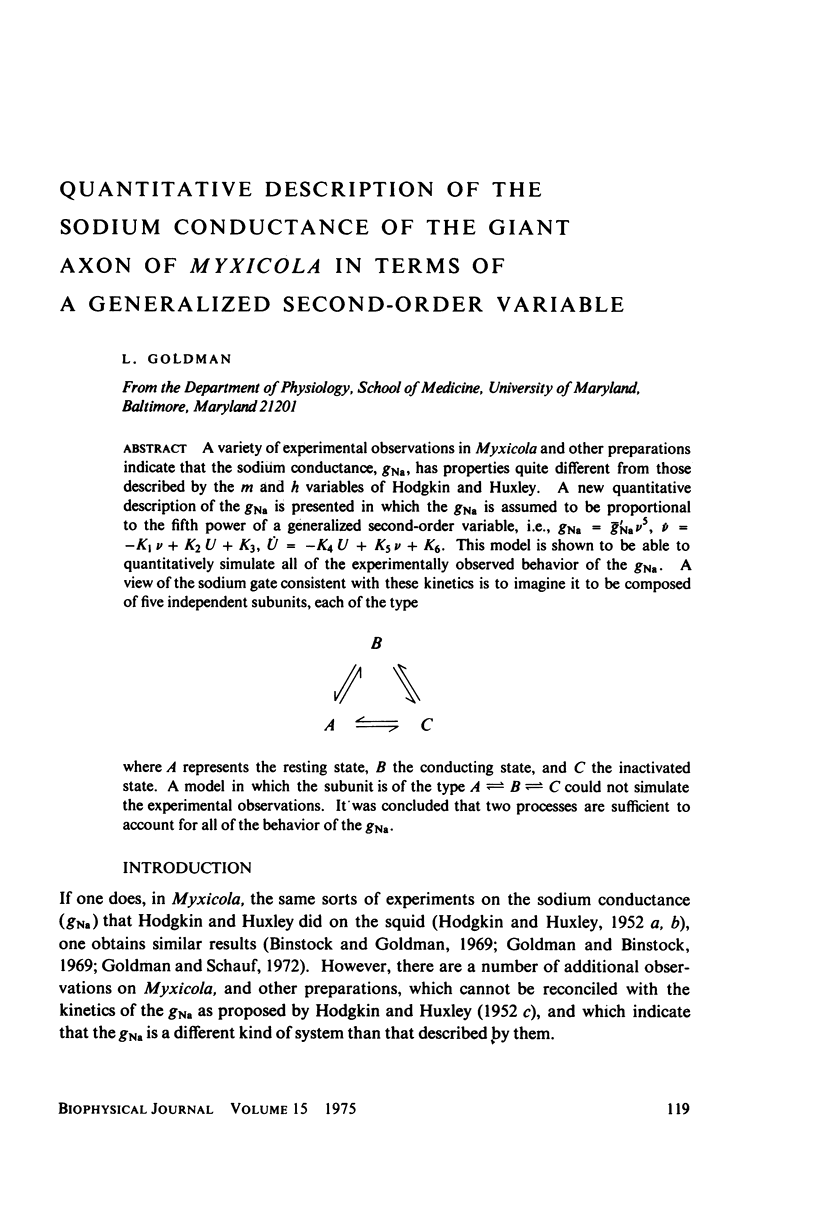











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. M., Bezanilla F., Rojas E. Destruction of sodium conductance inactivation in squid axons perfused with pronase. J Gen Physiol. 1973 Oct;62(4):375–391. doi: 10.1085/jgp.62.4.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F., Armstrong C. M. Gating currents of the sodium channels: three ways to block them. Science. 1974 Feb 22;183(4126):753–754. doi: 10.1126/science.183.4126.753. [DOI] [PubMed] [Google Scholar]
- Binstock L., Goldman L. Current- and voltage-clamped studies on Myxicola giant axons. Effect of tetrodotoxin. J Gen Physiol. 1969 Dec;54(6):730–740. doi: 10.1085/jgp.54.6.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Hodgkin A. L., Meves H. The effect of changing the internal solution on sodium inactivation and related phenomena in giant axons. J Physiol. 1965 Oct;180(4):821–836. doi: 10.1113/jphysiol.1965.sp007733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Evidence for two types of sodium conductance in axons perfused with sodium fluoride solution. J Physiol. 1970 Dec;211(3):653–678. doi: 10.1113/jphysiol.1970.sp009298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B. INACTIVATION OF THE SODIUM-CARRYING MECHANISM IN MYELINATED NERVE FIBRES OF XENOPUS LAEVIS. J Physiol. 1963 Nov;169:445–451. doi: 10.1113/jphysiol.1963.sp007271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B. Steady state inactivation of sodium permeability in myelinated nerve fibres of Xenopus laevis. J Physiol. 1959 Oct;148:671–676. doi: 10.1113/jphysiol.1959.sp006316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDMANN D. E. A MOLECULAR STRUCTURAL BASIS FOR THE EXCITATION PROPERTIES OF AXONS. Biophys J. 1964 May;4:167–188. doi: 10.1016/s0006-3495(64)86776-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman L., Binstock L. Current separations in Myxicola giant axons. J Gen Physiol. 1969 Dec;54(6):741–754. doi: 10.1085/jgp.54.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman L., Schauf C. L. Inactivation of the sodium current in Myxicola giant axons. Evidence for coupling to the activation process. J Gen Physiol. 1972 Jun;59(6):659–675. doi: 10.1085/jgp.59.6.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman L., Schauf C. L. Quantitative description of sodium and potassium currents and computed action potentials in Myxicola giant axons. J Gen Physiol. 1973 Mar;61(3):361–384. doi: 10.1085/jgp.61.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):449–472. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):497–506. doi: 10.1113/jphysiol.1952.sp004719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOYT R. C. THE SQUID GIANT AXON. MATHEMATICAL MODELS. Biophys J. 1963 Sep;3:399–431. doi: 10.1016/s0006-3495(63)86829-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas H. G., Kern R., Einwächter H. M., Tarr M. Kinetics of Na inactivation in frog atria. Pflugers Arch. 1971;323(2):141–157. doi: 10.1007/BF00586445. [DOI] [PubMed] [Google Scholar]
- Hoyt R. C., Adelman W. J., Jr Sodium inactivation. Experimental test of two models. Biophys J. 1970 Jul;10(7):610–617. doi: 10.1016/S0006-3495(70)86323-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt R. C. Sodium inactivation in nerve fibers. Biophys J. 1968 Oct;8(10):1074–1097. doi: 10.1016/S0006-3495(68)86540-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt R. C., Strieb J. D. A stored charge model for the sodium channel. Biophys J. 1971 Nov;11(11):868–885. doi: 10.1016/S0006-3495(71)86261-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULLINS L. J. An analysis of conductance changes in squid axon. J Gen Physiol. 1959 May 20;42(5):1013–1035. doi: 10.1085/jgp.42.5.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore L. E., Jakobsson E. Interpretation of the sodium permeability changes of myelinated nerve in terms of linear relaxation theory. J Theor Biol. 1971 Oct;33(1):77–89. doi: 10.1016/0022-5193(71)90217-7. [DOI] [PubMed] [Google Scholar]
- Offner F. F. The excitable membrane. A physiochemical model. Biophys J. 1972 Dec;12(12):1583–1629. doi: 10.1016/S0006-3495(72)86185-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peganov E. M. O kinetike protsessa inaktivatsii natrievykh kanalov v perekhvate Ranv'e liagushki. Biull Eksp Biol Med. 1973 Nov;76(11):5–9. [PubMed] [Google Scholar]
- Peganov E. M., Timin E. N., Khodorov B. I. O vzaimosviazi protsessov natrievoi aktivatsii i inaktivatsii. Biull Eksp Biol Med. 1973 Oct;75(10):7–11. [PubMed] [Google Scholar]
- Schauf C. L. Sodium currents in Myxicola axons. Nonexponential recovery from the inactive state. Biophys J. 1974 Feb;14(2):151–154. doi: 10.1016/S0006-3495(74)70006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tredgold R. H. A possible mechanism for the negative resistance characteristic of axon membranes. Nat New Biol. 1973 Apr 18;242(120):209–210. doi: 10.1038/newbio242209a0. [DOI] [PubMed] [Google Scholar]



