Abstract
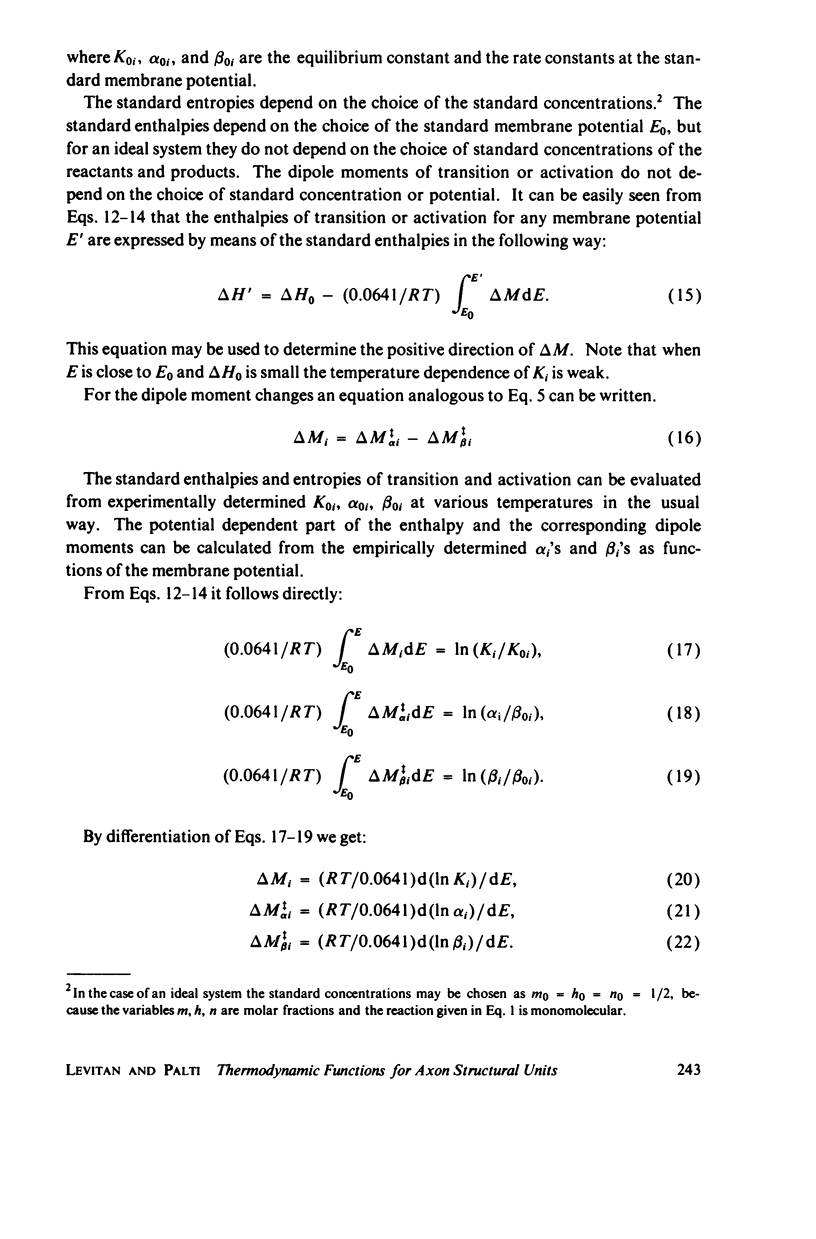
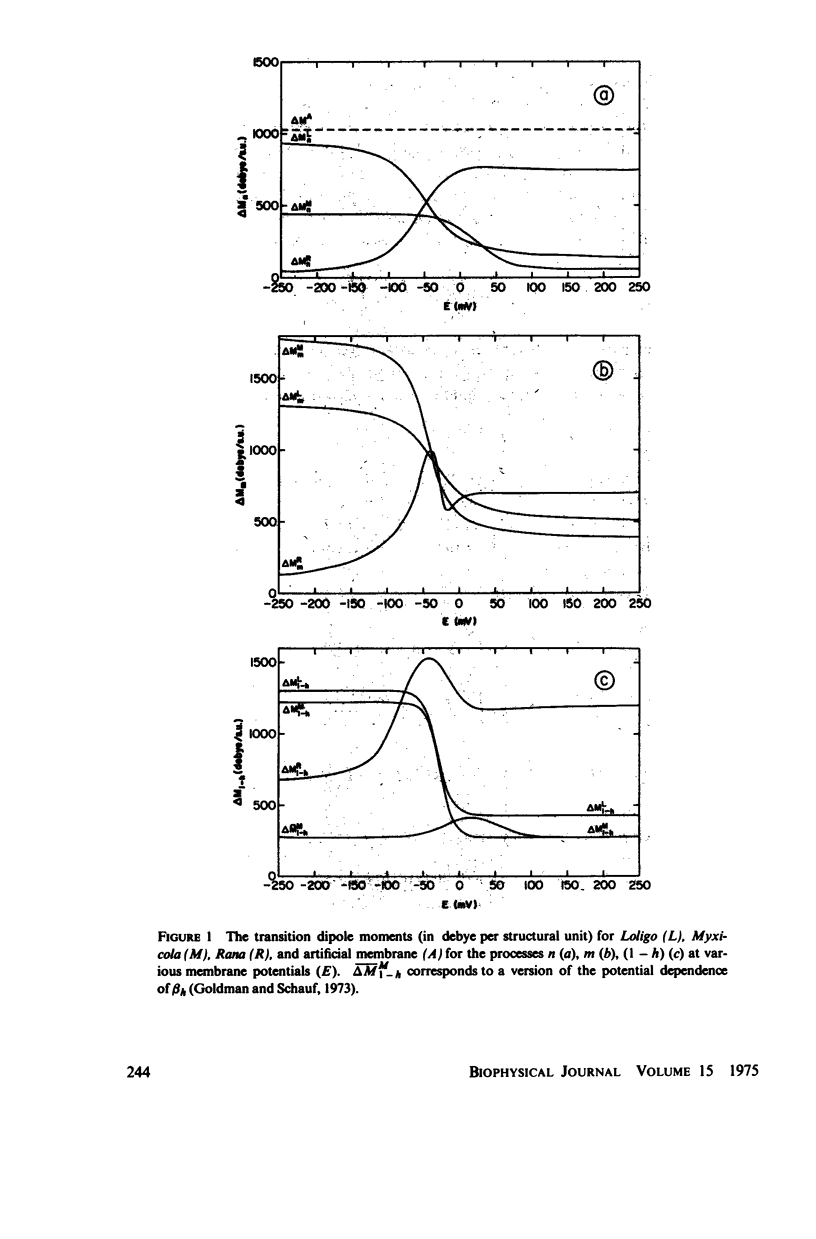
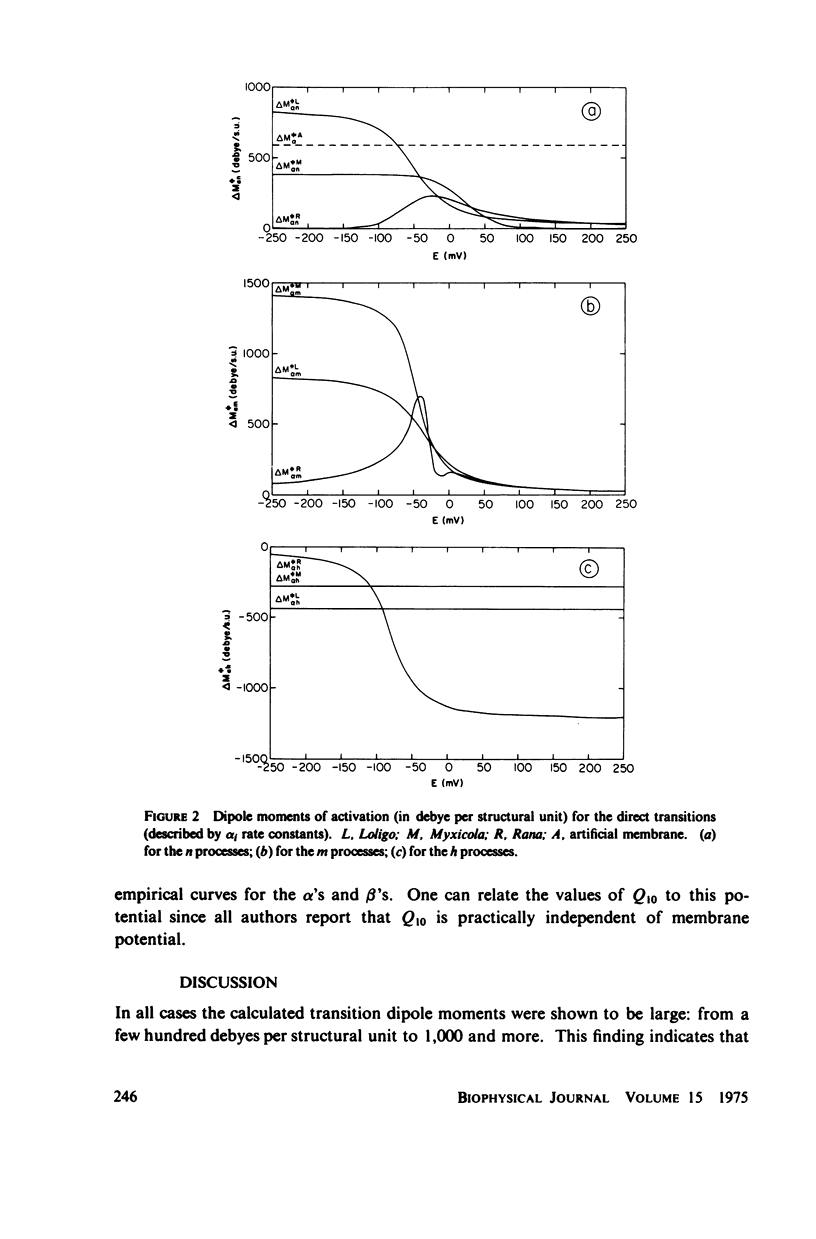
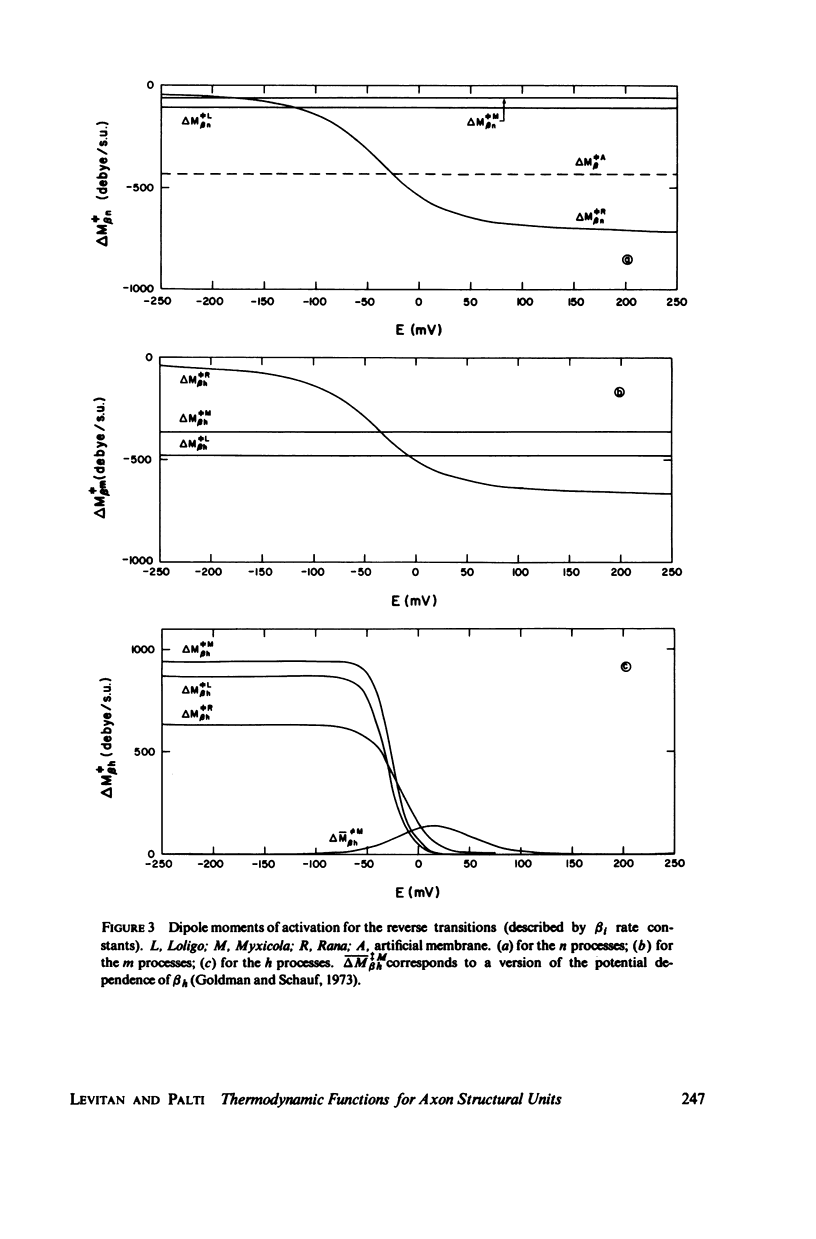
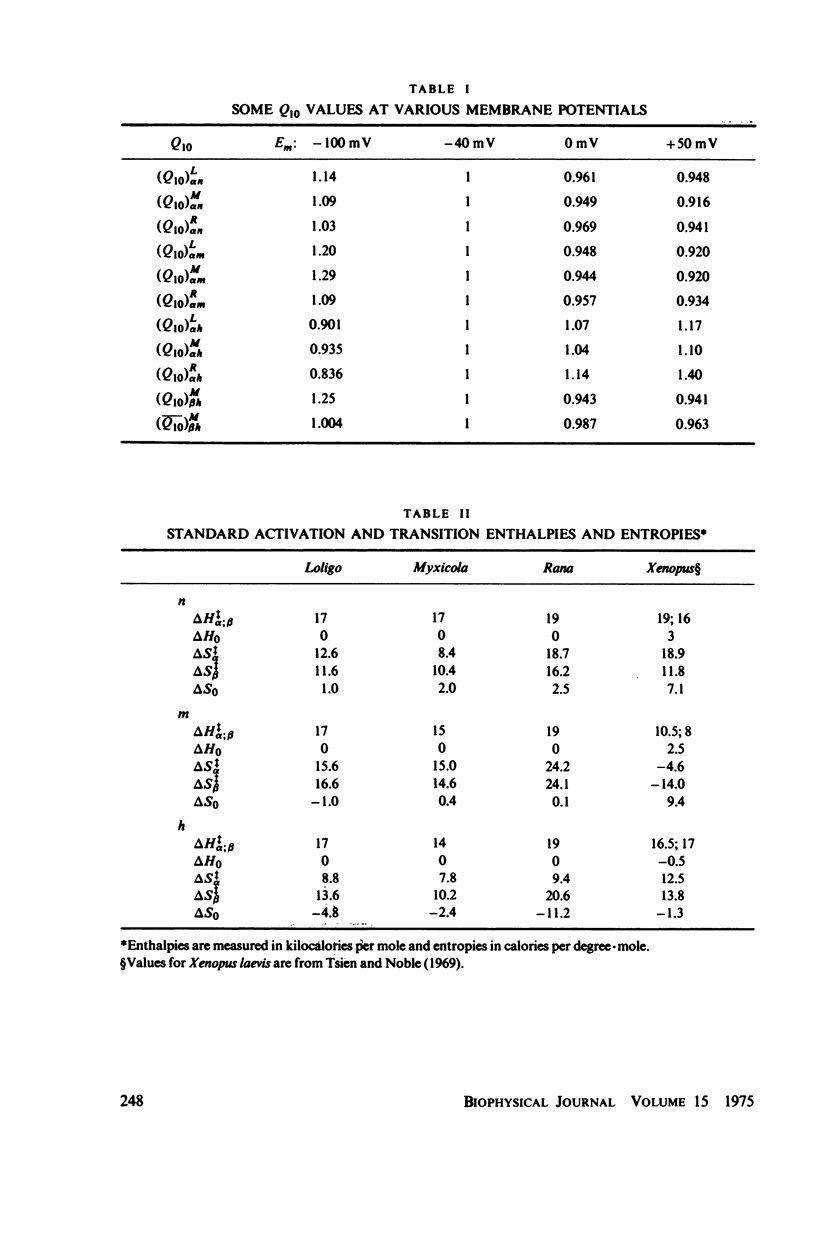
Dipole moment, enthalpy, and entropy changes were calculated for hypothetical structural units which control the opening and closing of ionic channels in axon membranes. The changes of these thermodynamic functions were calculated both for activation (transition to intermediate complex) and for the structural transformation as a whole. The calculations are based on the experimentally determined Q10 values and the empirical formulae for the rate constants (alpha's and beta's) as functions of membrane potentials in Hodgkin-Huxley type models. From the calculated thermodynamic functions we suggest that the specific structural units of the axon membranes are probably of macromolecular (possible protein-like) dimensions with large dipole moments (hundreds of debyes). The calculated dipole moment changes of a single structural unit indicate that in many cases these dipole moments saturate at strong depolarizations or hyperpolarizations. The transitions in structural units show substantial activation enthalpies and entropies but the net enthalpy and entropy changes are practically negligible for the transition as a whole, i.e. the structural units presumably undergo displacements. While the calculated dipole moment changes associated with structural transformations in Loligo and Myxicola show similar potential dependencies, those for Rana usually show a different behavior. The relevance of the dipole moment changes to gating currents is discussed.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. M., Bezanilla F. Charge movement associated with the opening and closing of the activation gates of the Na channels. J Gen Physiol. 1974 May;63(5):533–552. doi: 10.1085/jgp.63.5.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F., Armstrong C. M. Gating currents of the sodium channels: three ways to block them. Science. 1974 Feb 22;183(4126):753–754. doi: 10.1126/science.183.4126.753. [DOI] [PubMed] [Google Scholar]
- Ehrenstein G., Blumenthal R., Latorre R., Lecar H. Kinetics of the opening and closing of individual excitability-inducing material channels in a lipid bilayer. J Gen Physiol. 1974 Jun;63(6):707–721. doi: 10.1085/jgp.63.6.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., MOORE L. E. THE EFFECT OF TEMPERATURE ON THE SODIUM AND POTASSIUM PERMEABILITY CHANGES IN MYELINATED NERVE FIBRES OF XENOPUS LAEVIS. J Physiol. 1963 Nov;169:431–437. doi: 10.1113/jphysiol.1963.sp007269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman L., Schauf C. L. Quantitative description of sodium and potassium currents and computed action potentials in Myxicola giant axons. J Gen Physiol. 1973 Mar;61(3):361–384. doi: 10.1085/jgp.61.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F., KATZ B. Measurement of current-voltage relations in the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):424–448. doi: 10.1113/jphysiol.1952.sp004716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. L., Chen Y. On the theory of ion transport across the nerve membrane. VI. Free energy and activation free energies of conformational change. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1723–1726. doi: 10.1073/pnas.69.7.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes R. D., Rojas E. Kinetics and steady-state properties of the charged system controlling sodium conductance in the squid giant axon. J Physiol. 1974 Jun;239(2):393–434. doi: 10.1113/jphysiol.1974.sp010575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore L. E. Effect of temperature and calcium ions on rate constants of myelinated nerve. Am J Physiol. 1971 Jul;221(1):131–137. doi: 10.1152/ajplegacy.1971.221.1.131. [DOI] [PubMed] [Google Scholar]
- Schauf C. L. Temperature dependence of the ionic current kinetics of Myxicola giant axons. J Physiol. 1973 Nov;235(1):197–205. doi: 10.1113/jphysiol.1973.sp010384. [DOI] [PMC free article] [PubMed] [Google Scholar]


