Abstract
Patterns of biological diversity should be interpreted in light of both contemporary and historical influences; however, to date, most attempts to explain diversity patterns have largely ignored history or have been unable to quantify the influence of historical processes. The historical effects on patterns of diversity have been hypothesized to be most important for taxonomic groups with poor dispersal abilities. We quantified the relative stability of rainforests over the late Quaternary period by modeling rainforest expansion and contraction in 21 biogeographic subregions in northeast Australia across four time periods. We demonstrate that historical habitat stability can be as important, and in endemic low-dispersal taxa even more important, than current habitat area in explaining spatial patterns of species richness. In contrast, patterns of endemic species richness for taxa with high dispersal capacity are best predicted by using current environmental parameters. We also show that contemporary patterns of species turnover across the region are best explained by historical patterns of habitat connectivity. These results clearly demonstrate that spatially explicit analyses of the historical processes of persistence and colonization are both effective and necessary for understanding observed patterns of biodiversity.
Keywords: Australian Wet Tropics, β-diversity, dispersal, habitat connectivity, historical habitat stability
Patterns in local species richness (α-diversity) and in the turnover (dissimilarity) of species composition across geographic space (β-diversity) are a function of environmental heterogeneity, speciation, extinction, and colonization (1–6). Stability in climate and vegetation over geological time scales should allow more species to arise and persist, resulting in greater α-diversity in more stable regions (2, 6, 7). For instance, Fjeldsa and colleagues (8) found that currently stable regions have high endemism for both old and recent taxa in tropical avifauna, but did not explicitly predict spatial patterns of paleoclimatic stability, and relate these regions to species richness.
Dispersal interacts with the historic stability of the habitat to affect α- and β-diversity in at least two ways (6, 9). First, across a given landscape, speciation is more likely in dispersal-limited groups because of reproductive isolation and/or consistent divergent selection across environmental gradients (10). Second, dispersal-limited species are more likely to experience local extinction and are less likely to recolonize an area should local extinction occur (11). In studies of β-diversity, isolation is typically quantified as the geographic distance among current suitable habitats. Further, although advances in landscape ecology have resulted in more environmentally realistic measures of isolation (12), research generally considers only current environments. Nevertheless, past climate fluctuations have often caused islands of suitable habitat to shift, expand, or disappear. The distance between currently suitable habitats may not reflect the resulting historical variability in habitat isolation and stability and, thus, may not be the best predictor of biodiversity.
Because of their geographic isolation and high endemism, the intensively studied tropical rainforests of the northeast Australian Wet Tropics (AWT) bioregion provide an unusual opportunity to evaluate the relative contribution of current and historical factors to current patterns of species diversity. Species endemic to the AWT are mostly confined to cool upland rainforests (>300 m above sea level) in 13 upland subregions, which currently are generally surrounded by lower drier subregions and can be likened to islands (13–15) (Fig. 1A). A combination of paleoecological and biogeographic evidence indicates substantial climate-induced contractions and shifts of upland rainforest vegetation during the Quaternary period (16–17). Specifically, rainforests contracted during restrictive cool-dry [18 thousand years ago (Kya); e.g., the Last Glacial Maximum period] and warm-wet (5–3.6 Kya) periods and expanded during the cool-wet period of the early Holocene period (8–6 Kya) and again under the current climates.
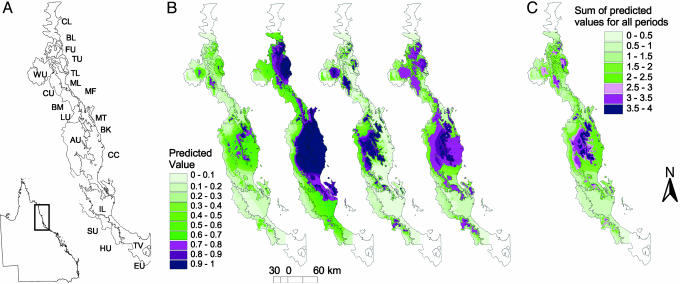
Fig. 1.
Maps showing predicted distribution of forest in AWT at different time periods. (A) Subregions contained in the AWT (CL, Cooktown Lowlands; FU, Finnegan Uplands; BL, Bloomfield–Helenvale Lowlands; TU, Thornton Uplands; TL, Thornton Lowlands; CU, Carbine Uplands; ML, Mossman Lowlands; BM, Black Mountain Corridor; MF, McAlister Foothills; LU, Lamb Uplands; AU, Atherton Uplands; BK, Bellenden–Ker/Bartle–Frere; KU, Kirrama Uplands; CC, Cairns–Cardwell Lowlands; MT, Malbon–Thompson Uplands; LE, Lee Uplands; SU, Spec Uplands; HU, Halifax Uplands; IL, Ingham Lowlands; TV, Townsville Lowlands; WU, Windsor Uplands). (B) Predicted distribution models of forest vegetation. (Left to Right): cool-dry, cool-wet, warm-wet, and current climates. (C) The stability surface, which is the combination of the four models.
We expected that (i) predictions of α- and β-diversity of rainforest species in the AWT would be improved by considering spatially explicit models of habitat history, and (ii) the influence of history is likely to vary among taxonomic groups on the basis of their dispersal ability. A priori, we identified five groups for analysis: birds, mammals, reptiles, aquatic-breeding frogs, and terrestrial-breeding frogs of the family Microhylidae in which birds have the highest dispersal capacity and microhylid frogs have the lowest dispersal capacity. The species richness of regionally endemic vertebrates in a given subregion has been related to the current area and shape of the rainforest within the subregion, where shape was hypothesized to be a surrogate of rainforest persistence (13, 18). Phylogeographic studies suggest that taxonomic groups varied in their responses to climate-driven changes in habitat distributions and that these changes primarily affected local extinction and recolonization dynamics rather than speciation (reviewed in ref. 18–20). Microhylid frogs appear to be an exception; they have radiated extensively within the AWT and likely speciated in situ, albeit well before the late Quaternary period (21, 22). In this article, we combine current and historical models of suitable habitats with species presence data to explore how historical habitat stability and connectedness influenced patterns of species diversity in vertebrate species with contrasting dispersal ability in rainforests of the AWT.
Results and Discussion
Our spatially explicit predictions of rainforest distribution and stability (Fig. 1 B and C) were consistent with paleomodels of both rainforest-restricted snails (23) and the subregional distribution of rainforest (24). Our models predicted that rainforest persisted south of the Black Mountain Corridor, primarily on the Bellenden–Ker Range and eastern Atherton Uplands, with smaller stable areas of rainforest on the Malbon–Thompson Range and the Lamb, Kirrima, and Spec Uplands. The predicted stable areas in the north occurred on the Carbine, Thornton, and Finnegan Uplands, and to a lesser extent the Windsor Uplands. Several coastal refugia, including the Bellenden–Ker and Thomson Ranges and the Thornton and Finnegan Uplands, had high historical stability relative to their current area, whereas the Lee Uplands had much lower predicted stability relative to its current area (Fig. 1C).
Species richness was positively correlated with both area and stability and was negatively correlated with shape (Table 1). On the basis of R2 values of univariate regressions, area was the best predictor of species richness for birds, mammals, and aquatic-breeding frogs, and stability was the best predictor for reptiles and microhylid frogs, as expected because of their dispersal ability. Nonetheless, the predictive ability of area and stability were similar for several groups. We determined that the summed Akaike weight was highest for area in birds, mammals, frogs, and reptiles, whereas stability has the highest information content in microhylid frogs.
Table 1. Predictors of species richness for rainforest-dependent endemic vertebrates aquatic-breeding frogs, frogs; terrestrial breeding microhylid frogs, Microhylids.
| Area
|
Shape
|
Stability
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Taxonomic group | R2 | AIC information content | Parameter estimates | Variances for parameter estimates | R2 | AIC information content | Parameter estimates | Variances for parameter estimates | R2 | AIC information content | Parameter estimates | Variances for parameter estimates | Total R2 |
| Birds | 0.64 | 0.97 | 4.98 | 0.93 | 0.32 | 0.95 | -16.51 | 56.99 | 0.40 | 0.30 | 0.04 | 0.49 | 0.80 |
| Mammals | 0.38 | 0.83 | 2.99 | 0.77 | 0.33 | 0.62 | -7.80 | 13.86 | 0.32 | 0.41 | 0.08 | 0.28 | 0.60 |
| Frogs | 0.52 | 0.92 | 3.14 | 0.97 | 0.19 | 0.66 | -7.01 | 7.07 | 0.51 | 0.56 | 0.81 | 0.69 | 0.78 |
| Reptiles | 0.50 | 0.92 | 4.50 | 1.80 | 0.22 | 0.66 | -11.32 | 20.38 | 0.59 | 0.67 | 1.63 | 1.33 | 0.82 |
| Microhylids | 0.59 | 0.54 | 0.66 | 0.62 | 0.14 | 0.31 | -2.57 | 2.24 | 0.60 | 0.90 | 1.41 | 0.37 | 0.68 |
Results are reported for Akaikes information criterion (AIC) regression and regressions with single and multiple regression (R2). Highest R2 values and AIC scores for individual parameters are in bold.
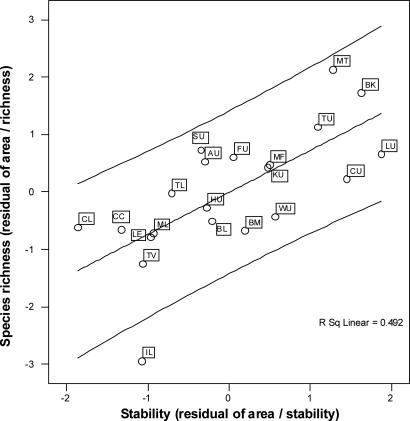
When all species were combined in a single analysis, stability explained a high and statistically significant proportion of the variability in endemic species richness independent of area (R2 = 0.49; P < 0.001; Fig. 2). Although the current habitat area is the most widely used predictor of species richness and the species–area relationship is considered to be one of the few “laws” in ecology (6), these results demonstrate that historical stability can be as important, and in low dispersal taxa even more important, than area in explaining spatial variation in species richness.
Fig. 2.
Plot of the residuals of an area/stability relationship against the residuals of an area/species-richness relationship. The plot demonstrates that there is a strong overall relationship between species richness and stability of rainforest habitat over time, which is independent of rainforest area. CL, Cooktown Lowlands; FU, Finnegan Uplands; BL, Bloomfield–Helenvale Lowlands; TU, Thornton Uplands; TL, Thornton Lowlands; CU, Carbine Uplands; ML, Mossman Lowlands; BM, Black Mountain Corridor; MF, McAlister Foothills; LU, Lamb Uplands; AU, Atherton Uplands; BK, Bellenden–Ker/Bartle–Frere; KU, Kirrama Uplands; CC, Cairns–Cardwell Lowlands; MT, Malbon–Thompson Uplands; LE, Lee Uplands; SU, Spec Uplands; HU, Halifax Uplands; IL, Ingham Lowlands; TV, Townsville Lowlands; WU, Windsor Uplands.
Species dissimilarity among subregions across all endemic vertebrates ranged from 0.385 to 0.989 (on a scale of 0–1), with the highest dissimilarity values between the rich uplands and lowlands depauperate in endemics. The proportion of dissimilarity explained by the Mantel regressions ranged from 18% to 61% (Table 2). In no cases did current environmental dissimilarity contribute significantly to the multivariate models, which is remarkable in that this parameter is the one most commonly used to model β-diversity (25, 26). The most striking result was that the spatial dissimilarity of microhylid frog assemblages was affected primarily by the degree of isolation under the restrictive warm-wet climate. The regression for this group had an R2 value more than twice that of any other group (Table 2).
Table 2. Results of a forward step-wise multiple Mantel between species dissimilarity (Sørenson index) for each taxonomic group and environmental factors.
| Standardized coefficients
|
||||
|---|---|---|---|---|
| Taxonomic group | Δ Area | Distance CD | Distance WW | Total R2 |
| Birds | 0.42 | 0.18* | ||
| Mammals | 0.54 | 0.30** | ||
| Aquatic-breeding frogs | 0.48 | 0.23** | ||
| Reptiles | 0.41 | 0.33 | 0.24** | |
| Terrestrial-breeding microhylid frogs | 0.78 | 0.61** | ||
Only significant environmental factors are shown. Δ area, pairwise difference in area; distance CD, environmentally weighted distance during cool-dry periods; distance WW, environmentally weighted distance during warm-wet periods.
Significant to 0.05
Significant to 0.01
The patterns of dissimilarity among assemblages of birds and aquatic-breeding frogs were influenced only by differences in current area, suggesting that these groups have equilibrated to current environmental factors as a consequence of their greater dispersal ability. This is certainly the case for birds that have a high dispersal capacity relative to other vertebrates and show little genetic structure among subregions (20, 24). Both mammals and reptiles have historical parameters in their models. For mammals, the importance of environmentally weighted distance under warm-wet climates may be driven by arboreal mammals, a heat-intolerant, narrowly endemic subset of mammals unlikely to disperse across unsuitable environments (15).
Because microhylid frogs have the lowest dispersal abilities of the five taxonomic groups and have patterns of species richness positively influenced by consistent rainfall throughout the year (21), the isolation of uplands by drier sclerophyll-dominated habitats should strongly influence the dissimilarity of their assemblages among regions. Microhylids are the only group to have radiated within the AWT and given the geographic adjacency of single-subregion endemics (22); therefore, it is likely that the current pattern of species diversity of microhylids reflects both vicariant speciation among historical refugia (as indexed by the environmentally weighted distance during restrictive periods) and persistence within them (as indexed by stability). The marked discrepancies between correlates of species dissimilarity in aquatic-breeding and microhylid frogs are reminiscent of reported differences in the determinants of patterns of species richness and nestedness for these groups (21).
Our results demonstrate that inclusion of spatial surrogates for historical processes (stability surfaces for persistence and speciation and environmentally weighted distances based on past climates for limitations to dispersal and recolonization) improves our ability to predict both α- and β-diversity, especially for dispersal-limited groups. Biologists have long been aware that history is important for understanding species distribution patterns and community composition (11, 14). Nonetheless, history has only recently been explicitly incorporated into analyses aimed at determining the drivers of species richness and turnover (2–4, 27). The current area of habitat has long been used to inform conservation planning, with larger continuous areas being conserved preferentially because they are likely to contain more species (6, 28). In this article, we show that the stability of suitable habitats through time is as important as area in determining patterns of species richness and spatial turnover of regional species assemblages. Moreover, stability can influence patterns of species diversity independent of area. There is a growing effort to predict the distribution of biodiversity globally (26, 27, 29), and tropical rainforests are a particular challenge because of their extraordinarily high diversity and complexity. Our results for the rainforest fauna of the AWT support the contention that historically stable refugia should have high priority for conservation (2, 8, 22).
Materials and Methods
Richness Data. We used an extensive database of species distribution records (updated from ref. 13) with reliable subregional distribution data for 69 of the 84 terrestrial vertebrate species that are endemic to the region. Five groups were analyzed: birds (13 spp.), mammals (11 spp.), reptiles (21 spp.), aquatic-breeding frogs (11 spp.), and terrestrial-breeding frogs of the family Microhylidae (13 spp.) in which birds have the highest and microhylid frogs the lowest dispersal capacity. Mammal species included seven arboreal, one terrestrial, and three that were both arboreal and terrestrial. Reptiles were dominated by lizards. We split frogs into two groups because of marked differences in ecology, size, and biogeographic patterns (30). The richness of endemic species across all taxonomic groups varied from 6 to 51 among subregions.
Predictive Habitat Models. We use a unique approach to determine whether historical variability in habitat suitability negatively impacts local species richness in the AWT. Using fine scale maps of past vegetation generated via predictive distribution modeling, we quantify the historic environmental stability at a subregional scale and relate variation in species richness to this measure of stability, as well as to current area and shape. Various methods exist to create predictive distribution models, but there is little rigorous comparison among methods (but see ref. 31), and alternative methods can produce divergent results under different climates (32). Therefore, we explored two different modeling methods to create distributional models, BIOCLIM (envelope-style method) and logistic regression. Both BIOCLIM and regression models produced qualitatively similar results, and our conclusions do not depend on the method used. We chose regression modeling because it generates a continuous surface of values and, hence, provided more information for creating environmentally weighted distances among regions.
To create distribution models of upland rainforest, we took 2,000 random points from areas designated as upland rainforest (microphyll, mesophyll, or notophyll) (33). Absence data (10,000 localities) were taken at random from the entire AWT region from areas that do not currently have upland rainforest. We down-weighted absence points (five absence points = one presence point) to minimize the influence of any single, incorrect point (for example, deforested areas could have a suitable climate for rainforest). We used three spatially interpolated environmental surfaces (80-m resolution): annual mean temperature (AMT), annual mean precipitation (AMP), and precipitation of the driest quarter (PDQ). We created a predicted map of upland rainforest using logistic regression where forest presence or absence was the dependent variable and AMT, AMP, and PDQ and each of these variables squared were independent variables. We then mapped this information spatially. We did not fit complex relationship variables to avoid model over-fitting, which could be problematic when extrapolating different climates.
To predict the distribution of rainforest under different climates, we projected the environmental envelope (i.e., regression equation) onto paleoclimatic surfaces for each paleoclimatic period. To create paleoclimatic surfaces, we altered the current climate on the basis of the predicted climate shifts of the three environmental variables as follows: cool-dry: AMT – 3.5°C, AMP × 0.5, and PDQ × 0.6 (i.e., cool-dry was 3.5°C cooler with 50% less AMP and 40% less PDQ); cool-wet: AMT – 2.0°C, AMP × 1.2, and PDQ × 2; and warm-wet: AMT + 2.0°C, AMP × 1.25, and PDQ × 1.5 (34). We evaluated current models with an independently generated set of 2,000 random points (1,000 each from forest and nonforest) using a receiving operator curve area, which measures the ability of a model to discriminate between forest and nonforest sites (35). We obtained a value of 0.78, indicating that our model performed well. We could not validate paleopredictions, but the fact that both regression and BIOCLIM models produced qualitatively similar results suggests that our conclusions do not depend on the method used.
We measured the constancy of rainforest habitat through time, that is, habitat stability, by summing models of potential distribution of upland rainforests across all four climatic periods. As a result, each pixel had a value from 0 (never predicted to be suitable) to 4 (perfectly predicted across all time periods). We calculated mean pixel stability per subregion to obtain a habitat stability value that is independent of area.
Regression Analyses. We used Akaike's information criterion methods and logistic regression to determine the capacity of area, shape, and stability to predict species richness for each taxonomic group. Akaike's information criterion methods allow model selection uncertainty to be incorporated in analyses by quantifying the importance of each variable across multiple models (36). To quantify the evidence for the importance of each variable, we summed the Akaike weights across all of the models in the set (in the three variable case, there are seven models) where a given variable occurred. These values can be directly compared, providing a means for ranking in terms of information content (36). Shape was quantified as the departure from circular and is calculated from the area (A) and perimeter length (P) of rainforest within each of the subregions as follows: shape index = P/2(π A)0.5, hence, the smaller the value, the rounder the shape. Both area and shape were log-transformed. Other variables, such as heterogeneity of rainforest types, were not included because they have little explanatory power when compared with shape and area (13).
Mantel Matrix Regression. To explore why differences in species composition exist among subregions, we developed a series of matrices representing current and historical processes of dispersal and local extinction. We used a forward step-wise Mantel regression test (permute! version 3.4; p-value required for variable to enter model is 0.1) (37), which computes the regression coefficients and associated R2 value of one or more independent matrix variables by using permutational methods (99 permutations were run). We created the matrix of species dissimilarity for each taxonomic group using Sørenson's similarity statistic (five matrices).
The following matrices were created to quantify variation among current subregions: climatic dissimilarity, Euclidean distance measured on eight standardized variables that describe mean and variation in climate parameters (mean and coefficient of variation of annual temperature and rainfall) and variables that might limit species distributions (temperature of the coldest and warmest month and rainfall of the driest and wettest quarter), and the absolute difference in log-transformed current area and shape. Given the species–area relationship and the expectation that at a regional scale there is a limited species pool, areas of similar size should have similar species compositions. To assess the importance of past climate, we used the absolute difference in mean pixel stability of subregional rainforests. Stability should reflect the likelihood of species persistence within subregions over interglacial time scales.
To study dispersal limitation, we created a matrix of pair-wise environmentally weighted distances (four matrices; one for cool-dry, warm-wet, cool-wet, and current conditions). The environmentally weighted distance during restrictive climate periods should reflect the potential for isolation during periods of rainforest contraction. Calculating environmentally weighted distances requires two steps: (i) construction of a cost (weighted grid) and (ii) calculation of a least-cost path between two points. We used the predicted rainforest distribution models to create cost grids by calculating one minus the estimated probability of forest for each pixel. Pixels with a value of 0.3 (corresponding to 70% probability of having rainforest) were set to a zero cost under the assumption that these pixels were effectively a continuous forest habitat. Hence, if there was a continuous corridor of forest between two regions, the environmentally weighted cost was zero, whereas if there was a very low probability of forest between regions, the corresponding cost was high. Subregions often included multiple, disconnected zero-cost areas (i.e., suitable habitat). We created an environmentally weighted distance matrix for each climate period by taking the mean value of all cost paths between patches of suitable habitat within each pair of subregions.
Acknowledgments
We thank S. Baines, R. Hijmans, S. Hubbell, B. Loiselle, M. Pigliucci, R. Sokal, and two anonymous reviewers for helpful comments on the manuscript. This work was supported by National Science Foundation Grant DEB-0416152.
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: AWT, Australian Wet Tropics; AMT, annual mean temperature; AMP, annual mean precipitation; PDQ, precipitation of the driest quarter.
References
- 1.Ricklefs, R. E. & Birmingham, E. (2001) Science 294, 1522. [DOI] [PubMed] [Google Scholar]
- 2.Jansson, R. (2003) Proc. R. Soc. London Ser. B 270, 583–590. [Google Scholar]
- 3.Graham, C. H., Ron, S. R., Santos, J. C., Schneider, C. J. & Moritz, C. (2004) Evol. Int. J. Org. Evol. 58, 1781–1793. [DOI] [PubMed] [Google Scholar]
- 4.Qian, H., Ricklefs, R. E. & White, P. S. (2005) Ecol. Lett. 8, 15–22. [Google Scholar]
- 5.Ricklefs, R. E. (2004) Ecol. Lett. 7, 1–15. [Google Scholar]
- 6.Rosenzweig, M. L. (1995) Species Diversity in Space and Time (Cambridge Univ. Press, Cambridge, U.K.).
- 7.Willis, J. C. (1922) Age and Current Area, A Study in Geographical Distribution and Origin in Species (Cambridge Univ. Press, Cambridge, U.K.).
- 8.Fjeldsa, J., Lambin, E. & Mertens, B. (1999) Ecography 21, 63–78. [Google Scholar]
- 9.Hubbell, S. P. (2001) The Unified Theory of Biodiversity and Biogeography (Princeton Univ. Press, Princeton).
- 10.Endler, J. A. (1977) Geographic Variation, Speciation and Clines (Princeton Univ. Press, Princeton). [PubMed]
- 11.MacArthur, R. H. & Wilson, E. O. (1967) The Theory of Island Biogeography (Princeton Univ. Press, Princeton).
- 12.Turner, M. G., Gardner, R. H. & O'Neill, R. V. (2001) Landscape Ecology in Theory and Practice (Springer, New York).
- 13.Williams, S. E. & Pearson, R. (1997) Proc. R. Soc. London Ser. B 264, 709–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams, S. E., Pearson, R. G. & Walsh, P. J. (1996) Pacific Conserv. Biol. 2, 327–362. [Google Scholar]
- 15.Winter, J. W. (1997) Wildl. Res. 24, 493–511. [Google Scholar]
- 16.Hopkins, M. S., Ash, J., Graham, A. W., Head, J. & Hewett, R. K. (1993). J. Biogeogr. 20, 357–372. [Google Scholar]
- 17.Kershaw, A. P. (1994) Palaeogeogr. Palaeoclimatol. Palaeoecol. 109, 399–412. [Google Scholar]
- 18.Schneider, C. J. & Williams, S. E. (2005) in Tropical Rainforests: Past, Present and Future, eds. Moritz, C., Bermingham, E. & Dick, C. (Univ. of Chicago Press, Chicago), pp. 401–424.
- 19.Moritz, C., Patton, J. L., Schneider, C. J. & Smith, T. B. (2001) Annu. Rev. Ecol. Syst. 31, 533–563. [Google Scholar]
- 20.Moritz, C., Hoskin, C., Hugall, A. & Graham, C. (2005) in Phylogeny and Conservation, eds. Purvis, A., Gittleman, J. & Brooks, T. (Cambridge Univ. Press, Cambridge, U.K.), pp. 243–267.
- 21.Williams, S. E. & Hero, J. M. (2001) Biol. Conserv. 98, 1–10. [Google Scholar]
- 22.Hoskin, C. J. (2004) Aust. J. Zool. 52, 237–269. [Google Scholar]
- 23.Hugall, A., Moritz, C., Moussalli, A. & Stanisic, J. (2002) Proc. Natl. Acad. Sci. USA 99, 6112–6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hilbert, D. W. & Ostendorf, B. (2001) Ecol. Modell. 146, 311–327. [Google Scholar]
- 25.Condit, R., Pitman, N., Leigh, E. G., Jr., Chave, J., Terborgh, J., Foster, R. B., Núñez, P., Aguilar, S., Valencia, R., Villa, G., et al. (2002) Science 295, 666–669. [DOI] [PubMed] [Google Scholar]
- 26.Ferrier, S., Powell, G. V. N., Richardson, K. S., Manion, G., Overton, J., Allnutt, T. F., Mantle, K., Burgess, N. D., Faith, D. P., Kier, G., et al. (2004) Bioscience 54, 1101–1109. [Google Scholar]
- 27.Graham, C. H., Smith, T. B. & Languy, M. (2005) J. Biogeogr. 32, 1371–1384. [Google Scholar]
- 28.Faith, P. D., Ferrier, S. & Walker, P. A. (2004) J. Biogeogr. 31, 1207–1217. [Google Scholar]
- 29.Rodrigues, A. S. L., Akçakaya, H. R., Andelman, S. J., Bakarr, M. I., Boitani, L., Brooks, T. M., Chanson, J. S., Fishpool, L. D. C., da Fonseca, G. A. B., Gaston, K. J., et al. (2004) Bioscience 54, 1092–1100. [Google Scholar]
- 30.Williams, S. E. & Hero J. M. (1998) Proc. R. Soc. London Ser. B 265, 597–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Segurado, P. & Araujo, M. B. (2004) J. Biogeogr. 31, 1555–1568. [Google Scholar]
- 32.Thuiller, W. (2003) Global Change Biol. 9, 1353–1362. [Google Scholar]
- 33.Webb, L. & Tracey, J. (1981) in Ecological Biogeography of Australian Rainforests: Pattern and Change, ed. Keast, J. A. (W. Junk, The Hague, The Netherlands), pp. 605–694.
- 34.Nix, H. A. (1991) in Rainforest Animals, Atlas of Vertebrates Endemic to Australia's Wet Tropics, eds. Nix, H. A. & Switzer, M. (Australian National Parks and Wildlife Service, Canberra), pp. 11–39.
- 35.Hanley, J. A. & McNeil, B. J. (1982) Radiology 143, 29–36. [DOI] [PubMed] [Google Scholar]
- 36.Burnham, K. P. & Anderson, D. R. (1998) Model Selection and Inference: A Practical Information Theoretical Approach (Springer, New York).
- 37.Legendre, P., Lapoint, F. J. & Casgrain, P. (1994) Evolution (Lawrence, Kans.) 48, 1487–1499. [DOI] [PubMed] [Google Scholar]