Abstract
Bves/pop1a is a unique, highly conserved integral membrane protein expressed in embryonic epithelia and striated muscle. Although studies have proposed a role in epithelial morphogenesis, the function of Bves/pop1a in development is completely unknown. Here we show that Xenopus laevis Bves (Xbves) RNA and protein are expressed in epithelia of the early embryo. Transfection of Xbves into nonadherent mouse L cells confers cell/cell adhesion. Global inhibition of Xbves function by morpholino injection into two-cell embryos arrests development at gastrulation by deregulating the epithelial movements of epiboly and involution. Clonal inhibition of Xbves activity within the A1 blastomere and its derivatives completely randomizes movement of its progeny within otherwise normally differentiating embryos. These data demonstrate that Bves/pop1a proteins play a critical role in epithelial morphogenesis and, specifically, in the cell movements essential for epithelial rearrangements that occur during X. laevis development.
Keywords: cell adhesion, gastrulation, embryo, convergent extension morpholino
Movement and reshaping of epithelia are essential during embryogenesis. In vertebrates, some of the first and most fundamental examples of epithelial morphogenesis are those that drive epiboly and gastrulation (1–3). In Xenopus laevis, these movements are governed, in part, by rearrangements of epithelial cells (4, 5). Epiboly in X. laevis is regulated by the intercalation of deep cells and thinning of epithelial cells (6). These movements governed by convergence and extension, which ultimately contribute to the closure of the yolk plug and neurulation, depend on many different signaling and adhesive systems that permit repositioning of epithelial sheets (4, 6). Although many players involved in these processes have been identified, there is still an incomplete understanding of the cell biological and biochemical processes that balance plasticity with structural integrity in epithelial sheets during morphogenesis (2, 7–14). Identification of new classes of molecules that regulate movement is critical for an understanding of early development and epithelial morphogenesis.
Bves is an integral membrane protein identified in avians and mammals (15–17). The Brand group subsequently cloned three genes and named them the popeye family (17). Bves, identified by T. Brand as Pop1a, is a product of the bves (pop1) gene, and Bves is the accepted name for the product of this gene (Mouse Genome Informatics, The Jackson Laboratory, HUGO Gene Nomenclature Committee, Bar Harbor, ME). The gene family has been renamed as the popdc family. Bves has a short extracellular N terminus with two conserved N-linked glycosylation sites, three transmembrane domains with two small intervening loops, and a long intracellular C terminus (17, 18). Although the sequence and structure of Bves are highly conserved between species, computer modeling detects no structural homology with other proteins. No functional motifs, outside the transmembrane domains, have been identified, and, thus, predictions of Bves function from proposed protein structure are speculative.
Bves traffics to points of cell/cell contact during early epithelial sheet formation in vitro (19–21). Transfection of chicken bves into L cells confers adhesive activity (19), whereas morpholino oligonucleotide knockdown disrupts epithelial sheet formation in vitro (21). In vivo expression studies detect the Bves in epithelia undergoing extensive remodeling during morphogenesis (16, 19, 21–23). Although Andree et al. (17, 24) have demonstrated that pop1-3 are highly expressed in cardiac myocytes, knockout of the pop1 gene in mice did not produce an embryonic phenotype, perhaps because of functional compensation by the other pop genes. Thus, no studies have demonstrated a function for any popdc family gene in vivo, particularly during early development.
Here we demonstrate the expression of X. laevis Bves (Xbves) in epithelia during the earliest phases of frog development and the conservation of its adhesive function. Global disruption of Xbves function arrests development at gastrulation with disrupted epithelial movement. Clonal analysis of Xbves knockdown coupled with lineage tracing demonstrates that Xbves-depleted cells lose normal patterns of movement during and after gastrulation and disperse randomly within normally developing embryos. Taken together, these studies demonstrate a fundamental role for Bves proteins in the regulation of amphibian gastrulation and in overall epithelial morphogenesis.
Results
Conservation of Protein Sequence, Adhesive Function, and Subcellular Localization of Xbves. Sequence analysis predicted a 338-aa protein termed Xbves that contained the canonical Bves/Pop structure, including a short N terminus with two invariant N-linked glycosylation sites, three hydrophobic domains separated two intervening loops, and a long C terminus (18). Xbves protein shared >90% amino acid similarity with mouse and chick Bves. Computational analyses did not identify known protein motifs or significant structural similarity to other proteins. To determine whether Xbves retained the adhesive function described for the chicken protein (19), Xbves cDNA was transfected into mouse L cells for cell adhesion analyses. As seen in Fig. 8A, which is published as supporting information on the PNAS web site, Xbves-transfected L cells readily adhered to each other, forming a statistically significant number of aggregates when compared to control cells (Fig. 8C, 66.83 ± 3.45 vs. 24.00 ± 4.18; P < 0.001 by Student's t test).
To determine whether Xbves traffics to points of cell/cell contact during epithelial sheet formation, X. laevis A6 kidney cells were plated a low density and allowed to form epithelial sheets. α-Xbves reactivity was observed at cell/cell boundaries and not at the free surface of these cells (Fig. 8D). In confluent sheets, Xbves was seen at the cell surface with a distribution overlapping that of E-cadherin at points of cell/cell contact (Fig. 9, which is published as supporting information on the PNAS web site).
Maternal and Early Stage Embryonic Expression of Xbves. The distribution of Xbves mRNA and protein during early embryogenesis was determined. Xbves transcripts were detected throughout the unfertilized egg (data not shown), consistent with EST analysis of oocyte libraries. The distribution of Xbves mRNA changed in the two- and four-cell embryos as hybridization was stronger in the animal region (Fig. 1 A–C). As cleavage continued, this region remained strongly positive (Fig. 1 D–F). During gastrulation (stages 10–12), all animal pole cells were positive for Xbves, and with the establishment of the blastopore, a clear distinction between surface cells and nonreactive yolk plug cells was apparent (Fig. 1 G and H). From stage 12.5 to 35, expression persisted in the dorsal regions of the embryo from the neural plate. Staining extended around the blastopore, neural plate, and somites but was reduced on the ventral surface (Fig. 1 I–N). Later, the posterior, ventral regions had reduced expression as Xbves became progressively restricted to the heart, somites, cement gland, and eyes by stage 35 (Fig. 1O). Because our data greatly varied from the previously published study (25), RT-PCR confirmed the presence of Xbves mRNA in the oocyte and cleavage, gastrulation, and neurulation stage embryos (Fig. 10A, which was published as supporting information on the PNAS web site). Whole-mount analyses were repeated by using three separate Xbves RNA probes, and the same general pattern of hybridization was observed (Fig. 10B).
Fig. 1.
Whole-mount in situ hybridization analysis of Xbves expression. A four-cell embryo is seen from the animal (A) and vegetal (B) poles and from the lateral side (C). A 16-cell embryo is seen from the animal (D) and vegetal (E) poles and from the lateral side (F). Xbves expression is observed in the surface epithelium in gastrulation stage embryos but is excluded from the yolk plug (G and H) and a more restrictive dorsal expression pattern in neurulation stage (I). Lateral view, dorsal view, and ventral view of embryos at stages 20 (J–L) and 25 (M and N) demonstrates Xbves distribution. Xbves expression in the heart (white arrow), eye (black arrow), cement gland, and somites of a stage 35 embryo (O). Control sense probe hybridization of four-cell (P), stage 11.5 (Q), and stage 35 embryos (R) are negative.
Immunohistochemistry showed that regions of protein distribution corresponded to those that express Xbves mRNA. For example, at stage 11.5, surface cells were strongly stained by α-Xbves, whereas yolk cells were unreactive (Fig. 2A; compare to Fig. 1 G and I). In addition, newly involuted cells were also positive, and higher magnification revealed that this reactivity was confided to regions of cell/cell contact and was absent from the apical surface of cells (Fig. 2 B and B′). At stage 19, protein expression persisted in the dorsal ectoderm/epidermis and the underlying neural tube and optic vesicle (Fig. 2 C and D). Other epithelial structures derived from mesoderm, such as the notochord and somites, were also positive for α-Xbves. Later, epithelial elements, such as the velar plate, pronephros, somatically derived striated muscle, eye, and heart, expressed Xbves (Fig. 11, which is published as supporting information on the PNAS web site).
Fig. 2.
Immunohistochemical analysis of Xbves expression in early embryos. (A) At stage 11.5, anti-Xbves (green) reacts with surface and involuted cells but is negative for endodermal cells of the yolk plug (YP). (B) High power view of region outlined by the box in A. Arrows show that Xbves is confined to regions of cell/cell contact and is missing from the apical regions of the cell. (B′) High power view of stage 8 embryos shows similar distribution of Xbves protein. (C) Anti-Xbves reacts with ectodermal and mesodermal tissues of the stage 19 embryo. The neural tube (NT), eye (E), and surface ectoderm (arrows) are specific epithelial structures labeled by this serum. The white box, magnified in C, shows staining of the notochord. (D) Xbves expression is detected at the cell surface of mesodermally derived notochord.
Disruption of Xbves Function Inhibits Epithelial Movements During Gastrulation but Not Mesoderm Formation.To determine whether Xbves regulates epithelial morphogenesis, Xbves morpholinos (MOs) were injected into both cells of the two-cell embryo. Xbves MO-injected embryos appeared to undergo normal cleavage (Fig. 3B) and are indistinguishable from control MO-injected and noninjected embryos until stage 9+. At stage 10, differences in yolk plug size and position of the advancing ectoderm suggested retardation or inhibition of the cell movements of epiboly. Xbves MO-injected embryos began to form a dorsal blastopore lip, but by stage 11, epithelial movement was greatly inhibited. Xbves MO-injected embryos had a large yolk plug, did not form a neural plate, and became necrotic thereafter (Fig. 3F). Control MO had no effect on gastrulation and neural plate formation (arrows, Fig. 3H).
Fig. 3.
Injection of Xbves morpholino into a two-cell X. laevis embryos inhibits gastrulation movements. (A, C, E, and G) Uninjected embryos serve as a control. Arrows indicate the blastopore (D) and the forming neural plate (E, G, and H). At stage 6, Xbves MO-injected embryos develop in an apparently normal fashion (B). By stage 11, experimental embryos are delayed or inhibited in yolk plug closure (D, arrow denotes the edge of the yolk plug), and by stage 14 (F), most embryos lack closed yolk plugs and have become necrotic. Control sense MO-injected embryos (H) complete gastrulation and undergo neurulation (neural plate, white arrows) at the same rate compared with uninjected embryos (G).
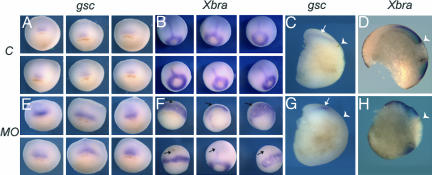
Mesoderm formation is critical for gastrulation but is independent of sustained epithelial movements necessary for development (26, 27). Thus, Xbves function was disrupted and expression of two mesodermal markers, goosecoid and Xbra (28, 29), was examined. At the onset of gastrulation, goosecoid and Xbra expression were similar in experimental and control embryos (Fig. 12B, which is published as supporting information on the PNAS web site). In controls, goosecoid-expressing cells were observed at stage 9/9.5 as a patch of cells near the dorsal lip, and bisection of stage 10.5 embryos showed that this expression shifted to the deep cell layers after involution (Fig. 4 A and C). Normal Xbra expression was characterized by transcription around the blastopore and in chordamesodermal cells moving anteriorly (arrows, Fig. 4B; refs. 30–32). Bisection of stage 12 control embryos demonstrated movements of involuting mesendoderm, formation of the archenteron, and clear definition of the yolk plug as indicated by Xbra expression (Fig. 4D).
Fig. 4.
In situ hybridization analysis of mesodermal markers goosecoid and xbra. Expression of goosecoid and xbra markers is unaffected by injection of embryos with control MO at stage 10.5 (A and B). (Six examples are given for each group.) Bisection of whole mount-stained embryos shows the diffuse subsurface distribution of goosecoid reactivity in gastrulated cells (C, arrow), whereas xbra-stained samples reveal the movement of mesendoderm away from the blastopore and the formation of the archenteron (D). In contrast, injection of Xbves MO leads to an abnormal accumulation of goosecoid staining immediately anterior to the dorsal lip of the blastopore (E) and the lack of xbra in midline mesendoderm (F, arrows). Note that goosecoid and xbra staining are restricted to the surface of the embryo in Xbves MO-treated samples (G, arrow, and H). The white arrowhead in C, D, G, and H denotes the position of the dorsal lip of the blastopore in all bisected embryos.
Beginning at stage 10/10.5, goosecoid hybridization of Xbves MO-injected embryos was more abundant in an area juxtaposed to the dorsal lip (Figs. 4E and 12). Bisection revealed that the intense staining was concentrated at the embryo surface (Fig. 4G). Xbra expression was observed in Xbves MO-injected embryos in the mesoderm around the blastopore at stage 9.5 (Fig. 12). However, at stage 10/10.5, Xbra staining in Xbves MO-injected embryos was broader and more irregular, and staining of the axial chordamesodermal rod was completely absent in the vast majority of the embryos (Figs. 4F and 12). Xbra-expressing cells in Xbves MO-injected animals remained at the surface, suggesting altered involution (Fig. 4H).
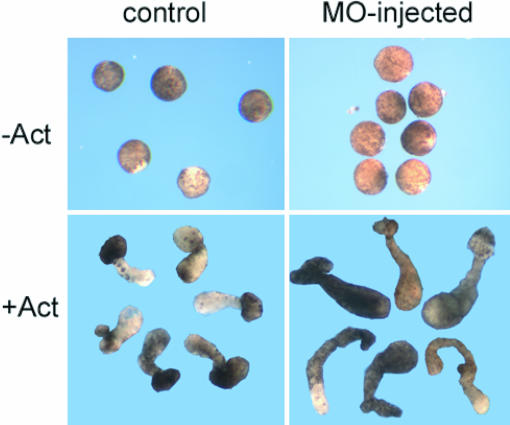
Animal cap assays are used to determine whether specific molecules influence gastrulation. Isolated embryo caps from control and Xbves MO-treated embryos were treated with Activin-A, a factor that induces convergence/extension causing an elongation of the dissected tissue. Control and Xbves MO embryos cultured in Activin-A exhibited epithelial movements and animal cap elongation, whereas control embryos did not elongate (Fig. 5). Interestingly, we observed a 178% greater elongation of Xbves MO-injected caps, suggesting that Xbves impacts gastrulation movements. The increase in extension is compatible with previous work showing that inhibition of Xbves function potentiates cell movement (discussed below).
Fig. 5.
Xbves depletion enhances extension of animal cap explants. Animal cap assays were performed as described in Materials and Methods. In the absence of Activin-A, no extension was observed in control or Xbves MO-injected samples. In the presence of Activin-A, control caps extended as predicted, whereas experimental caps exhibited even greater extension (P < 0.005; Student's t test) during the course of study.
RNA Rescue and Protein Knockdown. To verify that the phenotype observed with MO injection was specific to the knockdown of Xbves protein, we coinjected MO with Xbves rescue RNA and monitored embryos for restoration of development. Rescue RNA-injected embryos developed normally, proceeding through gastrulation to tailbud and tadpole stages with only minor tail malformations and a slightly arched dorsum (Fig. 13 G–L, which is published as supporting information on the PNAS web site). Next, we analyzed control MO- and Xbves MO-injected embryos for a reduction in Xbves protein expression by using immunofluorescence. Fig. 13 illustrates that control MO-injected embryos expressed Xbves protein at high levels and trafficked the protein to the cell surface (Fig. 13 M and N). In contrast, Xbves MO-injected embryos had significantly reduced levels of Xbves with little, if any, membrane staining (Fig. 13 O and P). Solid-state ELISA was also used to quantify the amount of Xbves protein depletion after MO treatment. As seen in Fig. 13Q, the relative activity of α-XBves was significantly reduced, >10-fold, in embryo extracts (P < 0.0073, ANOVA), whereas α-actin reactivity was unaffected (P > 0.05, ANOVA). It is interesting to note that Xbves knockdown also results in diminished levels of E-cadherin and occludin, both components of cell junctions (data not shown).
Progeny of Xbves-Depleted Blastomeres Display Rogue Cell Movements Within Developing Embryos.We next determined whether depletion of Xbves protein within a marked clone of cells would alter their movement relative to normally developing cells. The A1 blastomere of the 32-cell embryo was injected with control and experimental MO along with a nuclear-targeted lineage tracer.
Gross morphology of control and experimental groups was indistinguishable, but striking differences in the distribution of A1 progeny between control and experimentally injected cells were observed. In the controls (uninjected or control MO-injected), labeled A1 descendents formed a tightly compacted continuous strip of cells at stage 12. This strip was narrowest at the blastopore, where cells had begun involution and broadest near its anterior margin (Fig. 6 A and D; see also Fig. 14A, which is published as supporting information on the PNAS web site). This tightly packed array of cells was observed in 73% of control embryos (117 of 161; Fig. 6 A and D; see also Fig. 15, which is published as supporting information on the PNAS web site). In contrast, progeny of Xbves MO-injected A1 blastomeres had a completely different distribution. Most clones had a highly irregular shape and did not extend to the blastopore. Some clones were located on the pole opposite the blastopore and did not possess a discernable narrowed leading edge (Figs. 6 B and E and 14). Other Xbves MO-injected clones had a diffuse appearance with many interspersed nonlabeled cells (Fig. 6 C and F). Morphometric analysis of these clones determined that 73% (55 of 72 embryos with 5 ng of injected MO) and 82% (29 of 35 embryos with 10 ng of injected MO) had an irregular shape that did not extend to the blastopore (Figs. 6 and 15). The average widest point of 34 randomly selected Xbves MO-injected clones was 855 μm, compared to 585 μm in control embryos (P < 0.0001; Student's t test).
Fig. 6.
Injection of Xbves MO into the A1 blastomere alters movements of progeny during gastrulation. Three embryos were imaged to show the blastopore (A–C) and reimaged after a 90° rotation (D–F). Injection of control MO with lacZ tracer into the A1 blastomere demonstrates the convergence of A1 progeny toward the blastopore at stage 12 (A and D). Progeny of Xbves MO-injected A1 blastomeres do not follow normal paths of movement (B, C, E, and F). Cross-sections of these embryos demonstrate that progeny of the control MO-injected A1 blastomeres remain closely associated with the embryo surface (G), whereas derivatives of Xbves MO-injected cells are often detected below the surface (H, arrows). Fig. 14 shows additional examples.
Histological analysis of stage 12 control and experimental groups revealed that lacZ-labeled cells had differing distributions relative to the embryo surface. As seen in Fig. 6G, control-labeled cells were closely compacted near the outer surface of the blastocoel roof. In contrast, a substantial number of Xbves MO-injected cells were located in deeper layers of the animal cap (Fig. 6H, arrows). The location of labeled nuclei relative to the surface was measured in 20-μm increments. Also, as seen in Fig. 16, which is published as supporting information on the PNAS web site, the distribution of cells was such that Xbves MO-injected blastomere progeny was displaced from the surface.
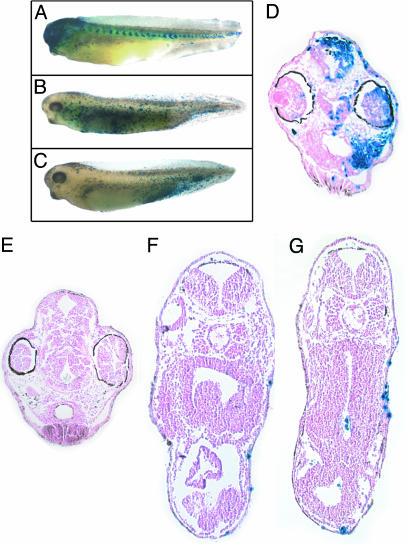
A1 progeny contribute to anteriorly located tissues in the head region, including the skin, connective tissue, and brain, as well as to anterior somites (33). To determine whether cell fate and location was altered by Xbves depletion, control and experimental embryos were allowed to develop to stage 37. Derivatives of lacZ/control MO-injected cells faithfully differentiated into predicted structures with little “scatter” of labeled cells (Fig. 7). Note that β-gal staining in the head totally obscured visualization of the eye and that somites were labeled (Fig. 7A). Sectioning of embryos demonstrated the extensive incorporation of lacZ-labeled cells in the eye, skin, brain, and connective tissue of the head, even in the deepest regions of the embryo. In contrast, the progeny of Xbves MO-injected blastomeres showed a wide and inconsistent range of distributions throughout the embryo. In most cases, β-gal-positive cells were dispersed without concentration in any particular structure and, notably, with no enrichment in the head or somites (Fig. 7 B and C). Additionally, a subset of embryos had labeled cells located exclusively in the caudal region, a position normally devoid of A1 progeny (compare to Fig. 7A). In sections, the β-gal-stained nuclei were scattered primarily in surface structures, including the epidermis and adjacent connective tissue space. Cells were almost always absent from deep structures such as the brain, spinal cord, and digestive system (Fig. 7 E–G). Importantly, viable cells were always observed in experimental animals, demonstrating that Xbves inactivation was not generally toxic to cells. These data suggest that inhibition of Xbves function resulted in loss of regulated cell movement and an apparently random distribution of progeny throughout the embryo.
Fig. 7.
Xbves MO injection randomized movement of A1 progeny. At stage 37, (A) A1 derivatives are observed in head structures and in somites of control-MO injected animals. (B and C) A1 progeny are randomly distributed throughout the embryo after Xbves MO injection. Note the absence of labeled cells from the head region with Xbves MO injection. (D) A cross section through the head region of control embryos reveals blue cells in various cranial structures and tissue types. (E) In sections through Xbves MO-injected embryos, few labeled, if any, cells are detected in the head region. (F and G) Trunk cross-sections show that most blue cells reside in the outer epidermis of the embryo.
Discussion
Expression and Adhesive Properties of Xbves. Previous data demonstrated Bves expression in epithelial structures of the postgastrulated embryo and in epithelial cell lines (15, 19–23). pop gene products are also highly expressed in developing and adult striated muscles (17, 22, 24, 34). Still, a comprehensive analysis of RNA and protein expression has not been reported. The current cDNA cloning, PCR, in situ, and immunochemical analyses detect Xbves mRNA and protein in the cleaving embryo. Xbves protein is detected in all, or nearly all, epithelia in the gastrulating embryo and is predominantly localized to the interface between epithelial cells. The broad pattern of Xbves expression in the early frog embryo is consistent with our previous studies that find this protein in epithelia undergoing significant shape changes during a myriad of morphogenetic processes in the embryo (19, 21, 22). Here, we also demonstrate conservation of Bves adhesive properties in Xbves. These data predict a broad role for Xbves in epithelial movements during gastrulation.
Inactivation of Xbves Function Inhibits Gastrulation Movements. To date, there are no functional studies of Bves proteins during development. Global MO knockdown of Xbves during early development leads to a general arrest of gastrulation characterized by the failure of epiboly, yolk plug closure, involution, and mesodermal patterning. Furthermore, by using a classic amphibian signaling system as a model, we demonstrate the convergence/extension movements of animal cap explants are altered and, in fact, are more robust after Xbves MO treatment. Although this finding is seemingly contradictory to the aforementioned data, this outcome was predicted by recently published work showing that Xbves depletion results in loss of tight junction integrity and an initially accelerated rate of epithelial movement in a wound-healing assay (21, 35). Thus, the potentiation of cap extension seen here due to Activin-A signaling may reflect the deregulation of cell adhesion at the junctional level. This finding, in turn, led us to postulate that cells with impaired Xbves function respond to Activin-A and/or extension signals with accelerated movement. Overall, our data suggest that the loss of proper cell–cell interactions within embryonic epithelia lead to misdirected morphogenic movements and arrest in development with either clonal or global Xbves knockdown. All of these anomalies have been previously attributed to defects in epithelial movement influenced by the essential roles of cell/cell and cell/matrix adhesion molecules in X. laevis gastrulation (2, 7, 13, 25, 36). Although these phenotypes vary, global inhibition of any of these molecules results in a cessation of epithelial movement and a failure to gastrulate. Thus, it is reasonable that inhibition of a regulator of epithelial adhesion, such as Xbves, may result in a similar phenotype.
Epithelial movement in X. laevis gastrulation is driven by convergence and extension (4, 6, 37, 38). Here, the convergence of epithelial cells along one axis results in the concomitant extension of cells along the perpendicular axis, resulting in the net movement or expansion of the surface cells over the yolk plug and the ingression of cells to form mesendoderm (4, 6, 13). This rearrangement of cells within the epithelial sheet is typified by the pyramidal shape of superficial epithelial cells labeled in control lacZ injections of the A1 blastomere (Fig. 6). When Xbves function is inhibited within the same population of cells, this precise rearrangement or reorganization of cells is severely disrupted, suggesting that the process of convergent/extension has been inhibited. Further, the displacement of Xbves MO-treated cells from the embryo surface indicates that these cells do not properly intercalate into the advancing epithelium. Taken together, these data suggest that Xbves is essential for the appropriate interaction and movement of neighboring epithelial cells during X. laevis gastrulation.
Xbves Plays a Role in Proper Cell Fate Decisions. Clonal disruption of Xbves function leads to the randomization of cell movement, that, in turn, may lead to alterations in cell differentiation. The production of mesendoderm in the frog depends on the involution of epithelial cells from the embryo surface (6, 32). In the absence of Xbves function in our clonal analysis studies, cells are apparently incapable of producing mesendoderm in significant amounts. We postulate that this result is due to the disruption of coordinated cell movement within epithelial sheets leaving the cells unable to reach deeper positions in the embryo. It is possible that Xbves-depleted morphant cells normally fated to mesendodermal lineages may regulate and assume an ectodermal phenotype or alternatively undergo apoptosis in the absence of proper differentiative signals. Although global inactivation of Xbves function is not incompatible with mesoderm production (Fig. 4), we cannot exclude the regulative capacity of morphant cells within a normally differentiating embryo. In either case, surviving cells appear to favor epidermal over neural fates within derivatives of the ectoderm. Furthermore, preliminary studies that examine the ultrastructural detail of Xbves-depleted embryos suggest that changes in cell shape/survival may be important factors in the outcome we observed (35). Thus, although its influence may be direct or indirect, proper Xbves function is critical for the morphogenetic movement, differentiation, and/or survival of cells fated to mesendodermal lineages.
Materials and Methods
Xenopus Methods. X. laevis eggs were harvested and fertilized by standard methods (39). Animal caps were dissected from devitellinated control or MO-injected embryos at stage 8.5–9 in Steinberg's solution with 0.01% BSA. Half of the embryos were placed in 10 ng/ml Activin A. Caps were cultured to stage 18–20 and scored for elongation.
cDNA Cloning and Antibodies. EST database searches revealed an expressed tag in a two-cell X. laevis library. This 500-bp cDNA was used as a probe to screen tailbud, dorsal lip, and oocyte libraries (gifts from Bruce Blumberg, University of California, Los Angeles). Thirteen independent cDNAs were cloned, aligned, and deposited in the National Center for Biotechnology Information (NCBI) database (AF 527799). These cDNAs were the only Xbves sequences isolated after screening 108 independent clones and were identical to all those reported in the NCBI database (September 2004) and by Hitz et al. (40). Only one Xbves transcript was identified, and no related family members were isolated. Still, our analyses are directed specifically toward the Xbves transcript and do not address possible function of related family members, should they exist. Two Xenopus peptide antisera designed to specifically recognize the Xbves protein (Ab1, CENWREIHHLVFHLANT; Ab2, KLYSLNDPTLGKKRKLDT) were generated in rabbits (Biosynthesis) and affinity purified (22). The antiserum used in this study does not react on a Western blot. α-E-cadherin was also used in these studies.
Cell Lines and Assays. A6 X. laevis kidney epithelial, CHO, and L6 cells were obtained from American Type Culture Collection (ATCC) and grown according to ATCC protocols. Mouse fibroblast L cells were stably transfected with control and full-length FLAG-tagged Xbves cloned into pCIneo vector (Promega), and adhesion assays were conducted according to Thoreson et al. (41). The ratio of cells in aggregates vs. the total cell number was tabulated and summarized by GraphPad (San Diego) prism 3.0. All values are reported as mean ± SEM.
RT-PCR. RNA preparation and RT-PCR analysis was performed according to Osler et al. (22) by using primer set 5′-ATGAAGGTGTCCTACCGAGGCCAT and 3′-CAGTGTGGGATCATTGAGCGAGTA (266-bp product) with an annealing temperature of 58°C and extension time of 1 minute. Reverse transcriptase was excluded from the negative control reactions.
MO Treatment and Rescue. A series of antisense MO were synthesized (Gene Tools, Carvalis, OR). The most efficacious MO in terms of eliminating protein synthesis in developing embryos was ATCTTTCTTATACCTGGATGTGCAG, which contains the reverse complement to the AT of the start codon and sequences immediately 5′. Control and experimental MO was dissolved in water and injected at 5 nl into blastomeres at varying concentrations of oligonucleotide. The sense complement had no influence on development up to 80 ng per blastomere. In one set of experiments, control or Xbves MO was injected into both cells of the two-cell embryo (40 ng per cell). An Xbves cDNA that lacks 5′ UTR (the first 17 bp of the MO) and begins with the start ATG codon was used to rescue MO treatment. mRNA was synthesized by mMESSAGE mMACHINE (Ambion, Austin, TX) and coinjected into both cells of the two-cell embryo (50 pg of rescue mRNA with 40 ng of MO). In a second set of experiments, 5 or 10 ng of control and Xbves MO plus 1 ng of lacZ tracer RNA were injected into the A1 blastomere of the 32-cell embryo. Animals were maintained for selected periods of time up to stage 35, and β-gal-expressing cells were identified (42). Whole-mount, lacZ-stained embryos were photographed and captured by Magnafire (National Institutes of Health).
Solid-State ELISA Protocol. Control and Xbves MO-injected embryos were collected at stage 12 and snap frozen. Thawed samples were then vortexed in Steinberg's solution, and proteins were collected by centrifugation. After one additional round of washing and centrifugation, proteins were extracted in 0.5 M NaCl with 0.5% Triton X-100 for 30 min on ice. After centrifugation, the supernatant was collected and the protein concentration determined. Fivefold serial dilutions of extracts beginning at 50 μg per well were applied to 96-well plates according to Gonzalez-Sanchez and Bader (43). Solid-state ELISA was conducted by using fixed amounts of primary [α-Bves (1:200) and α-actin (1:100)] and alkaline phosphatase-labeled goat anti-rabbit antibodies (1:5,000) by using standard methods (43). Four reactions were analyzed for each data point.
In Situ, Immunochemical, and Histological Analyses. Three separate Xbves probes (bp 70–670, 870-1210, and full-length 1–1738), Xbra (29), and goosecoid (28) were used for hybridization (44). To characterize α-Xbves antisera, A6 cells and CHO cells were transiently transfected with pCIneo/FLAG/Xbves (Fig. 9). Immunofluorescence analysis was standard (11, 22). Nuclear lacZ staining was conducted according to Sive et al. (45). For clonal analysis of A1 progeny, images of cross-sectioned stage 12 embryos were analyzed to determine the location of labeled nuclei relative to the embryo surface. Two hundred fifty or more randomly selected blue nuclei were counted for each group and were scored as 0–20, 20–40, 40–60, and >60 μm from the surface, and significance was determined by χ2 analysis.
Supplementary Material
Acknowledgments
We thank Kristin Price for her expert technical assistance; Shuji Takahashi, Yuki Ohi, and J. J. Westmoreland for helpful advice with Xenopus protocols and methodology; Angela Mattke, Hillary Hager, and Farideh Bowles for technical aid; and members of our laboratory for critical assessment of these studies. This work was supported by National Institutes of Health Grants P01 HL-76105, R01 HL-37675, R01GM-56238, and R03 EY-15211 and by American Heart Association Grant 0415184B.
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviation: MO, morpholino.
References
- 1.Keller, R. E. (1980) J. Embryol. Exp. Morphol. 60, 201–234. [PubMed] [Google Scholar]
- 2.Heasman, J., Crawford, A., Goldstone, K., Garner-Hamrick, P., Gumbiner, B., McCrea, P., Kintner, C., Noro, C. Y. & Wylie, C. (1994) Cell 79, 791–803. [DOI] [PubMed] [Google Scholar]
- 3.Nieuwkoop, P. D. (1973) Adv. Morphog. 10, 1–39. [DOI] [PubMed] [Google Scholar]
- 4.Wallingford, J. B., Fraser, S. E. & Harland, R. M. (2002) Dev. Cell 2, 695–706. [DOI] [PubMed] [Google Scholar]
- 5.Keller, R., Davidson, L. A. & Shook, D. R. (2003) Differentiation 71, 171–205. [DOI] [PubMed] [Google Scholar]
- 6.Keller, R. (1991) Methods Cell Biol. 36, 61–113. [DOI] [PubMed] [Google Scholar]
- 7.Brieher, W. M. & Gumbiner, B. M. (1994) J. Cell Biol. 126, 519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broders, F. & Thiery, J. P. (1995) Cell Adhes. Commun. 3, 419–440. [DOI] [PubMed] [Google Scholar]
- 9.Bradley, R. S., Espeseth, A. & Kintner, C. (1998) Curr. Biol. 8, 325–334. [DOI] [PubMed] [Google Scholar]
- 10.Cordenonsi, M., Mazzon, E., De Rigo, L., Baraldo, S., Meggio, F. & Citi, S. (1997) J. Cell Sci. 110, 3131–3139. [DOI] [PubMed] [Google Scholar]
- 11.Fagotto, F. & Gumbiner, B. M. (1994) Development (Cambridge, U.K.) 120, 3667–3679. [DOI] [PubMed] [Google Scholar]
- 12.Heasman, J., Kofron, M. & Wylie, C. (2000) Dev. Biol. 222, 124–134. [DOI] [PubMed] [Google Scholar]
- 13.Marsden, M. & DeSimone, D. W. (2001) Development (Cambridge, U.K.) 128, 3635–3647. [DOI] [PubMed] [Google Scholar]
- 14.Wallingford, J. B., Vogeli, K. M. & Harland, R. M. (2001) Int. J. Dev. Biol. 45, 225–227. [PubMed] [Google Scholar]
- 15.Reese, D. E., Zavaljevski, M., Streiff, N. L. & Bader, D. (1999) Dev. Biol. 209, 159–171. [DOI] [PubMed] [Google Scholar]
- 16.Reese, D. E. & Bader, D. M. (1999) Mamm. Genome 10, 913–915. [DOI] [PubMed] [Google Scholar]
- 17.Andree, B., Hillemann, T., Kessler-Icekson, G., Schmitt-John, T., Jockusch, H., Arnold, H. H. & Brand, T. (2000) Dev. Biol. 223, 371–382. [DOI] [PubMed] [Google Scholar]
- 18.Knight, R. F., Bader, D. M. & Backstrom, J. R. (2003) J. Biol. Chem. 278, 32872–32879. [DOI] [PubMed] [Google Scholar]
- 19.Wada, A. M., Reese, D. E. & Bader, D. M. (2001) Development (Cambridge, U.K.) 128, 2085–2093. [DOI] [PubMed] [Google Scholar]
- 20.Wada, A. M., Smith, T. K., Osler, M. E., Reese, D. E. & Bader, D. M. (2003) Circ. Res. 92, 525–531. [DOI] [PubMed] [Google Scholar]
- 21.Ripley, A. N., Chang, M. S. & Bader, D. M. (2004) Invest. Ophthalmol. Visual Sci. 45, 2475–2483. [DOI] [PubMed] [Google Scholar]
- 22.Osler, M. E. & Bader, D. M. (2004) Dev. Dyn. 229, 658–667. [DOI] [PubMed] [Google Scholar]
- 23.Vasavada, T. K., DiAngelo, J. R. & Duncan, M. K. (2004) J. Histochem. Cytochem. 52, 371–378. [DOI] [PubMed] [Google Scholar]
- 24.Andree, B., Fleige, A., Arnold, H. H. & Brand, T. (2002) Mol. Cell. Biol. 22, 1504–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hitz, M. P., Pandur, P., Brand, T. & Kuhl, M. (2002) Mech. Dev. 115, 123–126. [DOI] [PubMed] [Google Scholar]
- 26.Niehrs, C., Keller, R., Cho, K. W. & De Robertis, E. M. (1993) Cell 72, 491–503. [DOI] [PubMed] [Google Scholar]
- 27.Vodicka, M. A. & Gerhart, J. C. (1995) Development (Cambridge, U.K.) 121, 3505–3518. [DOI] [PubMed] [Google Scholar]
- 28.Cho, K. W., Blumberg, B., Steinbeisser, H. & De Robertis, E. M. (1991) Cell 67, 1111–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith, J. C., Price, B. M., Green, J. B., Weigel, D. & Herrmann, B. G. (1991) Cell 67, 79–87. [DOI] [PubMed] [Google Scholar]
- 30.Cunliffe, V. & Smith, J. C. (1992) Nature 358, 427–430. [DOI] [PubMed] [Google Scholar]
- 31.Green, J. B., New, H. V. & Smith, J. C. (1992) Cell 71, 731–739. [DOI] [PubMed] [Google Scholar]
- 32.Kwan, K. M. & Kirschner, M. W. (2003) Development (Cambridge, U.K.) 130, 1961–1972. [DOI] [PubMed] [Google Scholar]
- 33.Moody, S. A. (1987) Dev. Biol. 122, 300–319. [DOI] [PubMed] [Google Scholar]
- 34.DiAngelo, J. R., Vasavada, T. K., Cain, W. & Duncan, M. K. (2001) Hybrid. Hybridomics 20, 377–381. [DOI] [PubMed] [Google Scholar]
- 35.Osler, M. E., Chang, M. S. & Bader, D. M. (2005) J. Cell Sci. 118, 4667–4678. [DOI] [PubMed] [Google Scholar]
- 36.Levine, E., Lee, C. H., Kintner, C. & Gumbiner, B. M. (1994) Development (Cambridge, U.K.) 120, 901–909. [DOI] [PubMed] [Google Scholar]
- 37.Heasman, J., Ginsberg, D., Geiger, B., Goldstone, K., Pratt, T., Yoshida-Noro, C. & Wylie, C. (1994) Development (Cambridge, U.K.) 120, 49–57. [DOI] [PubMed] [Google Scholar]
- 38.Keller, R. & Danilchik, M. (1988) Development (Cambridge, U.K.) 103, 193–209. [DOI] [PubMed] [Google Scholar]
- 39.Wallingford, J. B., Ewald, A. J., Harland, R. M. & Fraser, S. E. (2001) Curr. Biol. 11, 652–661. [DOI] [PubMed] [Google Scholar]
- 40.Danilchick, M., Peng, H. B. & Kay, B. K. (1991) Methods Cell Biol. 36, 679–681. [PubMed] [Google Scholar]
- 41.Thoreson, M. A., Anastasiadis, P. Z., Daniel, J. M., Ireton, R. C., Wheelock, M. J., Johnson, K. R., Hummingbird, D. K. & Reynolds, A. B. (2000) J. Cell Biol. 148, 189–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morasso, M. I., Sargent, T. D. & Jamrich, M. (1994) Mech. Dev. 46, 63–70. [DOI] [PubMed] [Google Scholar]
- 43.Gonzalez-Sanchez, A. & Bader, D. (1984) Dev. Biol. 103, 151–158. [DOI] [PubMed] [Google Scholar]
- 44.Harland, R. M. (1991) Methods Cell Biol. 36, 685–695. [DOI] [PubMed] [Google Scholar]
- 45.Sive, H. L., Grainger, R. M. & Harland, R. M. (2000) Early Development of Xenopus laevis: A Laboratory Manual (Cold Spring Harbor Lab. Press, Plainview, NY).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.