Abstract
Toxoplasmosis is a healthcare problem in pregnant women and immunocompromised patients. Like humans, rats usually develop a subclinical chronic infection. LEW rats exhibit total resistance to Toxoplasma gondii infection, which is expressed in a dominant mode. A genome-wide search carried out in a cohort of F2 progeny of susceptible BN and resistant LEW rats led to identify on chromosome 10 a major locus of control, which we called Toxo1. Using reciprocal BN and LEW lines congenic for chromosome 10 genomic regions from the other strain, Toxo1 was found to govern the issue of T. gondii infection whatever the remaining genome. Analyzes of rats characterized by genomic recombination within Toxo1, reduced the interval down to a 1.7-cM region syntenic to human 17p13. In vitro studies showed that the Toxo1-mediated refractoriness to T. gondii infection is associated with the ability of the macrophage to impede the proliferation of the parasite within the parasitophorous vacuole. In contrast, proliferation was observed in fibroblasts whatever the genomic origin of Toxo1. Furthermore, ex vivo studies indicate that macrophage controls parasitic infection spreading by a Toxo1-mediated mechanism. This forward genetics approach should ultimately unravel a major pathway of innate resistance to toxoplasmosis and possibly to other apicomplexan parasitic diseases.
The protozoan Toxoplasma gondii is an obligate intracellular parasite that infects humans and a broad spectrum of vertebrate hosts. It is found worldwide, and the infection is common as indicated by a high prevalence of specific Ab among almost all human populations. T. gondii infection occurs by oral ingestion of either cysts from infected animal tissues, or oocysts excreted by cats. In healthy individuals, T. gondii establishes a chronic asymptomatic infection characterized by a specific immune response and the encystment of dormant bradyzoites into host tissues. A serious threat to human health can occur under congenital infection or reactivation of a latent infection in immunodeficient patients (1).
Epidemiological studies have indicated that the genetic make-up of the host and of the parasite are involved in the phenotypic expression of toxoplasmosis (2–4). Genetic studies in humans are hampered by both population heterogeneity and environment variability. In experimental conditions, genetic and environmental factors are under control. Results from genetic studies in animal models can be applied to human pathology through comparative genomics (5, 6). Rats, like humans, usually develop subclinical toxoplasmosis (7); this contrasts with the severity of the disease developed in most strains of mice. Surprisingly, the LEW rat strain exhibits a complete resistance to Toxoplasma infection (8). Indeed, unlike susceptible BN and F344 rats, LEW rats do not show trace of parasitic infection as shown by negative serology and lack of brain cysts. F1 hybrid (LEW × BN) and (LEW × F344) rats are resistant to T. gondii, indicating a dominant effect of the involved gene(s) (9). We carried out genetic studies in LEW and BN rats, which are two extreme strains from an immunological point of view (10). Here, we demonstrate that a single chromosomal region of 1.7 cM on chromosome 10 (c10), fully controls the refractoriness of the LEW rat to T. gondii infection. In vitro and ex vivo studies showed that the Toxo1-mediated refractoriness of the LEW rat to T. gondii infection is associated with the ability of macrophages, to impede the proliferation of the parasite within the parasitophorous vacuole and to control the spreading of the parasitic infection.
Results
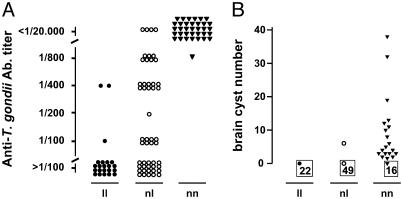
Linkage Analysis in (LEW × BN) F2 Rats Identifies a Locus on Chromosome 10 That Controls T. gondii Infection. 107 (LEW × BN) F2 rats were infected and studied. Anti-T. gondii Ab response was observed in 68 rats; it was high in 38 rats (titer ≤1/20,000). Brain cysts were detected in 20 rats. The sex of the rats had no effect on these traits. Linkage analysis identified a single significant (11) locus spanning a 12-cM interval between D10Arb4 and D10Wox24 on c10, we named Toxo1 (Table 3, which is published as supporting information on the PNAS web site). Segregation of anti-T. gondii Ab titers and of brain cyst numbers according to the genotypes of F2 rats at D10Rat116 are shown in Fig. 1. All rats sharing the two LEW alleles (ll) showed no detectable or a weak anti-T. gondii Ab response. Conversely, all rats sharing the two BN alleles (nn) but one had high anti-T. gondii Ab titers (≤1/20,000). An intermediate response was observed in the 50 heterozygous rats (nl). The differences between the groups were significant, thus indicating an additive effect of the gene(s) product(s) on anti-T. gondii Ab production. Brain cysts were observed in 19 of the 35 homozygous BN (nn) rats, in only one rat heterozygous BN/LEW (nl), and in none of the 22 homozygous LEW (ll). These results indicate that Toxo1 plays a major role in the control of T. gondii infection with a dominant or additive mode of action.
Fig. 1.
Susceptibility to T. gondii infection in (LEW × BN) F2 rats according to their genotype at the D10Rat116 microsatellite marker. A total of 107 rats were studied, of which 22 were homozygous LEW (ll), 50 were heterozygous BN/LEW (nl), and 35 were homozygous BN (nn). (A) Anti-T. gondii Ab titers measured by immunofluorescence. Titers represent the last dilution of the serum at which positive results were observed. Titers >1/100 were considered as negative; titers ≤1/20.000 as strongly positive. ll vs. nl, nl vs. nn, nn vs. ll: P < 0.001. (B) Number of brain cysts. Framed numbers represent the rats that develop no brain cyst. ll vs. nn and nl vs. nn, P < 0.001.
Studies in Reciprocal LEW/BN Congenic Lines Demonstrate That Toxo1 Fully Controls the Outcome of T. gondii Infection. Twelve reciprocal LEW/BN congenic lines, of which nine had a recombination within Toxo1 or at its boundaries, were studied to confirm and refine the localization of Toxo1 (Fig. 3, which is published as supporting information on the PNAS web site). Results of genetic dissection of the traits are shown in Table 1. Rats from five LEW.BNc10 congenic lines (lineages B, C, F, G, and I) became susceptible to infection. Conversely, rats from three BN.LEWc10 congenic lines (lineages C, E, and J) became refractory. In the other congenic lines, the introgression of genomic segments from the other strain had no effect on the outcome of infection, compared to the parental strain. These results confirm the presence of Toxo1 on c10 and pinpoint its localization to an interval of 4.4 cM between D10Rat116 and D10Wox24. Most importantly, these results demonstrate that Toxo1 fully controls the outcome of T. gondii infection. Indeed, infection is hindered by the Toxo1 LEW allele, even when the rest of the genome belongs to the susceptible BN strain. Conversely, infection does occur when animals are homozygous for the Toxo1 allele of BN origin, even when the rest of their genome belongs to the refractory LEW strain.
Table 1. Genetic dissection of T. gondii infection phenotypes in congenic lines.
| Microsatellite markers
|
Phenotypes
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Congenic lines | D10Mco17 cM¶ | D10Arb4 0.6 | D10Mco14 0.2 | D10Rat32 4.4 | D10Rat116 3.0 | D10Mgh7 0.7 | D1Rat297* 0.4 | D10Rat80 0.6 | D10Mit2 2.5 | D10Wox24 0.2 | D10Rat133 0.6 | D10Rat58 0.6 | D10Rat221 0.6 | Anti-T. gondii† | Brain cysts‡ | n§ |
| LEW.BNc10-D | II∥ | II | II | II | II | II | II | II | II | II | II | II | II | >1/500 | - (0) | 4 |
| LEW.BNc10-H | nn∥ | nn | nn | II | II | II | II | II | II | II | II | II | II | >1/500 | - (0) | 3 |
| LEW.BNc10-B | nn | nn | nn | nn | nn | nn | nn | nn | nn | nn | nn | nn | nn | ≤1/20,000 | + (2) | 3 |
| LEW.BNc10-C | II | nn | nn | nn | nn | nn | nn | nn | nn | nn | nn | II | II | ≤1/20,000 | + (3) | 4 |
| LEW.BNc10-F | II | II | II | II | II | nn | nn | nn | nn | nn | nn | nn | nn | ≤1/20,000 | + (4) | 4 |
| LEW.BNc10-G | II | II | II | nn | nn | nn | nn | nn | nn | nn | nn | II | II | ≤1/20,000 | + (3) | 4 |
| LEW.BNc10-I | nn | nn | nn | nn | nn | nn | nn | nn | nn | nn | nn | II | II | ≤1/20,000 | + (3) | 3 |
| BN.LEWc10-A | nn | nn | nn | nn | nn | nn | nn | nn | nn | nn | nn | nn | nn | ≤1/20,000 | + (3) | 5 |
| BN.LEWc10-F | II | II | II | nn | nn | nn | nn | nn | nn | nn | nn | nn | nn | ≤1/20,000 | + (2) | 5 |
| BN.LEWc10-C | nn | nn | nn | II | II | II | II | II | II | II | II | II | II | >1/500 | - (0) | 5 |
| BN.LEWc10-E | II | II | II | II | II | II | II | II | nn | nn | nn | nn | nn | >1/500 | - (0) | 5 |
| BN.LEWc10-J | nn | nn | nn | II | II | II | II | II | II | II | II | nn | nn | >1/500 | - (0) | 5 |
| Toxo1** | | | *** | *** | *** | | | |||||||||||
This marker, described as D1Rat297, is located on c10.
Anti-T. gondii Ab titers quantitated by immunofluorescence; all rats of the group showed the indicated value.
Presence (+) or absence (-) of brain cysts; brackets: number of rats showing brain cysts.
Number of tested rats. Rats were infected with 20 cysts of the Pru-β-gal strain.
Distance in cM (Kosambi units) between two adjacent markers
Genotypes: nn, II: homozygous BN (bold) or LEW.
Markers belonging to (***), and boundary markers of Toxo1 (|).
Studies in F2 (LEW × BN) and Congenic Rats Harboring Recombination Within Toxo1 Narrow Down the Localization of the Gene(s) to a 1.7-cM Interval. The fact that the gene(s) carried by Toxo1 control(s) the outcome of T. gondii infection independently of the genetic background, made it possible to refine further its localization in F2 (LEW × BN) rats and in progenies of rats issued from backcrosses of the BN.LEWc10-C line that showed recombination within the locus. Rats recombining within the locus were not informative when recombination converted the homozygous LEW (ll) genotype to a heterozygous LEW/BN (nl) genotype or vice versa. As expected, all of these rats (n = 7) showed phenotypes of resistance to T. gondii infection (data not shown). By contrast, recombination converting a homozygous BN genotype (nn) associated with susceptibility to a heterozygous BN/LEW genotype (nl) associated with resistance were informative. As shown in Table 2, the susceptibility of the F2 rats 1, 9, and 106 and the resistance of the F2 rats 5 and 76 and of the BN.LEWc10-Cb and -Cc rats confirmed and refined the localization of Toxo1. The resistance of the BN.LEWc10-Cc rats with a recombination from nn to nl genotype between D10Rat116 and D10Mgh7 confirms D10Rat116 as the centromeric boundary of Toxo1. The susceptibility to T. gondii infection of the F2 rat 9 that carries an nl genotype for D10Rat80 allowed the exclusion of the D10Rat80 marker from the locus. Thus, Toxo1 was narrowed down to a 1.7-cM interval between D10Rat116 and D10Rat80, which expands to 7.6 megabases in the q24 region of c10 rat (www.ensembl.org). This region contains 86 identified or putative rat genes, of which 49 have orthologous counterparts in human (17p13.2–13.1) and/or mouse (11B3-B4) genomes and 25 are olfactory receptor genes (Table 4, which is published as supporting information on the PNAS web site). A three-way comparative map (human–rat–mouse) reveals conservation of gene order and of distances between genes, with the notable exception of a chromosomal inversion of ≈2.1 megabases between TEKT1 and ALOX15 in the human 17p13, as compared to the rat and mouse conserved syntenic regions (Fig. 4, which is published as supporting information on the PNAS web site).
Table 2. Genetic dissection of T. gondii infection phenotypes in F2 (LEW × BN) and congenic rats recombinant within Toxo1.
| Microsatellite markers
|
Phenotypes
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| D10Rat32 cM* | D10Rat31 1.3 | D10Rat116 1.7 | D10Mgh7 0.7 | D1Rat297* 0.4 | D10Rat80 0.6 | D10Mit2 2.5 | Anti- T. gondii* | Brain cysts† | |
| F2 rat‡ | |||||||||
| 1 | nn¶ | nn | nn | nn | nn | nn | nl | ≤1/20,000 | 0 |
| 9 | nn | nn | nn | nn | nn | nl | nl | ≤1/20,000 | 11 |
| 106 | nn | nn | nn | nn | nn | nn | nl | ≤1/20,000 | 8 |
| 5 | nl | nl | nl | nl | nl | nl | nn | >1/100 | 0 |
| 76 | nl | nl | nl | nl | nl | nl | nn | >1/100 | 0 |
| BN.LEWc10-Cb§ | nn | nn | nl | nl | nl | nl | nl | >1/500 | 0 |
| BN.LEWc10-Cc§ | nn | nn | nn | nl | nl | nl | nl | >1/500 | 0 |
| Toxo 1* | | | *** | *** | | | |||||
See Table 1 for description.
Number of brain cysts
Five F2 (LEW × BN) rats characterized by informative points of recombination within Toxo 1 as defined in Table 1. Rats were ordered according to their genotypes.
Recombinant rats bred from (BN.LEWc10-C × BN) backcrosses characterized by points of recombination within Toxo 1; BN.LEWc10-Cb: n = 5; BN.LEWc10-Cc: n = 4.
Genotypes: nn, II: homozygous BN (bold) or LEW; nl: heterozygous BN/LEW.
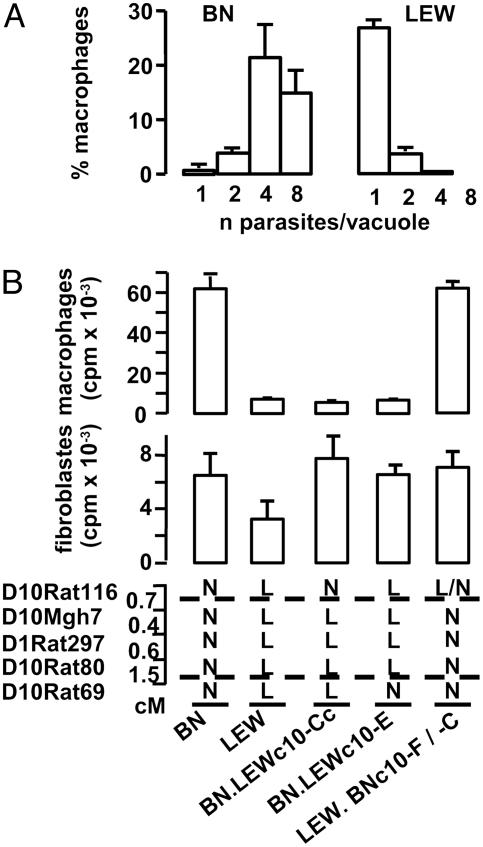
Toxo1 Controls the Proliferation of Toxoplasma Within Macrophages but Not Within Fibroblasts. Previous experiments have shown that the LEW refractoriness to T. gondii infection is mediated by hematopoietic cells (9). The role played by MΦ in the immune defense against T. gondii (12) and its possible involvement in the parasite spreading during the early stages of infection (13) led us to investigate both penetration and proliferation of the parasite in rat MΦ in vitro. When parasites were incubated for 2.5 min with naive MΦ, the average number of parasites that had invaded 100 MΦ were similar in BN and in LEW preparations (BN: 18.2 ± 1.4; LEW: 18.0 ± 1.0; three independent experiments). The parasite-containing vacuoles were found to be GRA1 positive and negative for the lysotraker dye, indicating that, in both cases, efficient active invasion and parasitophorous vacuole formation are taking place. After 1 h of contact, infected MΦ were washed to remove extracellular Toxoplasma and cultured for 20 h to study intracellular proliferation of the parasite. A dramatic difference in the fate of Toxoplasma was observed. Within BN MΦ, the majority of vacuoles contained four or eight parasites, whereas within LEW MΦ, the majority of vacuoles contained a single parasite (Fig. 2A). In both cases, the parasite-containing vacuoles were GRA1 positive. We further investigated whether these differences were under Toxo1 control, and whether they were specific of the MΦ population. For this purpose, MΦ and fibroblast monolayers from BN, LEW, and congenic lines were infected with tachyzoites from the RH strain and their intracellular growth was measured by monitoring [3H]uracil incorporation. Results are shown in Fig. 2B. Within LEW MΦ, a 90% inhibition of the parasite growth rate was observed compared to their proliferation within BN MΦ. Results from MΦ prepared from congenic lines indicated that this inhibition is directed by Toxo1. Indeed, in the two BN.LEWc10-Cc and -E lines, the Toxo1 LEW genome is responsible for an inhibition of the parasite proliferation identical to that of the resistant LEW parental strain. Conversely, in the LEW.BNc10-F lines, the Toxo1 BN genome is responsible for the restoration of the ability of the parasite to proliferate efficiently in MΦ as in the susceptible BN parental strain. In contrast, parasite proliferation was observed in all fibroblast preparations (Fig. 2B) as well as in fibroblast cell lines from BN and LEW origin (data not shown), thus indicating that the Toxo1-mediated inhibition of the parasite proliferation in MΦ was not a general phenomenon.
Fig. 2.
Toxo1 controls the proliferation of T. gondii within the macrophages. (A) BN or LEW MΦ were mixed with T. gondii for 1 h, washed, and cultured for 20 h. The figure represents the repartition of infected MΦ according to the number of parasites per parasitophorous vacuole. The columns and the bars show the mean result and the standard deviation of three independent experiments. (B) The intracellular growth of T. gondii on macrophage and fibroblast monolayers from BN, LEW, and congenic lines (BN.LEWc10-E, BN.LEWc10-Cc, LEW.BNc10-F, LEW.BNc10-C) was measured by monitoring [3H]uracil incorporation into T. gondii RNA. From the two different LEW.BNc10 lines of the same BN genotype at Toxo1, the -F line was used for macrophage studies and the -C line was used for fibroblast studies. The columns and the bars show the mean result and the standard deviation of triplicates in one rat. These results are representative of two (fibroblasts) and three (macrophages) independent experiments. As a whole, studies on macrophages were performed on six BN, four LEW, and four rats of each congenic line with similar results. Dotted lines indicate the limits of Toxo1 (boundary markers: D10Rat116 and D10Rat80). N, homozygous BN; L, homozygous LEW.
Toxo1 Controls Local Spreading of the Parasite After i.p. Infection. The observed Toxo1-dependent dramatic effect on parasite proliferation within MΦ in vitro, does not per se explain the total inhibition of the spreading observed in the resistant rat in vivo. The effect of Toxo1 on the parasite spreading was investigated by inoculating 5 × 108 YFP2 tachyzoites i.p. to LEW, BN, and congenic lines. One hour later, the peritoneal fluid of each infected rat was collected, and the majority of parasites (≈1.6 × 107) were found in extracellular position. No striking difference was found in their viability. They were then submitted to an in vitro invasion assay onto fibroblasts. A 98% inhibition of the invasion rate was observed for parasites collected from LEW peritoneal exudate as compared to the invasion rate determined for parasites collected from BN exudate. In the LEW.BNc10-F line, a 52% restoration of the ability of the parasite to invade efficiently fibroblasts compared to the susceptible BN parental strain was observed, indicating that this effect is under the control of Toxo1 (Fig. 5, which is published as supporting information on the PNAS web site).
Discussion
Genetic studies on the susceptibility of rodents to toxoplasmosis have been confined to the mouse. Survival of mice and number of cysts were found to be regulated by several loci (14, 15). These studies yielded valuable information on toxoplasmosis pathophysiology. However, in the mouse, infection often leads to an acute lethal disease. With respect to the clinical course and in utero transmission, the rat mimics more closely the human situation (7). In contrast to BN or F344 rats, LEW rats are refractory to T. gondii infection (8). This feature is not parasite strain-specific because it has been observed after oral infection with three different strains (Prugniaud, NED, CT1) and after i.p. infection with one additional strain (RH) (8). Using this unique rodent model, we found that a single locus, which we named Toxo1, directs toxoplasmosis outcome. In addition, we demonstrated that Toxo1 controls both proliferation of the parasite within its parasitophorous vacuole and spreading by MΦ-dependent mechanisms.
Hosts usually contain dissemination of the parasite and are protected from symptomatic toxoplasmosis via a vigorous immune response (13). Parasites become encysted and persist in host tissues as dormant bradyzoites, as observed in the F2 rats bearing two BN alleles at Toxo1 that develop a highly specific Ab response and brain cysts. The fact that not all rats show cysts in their brain is probably related to the relative resistance of BN rats to encystment (8), a trait likely to be under polygenic control (14, 15). In contrast, F2 rats homozygous for the LEW allele of Toxo1 show the characteristic LEW refractory state. Quite surprisingly, this locus alone confers a complete resistance to T. gondii infection, regardless of the remaining genome. The F2 BN/LEW rats heterozygous at Toxo1 showed intermediary Ab responses, and all but one were all free of brain cysts. Therefore, there is an additive effect of the gene products on the anti-T. gondii Ab response, and the resistance of LEW rats to brain cyst formation follows a dominant expression pattern.
Host genetic factors play a major role in the outcome of infections in humans. In most instances, the susceptibility to pathogens is likely to be controlled by several polymorphic genes (16). Instances of natural mutations of single germ-line genes associated with susceptibility or resistance to infectious diseases have been reported (17). In humans, the association of resistance to Plasmodium vivax with DARC gene mutations (18) and to HIV infection with CCR5 gene mutations (19) are examples of such a mechanism of simple inheritance. In the mouse, the Nramp1 gene was identified by forward genetics as being responsible for the resistance to infections by intracellular pathogens (20). The identification of Toxo1 provides a unique opportunity for insight in toxoplasmosis pathophysiology.
The mechanism of resistance of the LEW rat to toxoplasmosis works very efficiently, soon after infection. Indeed, from day 2 to 5 after infection, parasites cannot be detected in the LEW rat, neither locally at the site of infection nor in both draining lymph nodes and blood (9). Moreover, LEW rats and most of the F2 rats bearing two LEW alleles at Toxo1 barely develop an immune response. Thus, in the LEW rat, the parasite is rapidly cleared by an highly efficient host barrier. Refractoriness of LEW rat to toxoplasmosis is mediated by hematopoietic cells and occurs after i.p. as well as after oral infection (9). Moreover, MΦ plays a pivotal role in the immune defense against T. gondii (12) and is possibly involved in the spreading of parasites during the early stages of infection (13). Therefore, we investigated the fate of the parasite in the presence of rat peritoneal MΦ in both in vitro and ex vivo experiments. First, we found that upon primary contact, T. gondii can invade MΦ as well as fibroblasts from both LEW and BN rats. Therefore, as predicted from the dominant mode of control, refractoriness is not due to the lack of a host receptor required for parasitic invasion. Second, in an ex vivo experiment, we showed that within the first hour after infection, Toxo1 from LEW completely abolished the invasive capabilities of resident extracellular parasites. Therefore, it seems that, shortly after primary invasion, Toxo1 controls the parasite spreading in directing subsequent ability to invade neighboring cells. Although these effects remain to be determined at the molecular and biochemical levels, they show that the overall function of Toxo1 involves a multistep process. Third, we demonstrated that, after penetration, Toxo1 from LEW inhibits intravacuolar replication within the MΦ, but not within the fibroblast. This observation indicates that Toxo1-mediated effects are not a general phenomenon but rather are likely to operate specifically in hematopoietic cells such as MΦ. The extent to which other cells of the innate immunity are implicated in the phenotype of resistance remains to be determined. Particularly, the role of dendritic cells, which are likely to be involved in the dissemination of parasitic infection (21) has to be investigated.
T. gondii is a protozoan parasite that has adopted an intracellular lifestyle for eluding host defense mechanisms (22). Invasion of T. gondii occurs by active penetration of cells (23), using a system of adhesion-based motility called “gliding” that characterizes parasites of the apicomplexan phylum (24). In MΦ as in other host cells, invasion results in the formation of a unique parasite-replicating compartment, the parasitophorous vacuole that avoids the phagolysosomal pathway (25–28). Here, we showed that proliferation of the parasite within its parasitophorous vacuole depends on a Toxo1-mediated host response. Replication proceeds in each mitotic cycle every 7–10 h until the host cell is lysed (≈48 h after infection). Therefore, the first cycle of replication can be blocked by some Toxo1-mediated response in MΦ activated by the parasite invasion. T. gondii infections are known to modulate reactive oxygen (29) and nitric oxide (NO) pathways (30) to ensure parasite survival within MΦ (31). We did not observe a reversion of the phenotype using inhibitors of the NO synthase, but did not investigate other oxygen-dependent pathways. Because, in LEW MΦ, parasites are still detected within the vacuole after 20 h of culture, a direct killing of the parasite soon after invasion is unlikely. Therefore, we suggest that the microbiostatic effect is due to a lack of parasitophorous vacuole expansion and/or maturation. Future studies should address the role of Toxo1 in controlling processing of the parasitophorous vacuole. Moreover, it is possible that, in vivo, the entire process of parasite eradication involves a complex multistep mechanism that could explain the partial effect of the anti-IFN-γ Ab treatment observed in a previous study (9).
According to the rat genome sequence draft, the identified 1.7-cM interval extends 7.6 megabases in the q24 region of rat c10. This conserved syntenic region contains 86 identified or putative rat genes. Among these genes, several are likely to be expressed in hematopoietic cells. In our model, at least one gene controlling host–parasite interaction at the level of the parasitophorous vacuole is involved. Among the genes that are known to be present in Toxo1, there is no obvious candidate gene. However, one cannot exclude the involvement of several genes within the locus as observed in other murine models (32, 33). Another gene(s) involved in the host immune response could well be implicated. Genes that code for arachidonate lipoxygenases, such as Alox15, which are present in Toxo1, are candidates for such a mechanism (13). Further genetic dissection using rats with recombinations within Toxo1 is needed to further narrow down this region and to identify the involved gene(s).
In conclusion, the forward genetics approach supported by our model should unravel a key pathophysiological pathway at work in T. gondii infection. This knowledge could be used in the therapeutics and prophylactics in human (1) and veterinary medicine (34). Pathogenesis associated with apicomplexan parasitic diseases is mainly due to tissue damage induced by uncontrolled parasite proliferation. Particularly, Toxoplasma encephalitis is due to parasite-mediated destruction of neuronal tissue, and malarial fevers and anemia are a consequence of red blood cell lysis. Thus, the identification of Toxo1 gene(s) and of the mechanisms used to control the replication of these parasites within the parasitophorous vacuole could be useful for understanding other apicomplexan parasitic diseases. It should also unravel mechanisms that determine host-species barrier in this important group of parasites.
Materials and Methods
Rats. Breeding and experimental procedures were carried out in accordance with European guidelines and approved by our local ethical committee. F1 (LEW × BN) male and female rats were obtained from Janvier Laboratory (Le Genest-Saint-Isle, France). F2 (LEW × BN) progeny was produced in our animal facilities (specific pathogen-free conditions). Congenic lines were produced according to the “speed congenics” procedure (35), for BN c10 alleles onto the LEW genome (LEW.BNc10 lines) and reciprocally (BN.LEWc10 lines). Rats were selected for introgression of the entire Toxo1 locus and for recombination within the locus. When the rats showed homozygosity for the set of markers used to screen the genetic background (eighth and ninth backcrosses), the homozygous state for the introgressed chromosomal regions was fixed by mating two heterozygous rats and selecting appropriate progenies. Congenic lines were maintained by brother–sister mating.
Genetic Markers and Genotype Analysis. Preparation of genomic DNA and genotyping were performed as described (36). For the initial genome screen, 109 polymorphic microsatellite markers were selected (http://rgd.mcw.edu) to cover the rat genetic map at ≈99% with an average spacing of ≈17 cM between markers. Linkage Kosambi maps were constructed in a previous study (36). After the initial linkage analysis, 11 c10 additional markers between D10Wox26 and D10Rat27 were used to refine the localization of Toxo1.
Parasites and Experimental Design of Infection in Vivo. Cysts from the recombinant T. gondii Prugniaud strain that expresses the Escherichia coli β-galactosidase gene (Pru-β-gal) were used (37). Two-month-old Swiss mice were infected orally with 10 Pru-β-gal cysts. Their brains were collected 3 months later and ground in a Potter. Cysts were counted in a Thoma's cell and diluted in PBS. Rats were infected orally with 20 cysts. One month later, blood was collected from the retroorbital sinus for detection of anti-toxoplasma Ab response by Western blotting and quantification by indirect immunofluorescence (9). Rats were killed 6 weeks after infection, and their brains were collected for counting Pru-β-gal cysts in brain suspension, after addition of β-gal detection reagent (9).
Parasites and Experimental Design of Infection in Vitro. The RH strain of T. gondii used for the proliferation assay in rat peritoneal macrophages (MΦ) was maintained by injecting regularly female Swiss mice i.p. with 104 tachyzoites in 0.5 ml of Hanks' balanced salt solution (HBSS) (38). The parasites were collected from the peritoneal cavities of mice on day 3 after infection by washing with 1 ml of HBSS, centrifuged at 500 × g for 15 min at 4°C, suspended in serum-free medium (SFM, GIBCO), and counted.
Rat resident peritoneal cells were obtained by injection of sterile 199 medium with HBSS into the peritoneal cavity. Collected cells were centrifuged, and the cell pellet was suspended in SFM. Cells were allowed to adhere onto 24-well culture plates for 1 h at 37°C and 5% CO2. Nonadherent cells were then removed by washing with PBS. A total 98% of the adherent cells thus obtained had the morphological appearance of MΦ after May–Grunwald Giemsa staining and were detected as positive for nonspecific esterase. Rat subepithelial fibroblasts were obtained from shaved skin patches maintained for 7–10 days in the “DMEM + 4,500 mg/liter Glucose + GlutaMAX + Pyruvate” medium (GIBCO) containing 10% FCS, 1% Hepes, 1% nonessential amino acids, 100 units/ml penicillin, 100 μg/ml streptomycin, and 50 μM 2-mercaptoethanol, at 37°C and 5% CO2. When fibroblasts that grew away from the dermal explants were confluent, they were washed, trypsinized, and counted.
Toxoplasma Proliferation Assay in Peritoneal Macrophages and Fibroblasts. The intracellular growth of T. gondii in monolayer cells was monitored by [3H]uracil incorporation into T. gondii RNA (38). Briefly, 106 MΦ in SFM were cultured for 1 h in 24-well Falcon plates, and 3.104 fibroblasts in DMEM/10% FCS overnight in 48-well Falcon plates, at 37°C and 5% CO2. After washing in SFM, macrophage monolayers were infected with 105 tachyzoites, and fibroblast monolayers were infected with 3.104 tachyzoites, for 1 h in SFM at 37°C and 5% CO2. After washing to eliminate extracellular parasites, the cells were cultured for 20 h in the presence of [3H]uracil (2 μCi per well, 1 Ci = 37 GBq). The monolayers were then washed twice with PBS and disrupted with 1 M NaOH. After neutralization by 1 M HCl, the radioactivity was assessed in a Amersham Pharmacia LKB 1217 beta-counter.
Studies of Toxoplasma Behavior in Macrophages and in Extracellular Fluids After i.p. Infection. For invasion studies, tachyzoites (RH strain) were delivered to MΦ monolayers at a ratio of 50:1 in 300 μl of DMEM supplemented with 1 mM glutamine, 500 units/ml penicillin, and 50 μg/ml streptomycin. After 20-min contact on ice, invasion was allowed by settling for 2.5 min in a 37°C water bath. Monolayers were washed extensively in PBS to remove nonadherent parasites. Pulse-infected cells were directly fixed in 2.5% formaldehyde in PBS. In other experiments, invasion was allowed for 60 min and infected cells were returned to culture for 20 h before fixation to evaluate parasite replication. Slides were incubated sequentially with primary antibodies, followed by FITC secondary antibody (Jackson ImmunoResearch). Parasites were stained with the anti-SAG1 mAb (TG05–54) in presence of 0.002% saponin. Vacuoles were stained with the anti-GRA1 mouse mAb (TG17.43.1). In some experiments, before fixation, slides were incubated with either the LysoTraker (Molecular Probes) red dye to evaluate compartment acidification or with propidium iodide to evaluate cell and/or parasite integrity. The average number of parasites per vacuole was determined from counts of 200–500 host cells in three independent experiments. Slides were mounted in Pro-long Anti-fade (Molecular Probes) and examined by using a Zeiss Axioplan 2 microscope equipped for epifluorescence and phase-contrast.
For ex vivo experiments, we used the recombinant T. gondii RH strain expressing a tandem yellow fluorescent protein (RH-YFP2), kindly provided by Boris Striepen (University of Georgia, Athens). i.p. infection was done with 5 × 108 tachyzoites. One hour later, peritoneal fluids were recovered, centrifuged, and resuspended in 600 μl of supernatant. Extracellular parasites were counted, and their viability was determined with propidium iodine. Then, a 2.5-min pulse invasion assay onto human foreskin fibroblast (HFF) cells was performed (300 μl per slide). Slides were fixed and invasion rate was determined by counting the number of YFP2 parasites per 100 cells. In this assay, two slides and a minimum of 1,000 cells were counted per sample.
Data Analyses. Association of individual markers with resistance to T. gondii infection was assessed by comparing the genotype distribution of rats showing or not anti-toxoplasma Ab or brain cysts, with Pearson's χ2 statistics using spss. The significance of differences found between groups of F2 (LEW × BN) rats with different genotypes at the locus markers, initially derived from a Kruskall–Wallis H test, was subsequently confirmed by the Mann–Whitney test.
Supplementary Material
Acknowledgments
We thank Patrick Aregui, Audrey Boyer, Marie-Andrée Daussion, Thierry Ruiz, Corine Senty, Magali Toulouse, and Aline Tridon (Service de Zootechnie, Institut Fédératif de Recherche 30) for careful handling of the rats; Mathias Titeux for help with the fibroblast cultures; Daniel Gonzales-Dunia (Institut National de la Santé et de la Recherche Médicale U.563, Toulouse F-31300 France) for providing LEW and BN fibroblast cell lines; Valérie Bans and Karine Musset for technical assistance; and Prof. Alain Hovnanian and Drs. Etienne Joly and Neil Ledger for critical reading of the manuscript. M.M. was supported by a grant from the Association de Recherche sur la Polyarthrite Rhumatoïde (ARP). J.-F.S. and C.C. were supported by grants from the Société de Néphrologie. J.-F.S. and P.C. were recipients of predoctoral grants from the Fondation pour la Recherche Médicale. This work was supported by the Institut National de la Santé et de la Recherche Médicale (INSERM), the Ministère Délégué à la Recherche et aux Nouvelles Technologies (Microbiology Program and “Environment et Santé” Program). Congenic lines have been created with grants of the Genopole Midi-Pyrénées, ARP, Etablissement français des Greffes, Ligue Contre le Cancer, Université Paul Sabatier, Conseil Général de Région Midi-Pyrénées, and Ministère de l'Aménagement du Territoire et de l'Environnement.
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: c10, chromosome 10; SFM, serum-free medium.
References
- 1.Carruthers, V. B. (2002) Acta Trop. 81, 111–122. [DOI] [PubMed] [Google Scholar]
- 2.Couvreur, J., Desmonts, G. & Girre, J. Y. (1976) J. Pediatr. 89, 235–240. [DOI] [PubMed] [Google Scholar]
- 3.Mack, D. G., Johnson, J. J., Roberts, F., Roberts, C. W., Estes, R. G., David, C., Grumet, F. C. & McLeod, R. (1999) Int. J. Parasitol. 29, 1351–1358. [DOI] [PubMed] [Google Scholar]
- 4.Sibley, L. D., Mordue, D. G., Su, C., Robben, P. M. & Howe, D. K. (2002) Philos. Trans. R. Soc. London Ser. B 357, 81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacob, H. J. & Kwitek, A. E. (2002) Nat. Rev. Genet. 3, 33–42. [DOI] [PubMed] [Google Scholar]
- 6.Korstanje, R. & Paigen, B. (2002) Nat. Genet. 31, 235–236. [DOI] [PubMed] [Google Scholar]
- 7.Dubey, J. P. & Frenkel, J. K. (1998) Vet. Parasitol. 77, 1–32. [DOI] [PubMed] [Google Scholar]
- 8.Kempf, M. C., Cesbron-Delauw, M. F., Deslee, D., Gross, U., Herrmann, T. & Sutton, P. (1999) Med. Microbiol. Immunol. 187, 137–142. [DOI] [PubMed] [Google Scholar]
- 9.Sergent, V., Cautain, B., Khalife, J., Deslee, D., Bastien, P., Dao, A., Dubremetz, J. F., Fournie, G. J., Saoudi, A. & Cesbron-Delauw, M. F. (2005) Infect. Immun. 73, 6990–6997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fournié, G. J., Cautain, B., Xystrakis, E., Damoiseaux, J., Mas, M., Lagrange, D., Bernard, I., Subra, J.-F., Pelletier, L., Druet, P. & Saoudi, A. (2001) Immunol. Rev 184, 145–160. [DOI] [PubMed] [Google Scholar]
- 11.Lander, E. & Kruglyak, L. (1995) Nat. Genet. 11, 241–247. [DOI] [PubMed] [Google Scholar]
- 12.McCabe, R. E. & Remington, J. S. (1986) Infect. Immun. 52, 151–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aliberti, J. (2005) Nat. Rev. Immunol. 5, 162–170. [DOI] [PubMed] [Google Scholar]
- 14.McLeod, R., Skamene, E., Brown, C. R., Eisenhauer, P. B. & Mack, D. G. (1989) J. Immunol. 143, 3031–3034. [PubMed] [Google Scholar]
- 15.Johnson, J., Suzuki, Y., Mack, D., Mui, E., Estes, R., David, C., Skamene, E., Forman, J. & McLeod, R. (2002) Int. J. Parasitol. 32, 179–185. [DOI] [PubMed] [Google Scholar]
- 16.Hill, A. V. (1998) Annu. Rev. Immunol. 16, 593–617. [DOI] [PubMed] [Google Scholar]
- 17.Casanova, J. L., Schurr, E., Abel, L. & Skamene, E. (2002) Trends Immunol. 23, 469–472. [DOI] [PubMed] [Google Scholar]
- 18.Tournamille, C., Colin, Y., Cartron, J. P. & Le Van Kim, C. (1995) Nat. Genet. 10, 224–228. [DOI] [PubMed] [Google Scholar]
- 19.Huang, Y., Paxton, W. A., Wolinsky, S. M., Neumann, A. U., Zhang, L., He, T., Kang, S., Ceradini, D., Jin, Z., Yazdanbakhsh, K., et al. (1996) Nat. Med. 2, 1240–1243. [DOI] [PubMed] [Google Scholar]
- 20.Forbes, J. R. & Gros, P. (2001) Trends Microbiol. 9, 397–403. [DOI] [PubMed] [Google Scholar]
- 21.Courret, N., Darche, S., Sonigo, P., Milon, G., Buzoni-Gatel, D. & Tardieux, I. (2005) Blood, in press. [DOI] [PMC free article] [PubMed]
- 22.Sacks, D. & Sher, A. (2002) Nat. Immunol. 3, 1041–1047. [DOI] [PubMed] [Google Scholar]
- 23.Morisaki, J. H., Heuser, J. E. & Sibley, L. D. (1995) J. Cell Sci. 108, 2457–2464. [DOI] [PubMed] [Google Scholar]
- 24.Sibley, L. D. (2004) Science 304, 248–253. [DOI] [PubMed] [Google Scholar]
- 25.Joiner, K. A., Beckers, C. J., Bermudes, D., Ossorio, P. N., Schwab, J. C. & Dubremetz, J. F. (1994) Ann. N.Y. Acad. Sci. 730, 1–6. [DOI] [PubMed] [Google Scholar]
- 26.Suss-Toby, E., Zimmerberg, J. & Ward, G. E. (1996) Proc. Natl. Acad. Sci. USA 93, 8413–8418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mordue, D. G. & Sibley, L. D. (1997) J. Immunol. 159, 4452–4459. [PubMed] [Google Scholar]
- 28.Mordue, D. G., Hakansson, S., Niesman, I. & Sibley, L. D. (1999) Exp. Parasitol. 92, 87–99. [DOI] [PubMed] [Google Scholar]
- 29.Takao, S., Smith, E. H., Wang, D., Chan, C. K., Bulkley, G. B. & Klein, A. S. (1996) Am. J. Physiol. 271, C1278–C1284. [DOI] [PubMed] [Google Scholar]
- 30.Fang, F. C. (1997) J. Clin. Invest. 99, 2818–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seabra, S. H., de Souza, W. & DaMatta, R. A. (2002) Exp. Parasitol. 100, 62–70. [DOI] [PubMed] [Google Scholar]
- 32.Morel, L., Blenman, K. R., Croker, B. P. & Wakeland, E. K. (2001) Proc. Natl. Acad. Sci. USA 98, 1787–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Podolin, P. L., Denny, P., Lord, C. J., Hill, N. J., Todd, J. A., Peterson, L. B., Wicker, L. S. & Lyons, P. A. (1997) J. Immunol. 159, 1835–1843. [PubMed] [Google Scholar]
- 34.Buxton, D. (1998) Vet. Res. 29, 289–310. [PubMed] [Google Scholar]
- 35.Wakeland, E., Morel, L., Achey, K., Yui, M. & Longmate, J. (1997) Immunol. Today 18, 472–477. [DOI] [PubMed] [Google Scholar]
- 36.Mas, M., Subra, J. F., Lagrange, D., Pilipenko-Appolinaire, S., Kermarrec, N., Gauguier, D., Druet, P. & Fournie, G. J. (2000) Eur. J. Immunol. 30, 1698–1705. [DOI] [PubMed] [Google Scholar]
- 37.Dao, A., Soete, M., Sergent, V., Deslee, D., Fortier, B. & Dubremetz, J. F. (2002) Parasitol. Res. 88, 69–72. [DOI] [PubMed] [Google Scholar]
- 38.Thardin, J. F., M'Rini, C., Beraud, M., Vandaele, J., Frisach, M. F., Bessieres, M. H., Seguela, J. P. & Pipy, B. (1993) Infect. Immun. 61, 1432–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.