Abstract
FREQUENCY (FRQ) is a critical element of the circadian system of Neurospora. The white collar genes are important both for light reception and circadian function. We show that the responsiveness of the light input pathway is circadianly regulated. This circadian modulation extends to light-inducible components and functions that are not rhythmic themselves in constant conditions. FRQ interacts genetically and physically with WHITE COLLAR-1, and physically with WHITE COLLAR-2. These findings begin to address how components of the circadian system interact with basic cellular functions, in this case with sensory transduction.
Keywords: circadian/frequency/light/Neurospora/white collar
Introduction
Circadian systems coordinate the temporal program of organisms in all phyla to accommodate and anticipate the daily changes of the environment (Pittendrigh, 1993). Experimentally, circadian rhythms are characterized by their self-sustained, ∼24 h oscillation in constant conditions. Rhythms are entrained (in nature to 24 h) by appropriate environmental signals (zeitgeber), of which light is the most studied (Roenneberg and Foster, 1997). An intact circadian system includes input pathways, a mechanism that generates rhythmicity (rhythm generator) and outputs. Physiological experiments show that light input pathways (LIPs) to the rhythm generator may themselves be under circadian control. For example, in the marine unicell Gonyaulax, one of the circadian LIPs is only active during the subjective night (for definition of subjective day and night, see legend to Figure 1; Roenneberg and Taylor, 1994; Roenneberg and Deng, 1997). There are also indications that light receptors of circadian systems are rhythmic, e.g. cryptochrome in Drosophila (Emery et al., 1998) or phytochrome B in plants (Bognar et al., 1999).
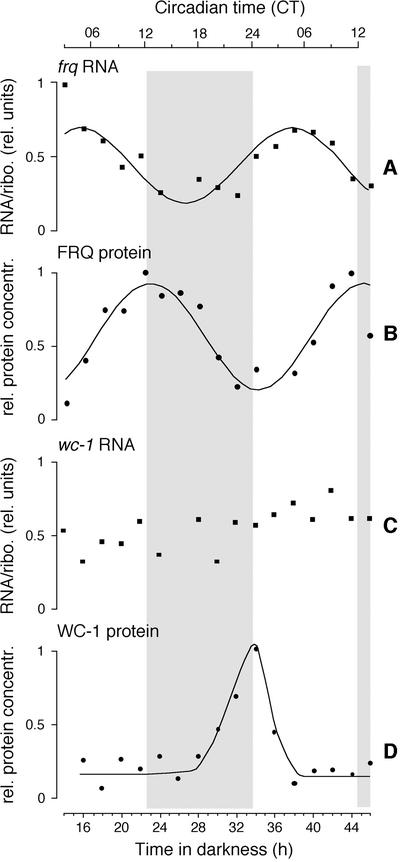
Fig. 1. Circadian time course of frq and wc-1 RNA and protein levels. frq+ was grown at 25°C in constant darkness. Samples were harvested at 2 h intervals and frq RNA (A), FRQ protein (B), wc-1 RNA (C) and WC-1 protein (D) levels were measured. Specific RNA signals were normalized to rRNA (RNA/ribo. rel. units) and specific protein signals were normalized to amido black-stained blots (rel. protein concentration). In rhythmic time series (A, B and C), the highest value was set equal to 1; in (C), the average signal was set equal to 0.5. See Materials and methods for curve fits. The initial light to dark transfer of the cells corresponds to dusk and is designated circadian time (CT) 12. See Materials and methods for determination of CT. Gray areas indicate subjective night (i.e. the half of the full circadian cycle that corresponds to darkness in a light:dark cycle); open areas are subjective day.
Thus, circadian light input is probably not a straightforward transduction of signals to the rhythm generator, but rather an active, circadianly regulated mechanism. In principle, input pathways can influence circadian rhythmicity by responding both to external zeitgebers and to the endogenous circadian system. Modeling shows that mutations in components of rhythmic input pathways change period as determined in constant conditions and contribute to self-sustainment. These theoretical results (Roenneberg and Merrow, 1998, 1999) are in accordance with reports showing that mutations in genes encoding input elements can change period or even result in arhythmicity in constant conditions (Millar et al., 1995; Somers et al., 1998; Iwasaki et al., 2000). Either of these observations would also be consistent with mutations in components that are central to the rhythm generator.
In all molecular/genetic model systems, a negative feedback loop is essential for self-sustained circadian rhythmicity in constant conditions and is generally considered to be central to the rhythm generator. These loops involve expression of genes to proteins (transcription, translation and modification, e.g. phosphorylation) which, in turn, inhibit their own expression. Such a transcription/translation feedback loop is also necessary for self-sustainment of circadian rhythms in Neurospora, involving the genes frequency (frq), white collar-1 (wc-1) and white collar-2 (wc-2) as central players (Dunlap, 1999). Initially, wc-1 and wc-2 were identified in screens for lack of light reception (Harding and Turner, 1981; Degli-Innocenti and Russo, 1984). They are both light-inducible, DNA-binding, putative transcription factors (Ballario et al., 1996; Linden and Macino, 1997), and WC-1 shares similarity with a class of proteins from diverse species that actively process electrons or photons (Huala et al., 1997). Also, WC-1 and WC-2 regulate basal levels of frq (Crosthwaite et al., 1997), which was identified in screens for circadian period mutants (Feldman and Hoyle, 1973). FRQ negatively regulates its own transcription in constant conditions (Aronson et al., 1994b), providing a mechanistic basis for continuous oscillations (self-sustained rhythmicity). However, circadian properties remain in the absence of the frq/FRQ transcription/translation feedback loop. FRQ-deficient strains are capable of circadian entrainment (a special, circadian form of synchronization) in temperature cycles, whereas light entrainment fails, indicating a functional role for FRQ in the LIP (Merrow et al., 1999) and opening up the possibility of additional circadian machinery that functions in the absence of FRQ (see also Lakin-Thomas and Brody, 2000).
Here, we characterize how FRQ is involved in light signal transduction. Two distinct physiological responses to light (conidial banding and carotenogenesis) represent two separate destinations of this light transduction pathway. The former has an absolute requirement for FRQ to respond to light, the latter only requires FRQ for circadian regulation and overall magnitude of light induction. We further show genetic and physical interaction of FRQ and the WC proteins, demonstrating that circadian regulation and light signal transduction are hard wired together.
Results
Regulation of LIP components
The components that have been characterized most thoroughly for their early involvement in light responses in Neurospora are WC-1 and WC-2. Both proteins are critical for normal circadian rhythmicity; however, wc-1 mRNA is regulated more robustly by light than wc-2 (Linden and Macino, 1997). Thus, for questions regarding the relationship between light and circadian regulation, we focused our studies on interactions of wc-1 and frq.
One of the important circadian features of frq RNA and protein is their rhythmicity in constant darkness (DD; Figure 1A and B; Aronson et al., 1994b; Garceau et al., 1997). We analyzed frq and wc-1 RNA and protein levels under these conditions. The RNA levels of wc-1 were variable, but not circadian (Figure 1C; Lee et al., 2000). WC-1 protein levels, however, changed with circadian time (Figure 1D; Lee et al., 2000). The period of the WC-1 oscillation is specific for different circadian period mutants (Lee et al., 2000). The differences for frq and wc-1 RNA and protein profiles in constant conditions show that their regulation is distinct. This is also indicated by the fact that maximum WC-1 protein levels coincide with the FRQ minimum (Figure 1B and D; Lee et al., 2000).
Regulation of FRQ and WC-1 is interdependent
Basal frq/FRQ levels are low in wc-1 and wc-2 mutants (Crosthwaite et al., 1997). Here, these levels were determined in a Δwc-1 strain (RIPed to a functional knockout; see Materials and methods and Talora et al., 1999), confirming that frq RNA and FRQ protein (Figure 2A and B) levels are substantially lower in the Δwc-1 than in a wc-1+ strain. These observations suggest that regulation of frq lies downstream of WC-1.
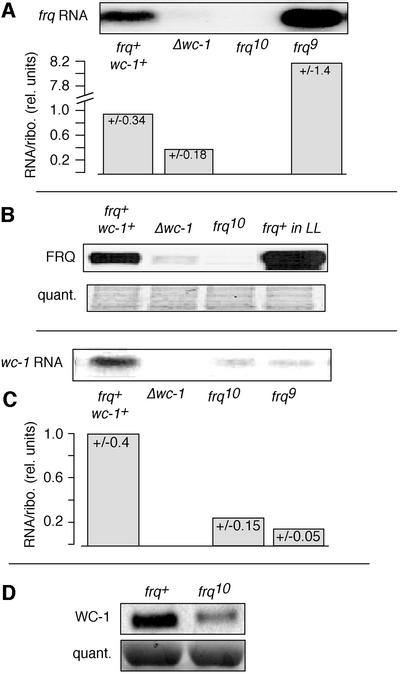
Fig. 2. Regulation of frq and wc-1. Mycelial pads from frq+, Δwc-1, frq10 and frq9 were incubated in darkness for 28 h and then RNA and protein extracts were prepared. (A) frq RNA is reduced in Δwc-1. frq RNA was detected by northern blotting (upper panel), quantified and normalized to rRNA levels (lower panel). The columns in the graphs represent averages of four experiments (standard deviations are given at the top of each bar). The controls frq10 and frq9 show no frq or elevated RNA, respectively, as expected. (B) FRQ protein is reduced in Δwc-1. FRQ protein was detected by western blotting. To control for equal loading of the gel, a portion of the blot was stained with amido black (lower panel). The controls frq10 and frq+LL (constant light) show no FRQ protein or elevated levels, respectively. (C) wc-1 RNA is reduced in frq10 and frq9. RNA was analyzed as described above. (D) WC-1 protein levels are reduced in frq10 as shown by western blotting. The amido black-stained membrane indicated equal loading (lower panel).
Recently, the activators of genes in the Drosophila circadian transcription/translation feedback loop were shown to depend on ‘downstream’ gene products for their expression (Bae et al., 1998; Glossop et al., 1999). A comparative analysis would suggest that this might be a common regulatory mechanism in circadian molecular networks. The rhythmicity of WC-1 (Figure 1D; Lee et al., 2000) suggests a similar interactive network between FRQ and the white collar gene products. We therefore determined wc-1 RNA levels in frq10 (a frq null strain, see Materials and methods for description). Basal RNA levels were lower than those observed in frq+ grown in DD (Figure 2C; in contrast to Lee et al., 2000), and WC-1 protein levels were reduced similarly (Figure 2D; and as in Lee et al., 2000). Thus, at least under some conditions (see Discussion), FRQ apparently is required to maintain basal levels of wc-1 RNA and protein in DD.
FRQ deficiency and light responsiveness
We probed the functional consequences of frq deficiency, and the resultant depressed WC-1 levels, on light-induced physiology by investigating several light-inducible outputs: conidial band formation, carotenogenesis and expression of specific RNAs. Figure 3A shows light regulation of conidial banding in a wild-type strain (tubes 1 and 2). As previously reported, frq null strains do not respond to light:dark (LD) cycles with succinct formation of conidial bands or their synchronization (Figure 3A, tube 3; Chang and Nakashima, 1997; Merrow et al., 1999; Lakin-Thomas and Brody, 2000; Roenneberg and Merrow, 2001). In contrast, temperature cycles entrain conidial band formation in frq9 (Merrow et al., 1999).
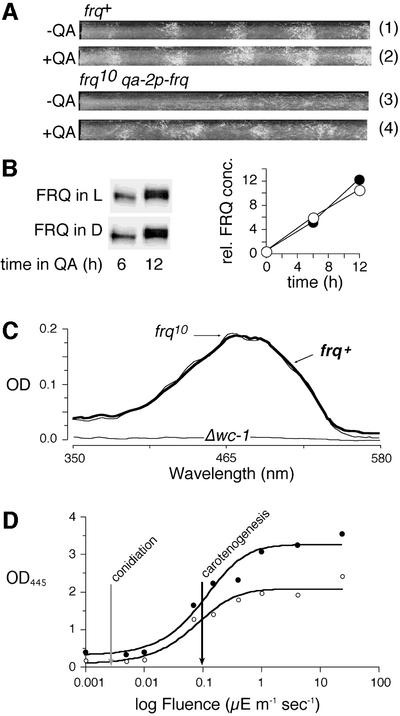
Fig. 3. A bifurcated light input pathway: FRQ is required for conidiation but not for carotenogenesis. (A) FRQ rescues light-regulated conidiation in frq10. Race tubes were inoculated with frq+ (bd) or frq10 qa-2p-frq (bd frq10 his3::his3qa-2p-frqhis), which expresses a His-tagged version of FRQ under the control of the inducible qa-2 promoter. The race tubes were incubated in 12 h light:12 h dark (LD 12:12) cycles with or without quinic acid (QA). Approximately 4.5 cycles are shown. (B) QA-induced expression of FRQ is unaffected by light. frq10 qa-2p-frq was harvested in either the light or dark portion of the third day of an LD 12:12 cycle. At time 0, FRQ expression was induced by QA. Mycelia were harvested after 6 and 12 h and FRQ was analyzed by western blotting (left panel). Signals were quantified by densitometry. Relative expression levels (rel. FRQ conc., right panel) are the average of triplicate samples, normalized for loading based on amido black-stained membranes. (C) Induction of carotenoids by light does not require FRQ. Carotenoids were induced in frq+ (thick line), frq10 (thin line) and Δwc-1 with 4 µE/m2/s of light for 5 h. Samples were extracted with hexane and absorption spectra were determined. The frq+ extract was diluted 2-fold relative to the other samples. (D) The fluence threshold of carotenogenesis is independent of FRQ. Carotenoids were induced in frq+ (filled circles) and frq10 (open circles) over the indicated range of fluences. Absorption of carotenoids was determined at 445 nm. Values >1.0 were measured as dilutions for accuracy. The black arrow indicates the fluence rate at which half-maximal light induction of carotenoids occurs for both strains. For comparison, the gray arrow indicates the fluence threshold for light-dependent conidial band formation in frq+ (Merrow et al., 1999; Roenneberg and Merrow, 2001).
To clarify whether FRQ, as such, is required for light-induced conidial band formation or whether this function depends on the intact negative feedback regulation of the frq/FRQ loop, we constitutively expressed FRQ in frq10 (using a qa-2p-frq fusion construct similar to that of Aronson et al., 1994b). Although under these conditions this response is not entirely normal, a light-regulated conidiation response was rescued (Figure 3A, tube 4); this strain remains arhythmic in DD (data not shown; and Aronson et al., 1994b). The timing of conidiation following the light signal (its phase angle) is similar to that for the frq+ strain. The reconstitution of light responsiveness could be mediated by light-induced rhythmicity of FRQ levels, even in the absence of transcriptional regulation. However, when FRQ was induced in the frq10 strain, there was no difference in phosphorylation state (judged by mobility in SDS–PAGE; Figure 3B, left panel) or protein accumulation (right panel) in light versus darkness (Figure 3B). Thus, expression of FRQ is required for light regulation of conidiation, even in the absence of negative feedback of FRQ on frq. Given that there is not an obvious qualitative or quantitative difference in FRQ protein induced in light or dark (Figure 3B), it is possible that rhythmicity of the protein is not essential for this response.
Because it was reported previously that the frq null strains do show light-induced gene expression (Arpaia et al., 1993, 1995), we investigated light-induced mycelial carotenogenesis (De Fabo et al., 1976). Figure 3C shows that this light response remained qualitatively intact in frq10: the absorption spectrum of hexane-extracted, light-induced tissue was the same in frq10 and frq+. However, final carotenoid concentrations were approximately half in frq10 compared with frq+ (the two superimposed spectra represent extracts of different dilutions; see Figure 3C legend). Note that light-dependent carotenoid synthesis is entirely absent in wc-1 mutants (Harding and Turner, 1981; Linden et al., 1999).
When fluence response curves for light induction of carotenogenesis in frq+ and frq10 were compared, the amplitude of the saturation response in frq10 was about half that of frq+ (Figure 3D). The sensitivity of both strains (fluence rate at half-maximal response, black arrow) was, however, identical. Comparison of the fluence threshold for carotenogenesis with that for light-driven synchronization of conidiation (Figure 3D, gray arrow; Merrow et al., 1999; Roenneberg and Merrow, 2001) suggests two, distinct, light-regulated pathways. Without FRQ, light-regulated conidial band formation is absent even in high light intensity LD cycles, but carotenoids are induced normally based on fluence threshold, although overall accumulation is about half. So, while both branches of this pathway are light blind without WC-1, they are each modulated differently by FRQ (see Figure 7A).
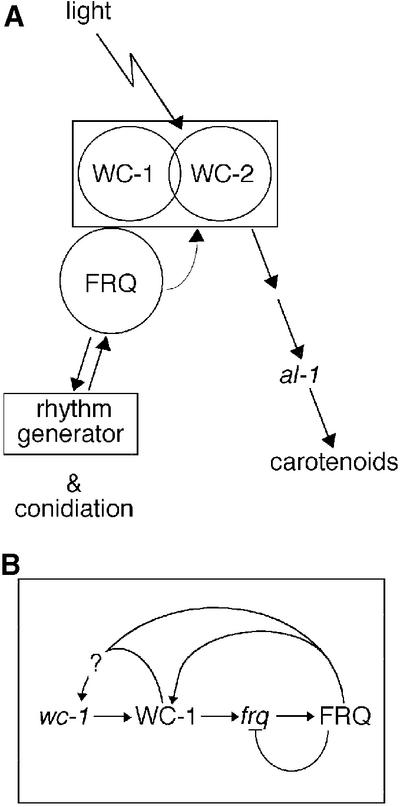
Fig. 7. The molecular components of the Neurospora circadian system and the light input pathway (LIP) are inseparable. (A) Our results are compatible with a bifurcation within the LIP, with one branch leading to light-dependent (or, in the absence of light, circadian) conidiation and the other to light-induced carotenogenesis. In terms of regulation by light, WC-1 is required for both branches, while FRQ is only essential for regulated conidiation. Both are important for circadian rhythmicity. In addition, the circadian system modulates the branch regulating carotenoid synthesis, possibly via FRQ. Within the LIP, the WCs and FRQ form a functional unit responsible for both light reception and circadian modulation. (B) Genetic interactions within the Neurospora LIP. Although not specified, a basal activation of wc-1 and frq is presumed, in addition to the inferred positive effect on wc-1 RNA levels by FRQ or WC-1 (see Figure 2). Also, WC-2 is involved in frq regulation (Crosthwaite et al., 1997), but this aspect was not addressed in this work.
Carotenoid production is the result of a complex, multi-step process. In contrast, one of the earliest detectable events after light exposure of Neurospora is induction of wc-1 RNA, which occurs in <2 min at high light intensities (P.Ballario, unpublished data). Induction of wc-1 RNA was, therefore, used to monitor early events in light signal reception and transduction, and contrasts the endpoint that carotenogenesis represents. The amplitudes (maximum response relative to baseline) of light-induced RNA in frq+ and frq10 appeared to be similar (Figure 4); however, the peak light-induced wc-1 levels were low in frq10, reaching, at most, the basal DD levels of wc-1 in frq+ (compare with Figure 2C). al-1 RNA was also induced weakly by light in frq10 (data not shown). al-1 encodes a downstream enzyme on the carotenogenesis pathway (phytoene dehydrogenase) (Schmidhauser et al., 1990). Given that RNA induction is a relatively rapid and discrete response compared with carotenogenesis, the disparity in strength of carotenoid and RNA induction is not directly comparable.
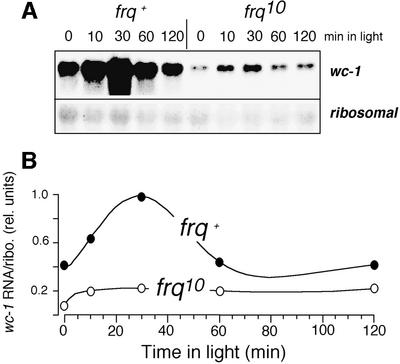
Fig. 4. Robust light induction of wc-1 RNA requires FRQ. frq+ and frq10 were incubated for 28 h in the dark and then exposed to 4 µE/m2/s of light. Samples were harvested over 2 h. (A) wc-1 and rRNAs were detected by northern blot analysis. (B) wc-1 RNA was quantified and normalized to the amount of rRNA. The maximal signal was set equal to 1. Data are the average of duplicate samples and representative of three experiments.
Circadian regulation of light responses
Light responses depend on FRQ and WC-1 (Figures 3A, C and D, and 4), and both proteins show a circadian rhythm in abundance (Figure 1B and D). We therefore investigated the physiological light responses described above at different times of the circadian cycle. Time courses measured over 2 h in the middle of the subjective day and night showed large differences in light-induced gene expression. While basal levels of wc-1 were indistinguishable (see also Figure 1C), the amplitude of the response in the subjective day was less than half when compared with the subjective night (Figure 5A).
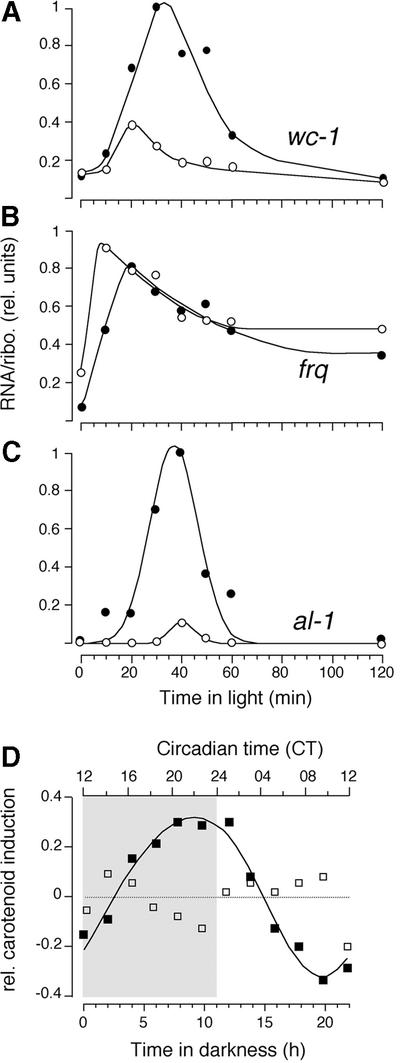
Fig. 5. Light responses are regulated by the circadian system. Time of day-specific light induction of wc-1 RNA (A), frq RNA (B) and al-1 RNA (C). Mycelial pads were incubated for 16 h (∼CT05, unfilled circles) and 27 h (∼CT17, filled circles) in the dark and then exposed to light (0.4 µE/m2/s). After the indicated time periods, RNA was prepared, analyzed by northern blotting and quantified based on rRNA values. The maximal signal was set equal to 1. (D) Light-induced carotenogenesis over the course of a circadian cycle. frq+ (filled squares) and frq10 (open squares) mycelia were transferred from light to dark. After the indicated time in darkness, samples were exposed to light for 5 h to induce carotenogenesis. Carotenoids were extracted with hexane and quantified by absorption at 445 nm. Circadian time of frq+ is shown on top; the gray background indicates subjective night.
frq RNA levels depend on WC-1 and WC-2, for both basal expression and rapid and robust light induction (Crosthwaite et al., 1997). In contrast to wc-1, frq RNA is induced to approximately the same maximal levels at the opposite circadian times (Figure 5B; Crosthwaite et al., 1995). Because frq levels are circadian (Figure 1A; Aronson et al., 1994b; Garceau et al., 1997), frq is already at different levels at the beginning of light incubation.
Finally, al-1 light induction resembles the pattern of wc-1 RNA induction, but the subjective night/day ratio is ∼10-fold (Figure 5C). Interestingly, wc-1 and al-1 RNA profiles, and to a lesser extent frq, show a transiency in prolonged light exposure (Schmidhauser et al., 1990; Arpaia et al., 1995; Linden et al., 1999), resembling classical adaptation responses. RNA levels are down-regulated within 1–2 h and, at least for wc-1, remain constant for 10 h of illumination (data not shown). The adaptation profile is apparent at both circadian times that were evaluated.
Lastly, we determined light-induced mycelial carotenogenesis at different circadian times. Light-dependent accumulation of carotenoids was also circadianly regulated, peaking before subjective dawn (notably, the circadian modulation of light-induced of carotenogenesis is absent in frq10, Figure 5D). Thus, circadian regulation of light responsiveness is evident at numerous levels, from some of the earliest known events of light detection to enzymatically regulated, downstream outputs.
FRQ is physically associated with the WC proteins
WC-1 and WC-2 are found in the same complex in vivo (Talora et al., 1999). We wondered if the involvement of FRQ in light responses could stem from a physical association with either of these proteins. We probed anti-WC-2 immunoprecipitates of cell extracts and found that FRQ binds to WC-2 (Figure 6A). The amount of FRQ in the complex correlated with its circadian accumulation in crude cell extracts (Figure 1B). For reference, FRQ in total cell extracts from frq+ is shown at two circadian times: one harvested at subjective dawn with low levels of highly phosphorylated FRQ, the other at late subjective day with large amounts of less phosphorylated FRQ (Figure 6A, left panel, right lanes). In this experiment, highly phosphorylated FRQ apparently does not participate in complex formation, although in some others it did. Using anti- WC-1, we were also able to immunoprecipitate FRQ from cell extracts of mycelia grown in constant light (LL; Figure 6B). Thus, FRQ is in complexes containing WC-2 and/or WC-1 in vivo and WC-2 is found in association with WC-1 (Talora et al., 1999).
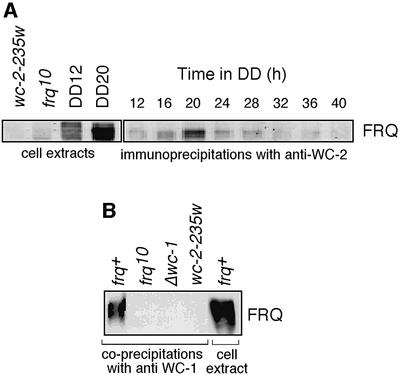
Fig. 6. Interaction of FRQ with WC-2 and WC-1. (A) Co-precipitation of FRQ with WC-2 (right panel) and control cell extracts (left panel). For immunoprecipitations, cell extracts were prepared from age-matched mycelial pads grown in DD for the indicated amounts of time. Samples were immunoprecipitated with anti-WC-2 serum (right panel), separated by SDS–PAGE and probed with antibodies against FRQ on western blots. Control cell extracts include those from wc-2-234w, frq10 and a strain wild-type at frq and wc-2 loci (frq+) at DD12 (∼CT01) and DD20 (∼CT10). These were probed for FRQ and indicate the relative abundance and phosphorylation state of FRQ protein at these times. (B) Co-precipitation of FRQ with WC-1. frq+, frq10, Δwc-1 and wc-2-234w were grown in constant light (LL). Extracts were prepared and subjected to immunoprecipitation with antibodies against WC-1. The precipitates were analyzed by SDS–PAGE and blots were probed with antibodies against FRQ. The cell extract of frq+ cultures represents 4% of the input in the co-precipitation experiment and is shown in the right lane as an estimate of the efficiency of the co-precipitation.
Discussion
We previously observed circadian entrainment by temperature cycles in Neurospora strains deficient in FRQ (Merrow et al., 1999). The same strains, however, fail to synchronize to light cycles (Figure 3A; Chang and Nakashima, 1997; Merrow et al., 1999; Lakin-Thomas and Brody, 2000), suggesting that FRQ is required in processing light signals, in addition to controlling essential circadian properties, such as self-sustained rhythmicity in DD. Here, we address several questions about the role of frq/FRQ in the LIP and the circadian system of Neurospora crassa. What is the nature of the interaction between the WC proteins and their complex (WCC) and FRQ? What is the role of FRQ in light transduction and how does it contribute to entrainment? Figure 7 is a diagrammatic view of the Neurospora LIP, specifically with respect to WC-1, WC-2 and FRQ at the protein level in Figure 7A and at the level of gene regulation of wc-1 and frq in Figure 7B.
Interdependent regulation of frq and wc-1 by their proteins
The circadian and light input pathways in Neurospora interact genetically. Robust frq RNA and protein expression depends on WC-1 and WC-2 proteins, which are essential for light responses in Neurospora (Figure 2A and B; Crosthwaite et al., 1997). However, frq expression is not simply downstream of the WC gene products: we find that basal, DD levels of wc-1 (Figure 2C) and WC-1 depend on FRQ (Figure 2D; Lee et al., 2000). Since FRQ levels are robustly circadian (Figure 1B; Garceau et al., 1997), one would expect rhythmic wc-1 RNA in DD, but this is not the case (Figure 1C). A recent report (Lee et al., 2000) also finds that wc-1 RNA is constitutive but further describes that basal wc-1 levels are similar in frq+ and frq10. This is in contrast to what we observe. The most obvious difference in the experimental protocols is the time at which the samples are harvested: after 28 h in darkness in the protocol described here, versus only 6 h in that presented by Lee et al. (2000). There could be residual light-induced wc-1 RNA in frq10 (Figure 4) that persists for at least 6 h in DD; sustainment of those RNA levels may depend on FRQ. WC-1 protein oscillates with a circadian period, out of phase with FRQ (Figure 1D; and as shown in Lee et al., 2000), and its regulation includes a post-transcriptional control that is initiated by FRQ expression (Lee et al., 2000). Thus, WC-1 regulates FRQ, and FRQ regulates WC-1, indicating the backbone of the circadian transcription/translation feedback loop in Neurospora (see Figure 7B).
A bifurcated light input pathway
The effect of FRQ on light transduction was investigated by comparing various light-induced responses. Carotenogenesis and conidiation each represent the result of extensive coordination of gene expression and metabolism. Both are regulated by light but, unlike conidiation, carotenogenesis is not circadianly rhythmic in DD. The WCs are essential for both of these light-regulated physiologies (Russo, 1988; Linden et al., 1999), while FRQ is only essential for light-regulated conidiation (Figure 3), indicating a bifurcation in the LIP (Figure 7A). The effect of FRQ on conidiation is specific for light transduction and not for development, per se, as conidiation proceeds normally in FRQ-less strains, though without photic or circadian regulation (Figure 3A, tube 3). FRQ has a different function in the branch leading to carotenogenesis: without FRQ, the quantities of light-induced RNAs and carotenoids are lower (Figures 3C and D, and 4).
Additional evidence for a bifurcation in the LIP lies in the fluence responses of the respective branches. While light synchronizes conidiation above a threshold of 2 nE/m2/s (gray arrow in Figure 3D; see Merrow et al., 1999; Roenneberg and Merrow, 2001), carotenogenesis is half-maximal at an ∼50-fold higher fluence rate (black arrow in Figure 3D).
Circadian response regulation
FRQ plays a critical role in the Neurospora circadian system, as well as in potentiating light-induced carotenogenesis. In addition, we have shown that light responses are stronger at specific circadian times compared with others (Figure 5D). The circadian response regulation is apparent within minutes (e.g. in RNA induction, Figure 5A and C). This represents a general phenomenon whereby evoked sensory processes, which are not rhythmic in constant conditions, are modulated by the circadian system, e.g. olfaction in Drosophila (Krishnan et al., 1999) or chlorophyll production and flowering in plants (Claus and Rau, 1956). In Neurospora, the strength of the light-induced response (in the non-circadianly expressed, evoked branch: wc-1, al-1 RNA and carotenogenesis) approximately correlates with the amount of WC-1 protein: no WC-1, no response; low levels of WC-1, weak response; and higher levels of WC-1, stronger response. Thus, FRQ may transduce the circadian modulation of light responses by dictating WC-1 levels (Figure 7A and B).
This correlation, however, is not entirely straightforward. There are some conditions where Neurospora is not stimulated by light despite abundant WC-1 protein. During prolonged light exposure, some RNA levels adapt (are down-regulated) although WC-1 levels remain the same (see Figures 4, and 5A and C; Talora et al., 1999). Furthermore, Neurospora is refractory to additional light increments for some hours after a dark to light transition (Schmidhauser et al., 1990; Arpaia et al., 1999).
How do FRQ and the WCs functionally participate in the various regulatory processes described above? The WC proteins are thought to possess both photoreceptor and transcription factor activity. FRQ function within the clock has been described with respect to transcriptional control. Indeed, all of these attributes could derive from transcription factor function as modulated by complex formation (Figure 6). Signal transduction by a photoreceptor has been shown to occur via transcriptional control. The plant photoreceptor phytochrome B forms a complex with and modulates the function of a DNA-bound transcription factor (Martinez-Garcia et al., 2000). Interestingly, this particular photoreceptor feeds into the Arabidopsis circadian system (Somers et al., 1998).
FRQ and light entrainment
The response of circadian systems to a given zeitgeber signal (e.g. a light pulse) is different at different circadian times. The quantification of these differential responses results in a phase response curve (PRC), a signature for a given circadian system and the respective zeitgeber. This quality is the basis for circadian synchronization (entrainment). FRQ is required for the Neurospora circadian system to respond to light. Induction of frq RNA by short light pulses has been invoked to explain the light PRC of Neurospora (Crosthwaite et al., 1995). When frq is increased to or above its DD maximum, then the circadian system is reset to the phase of FRQmax in DD (Figure 1B). Maximum phase shifts are thus achieved when a light pulse is given at the FRQ minimum (Crosthwaite et al., 1995).
Entrainment is distinct from a driven synchronization whereby a system is turned on or off directly by an exogenous signal. We have shown that light drives conidiation rather than entraining it in a PRC-dependent fashion (Merrow et al., 1999). In LD cycles, conidiation occurs with a constant lag after lights-off, regardless of zeitgeber period (i.e. in full photoperiods the response is the same for all tested circadian times). This feature is apparent even at the lowest light fluences that synchronize conidiation (2 nE/m2/s, comparable with moonlight, see Figure 3D). In spite of this driven synchronization, the lag between lights-off and conidiation onset is strain specific. It correlates with the period of the specific frq allele in DD, indicating that it depends on the activity of FRQ in the dark phase of the LD cycle. Although light pulse-induced frq levels correlate well with phase shifting (Crosthwaite et al., 1995), synchronization of conidiation in full LD cycles appears to function via FRQ protein, independently of its transcription. In the experiments shown in Figure 3A and B (constitutive frq expression from the qa-2 promoter), the lag between lights-off and conidiation is similar to that for frq+ (note that the qa promoter per se is non-responsive to light; Crosthwaite et al., 1995). One would predict that constitutive expression of different frq alleles (similar to the experiments in Figure 3A and B) would also give a strain-specific lag. With regard to mechanism, our experiments show no qualitative or quantitative effects of light on constitutively expressed FRQ protein after 6 or 12 h incubation (Figure 3B); however, acute light effects on FRQ and/or on the formation of the WCC–FRQ complex are possible, and might contribute to the rhythmicity.
Whether circadian systems are entrained primarily by the prolonged presence of light in LD cycles (parametric entrainment) or by acute changes in light (non-parametric entrainment) has long been a point of discussion (Beersma et al., 1999). In Drosophila, cryptochrome has been implicated in mediating non-parametric, but not parametric, entrainment (Stanewsky et al., 1998). Our results indicate that synchronization by full photoperiods does not require frq transcription, although phase shifting by short light pulses correlates well with frq induction (Crosthwaite et al., 1995). Thus, frq transcription may be involved in non-parametric light effects, and FRQ protein, together with the WCC complex, may mediate parametric light effects.
So, where does FRQ act within the circadian system: upstream of, central to or downstream of the rhythm generator? Our results show that the participation of FRQ is more complex. Previous results suggest that FRQ is not necessarily central to the rhythm generator (Merrow et al., 1999; Lakin-Thomas and Brody, 2000). Given the fact that conidiation is not regulated by light in its absence, FRQ appears to function as a gateway through which the light signal must pass, placing it upstream of the rhythm generator. Yet without FRQ, light responsiveness (e.g. of carotenogenesis) is not circadianly regulated, placing FRQ downstream of the rhythm generator.
The close association between light input and molecular components of circadian systems is one of the functional parallels characterized from cyanobacteria to mice (Crosthwaite et al., 1997; Shigeyoshi et al., 1997; Emery et al., 1998; Stanewsky et al., 1998; Bognar et al., 1999; Ceriani et al., 1999; Iwasaki et al., 2000; Roenneberg and Merrow, 2000). In addition, the Neurospora transcription/translation feedback loop, as described here (and in Lee et al., 2000) shares many features with those of Drosophila (Lee et al., 1998; Glossop et al., 1999) and mice (Shearman et al., 2000). In Neurospora, it also serves as the LIP.
Materials and methods
Strains and media
frq+ and frq9 are standard laboratory strains with the bd mutation in their background (Loros et al., 1986; Merrow et al., 1999). frq10 is a knockout of the frq locus (Aronson et al., 1994a) and is also on the bd background. The Δwc-1 strain is a functional knockout, generated by repeat-induced point (RIP) mutation (Talora et al., 1999), which was crossed with bd (bd Δwc-1 4–7). RIP is a method for gene inactivation in Neurospora, whereby the presence of a duplicate copy of DNA signals methylation and inactivation of both copies when the strain is put through a sexual cross (Selker and Garrett, 1988). This Δwc-1 strain makes no RNA or protein. wc-2-234W is a loss-of-function mutant, which produces a truncated protein (Linden and Macino, 1997), similarly to the frq9 strain (data not shown). frq10 qa-2p-frq describes a strain with the genotype bd frq10 his3::his3qa-2p-frqhis. To generate this, the bd frq10 his3 strain was transformed at the his-3 locus with a his-tagged copy of the frq open reading frame fused with the Neurospora qa-2 promoter (for a similar construction see Aronson et al., 1994b).
Race tubes contained 1× Vogel’s medium (Vogel, 1964) with 0.5% l-arginine, 10 ng/ml biotin and 2% agar (i.e. no glucose). In some race tubes and liquid cultures (–) quinic acid adjusted to pH 6.0 was added to 10–3 M. For light induction experiments (Figures 4 and 5A–C), as well as for the experiments shown in Figure 2, stationary, liquid cultures were grown in 1× Vogel’s medium with 2% glucose, 0.5% l-arginine and 10 ng/ml biotin. For the circadian time course (Figure 1), the same medium was used but with shaking cultures. For induction of carotenoids (Figure 3C–D and 5D), arginine was omitted from this medium.
Race tube experiments and circadian time courses
Race tubes were inoculated, germinated in constant light for ∼1 day and transferred to an LD 12:12 cycle at 25°C. The fluence rate was 2 µE/m2/s, delivered by cool white fluorescent bulbs (Osram, Germany). Race tubes were analyzed with the Chrono program (Roenneberg and Taylor, 2000). Circadian time courses were performed essentially as described (Aronson et al., 1994b) except for sample intervals (2 h). The time when cultures were transferred to constant darkness is, by definition, CT12 (dusk). Thus in these experiments, the actual time in DD (tDD) is transformed to CT by the following formula: CT = tDD × (24/FRP) + 12. For example, for Neurospora (frq+) after 16 h in DD, CT = 16 × (24/22) + 12 = 29.44. By convention, CT is always expressed as 0–24 circadian hours, so for this example, 29.44 – 24 = 5.44.
Light-induced mycelial carotenogenesis
Conidia (3 × 106) were inoculated into 20 ml of carotenogenesis medium in 8.5 cm Petri dishes. Sample cultures were evaluated by microscopy for the absence of conidial development. For the experiment shown in Figure 3C, mycelial pads were generated over 2 days incubation at 30°C in constant darkness. For the experiment shown in Figure 3D, pads were cultivated in constant light, then transferred to darkness prior to the dark to light transfers at the indicated times. The transfers to DD were staggered to control for age of the cultures. Excess medium was drained from the dishes for both dark control and light-exposed samples. After 5 h of light exposure, samples were harvested and dried by vacuum filtration. Carotenoid content was determined essentially as described earlier (Linden et al., 1997). A 150 µg aliquot of tissue was disrupted in a Ribolyzer (Hybaid-AGS, Germany) in 500 µl of methanol, 750 µl of hexane, 400 µg of sand and 400 µg of glass beads. The supernatant was collected following centrifugation and the spectral absorption was determined at 445 nm for the experiment shown in Figure 3D. For the experiment shown in Figure 3C, background was subtracted based on the levels of al-1 mycelial carotenoids, while for Figure 3D the background was determined from the DD levels of mycelial carotenoids in the two strains. For the experiment shown in Figure 5D, because carotenoid induction increases gradually over time in constant darkness, a non-rhythmic trend was subtracted from the time series of carotenoid induction.
Light induction experiments
In the experiments shown in Figures 4 and 5, 25 ml of medium in 250 ml flasks was inoculated with 3.5 × 106 conidia. These cultures were held in constant light at 30°C for ∼1–2 days prior to transfer to constant darkness (DD) at 25°C. Following the indicated time in darkness, age-matched cultures were exposed to continuous light (fluences indicated in the figure legends) until they were harvested by filtration and frozen in liquid nitrogen.
RNA analyses
RNA was prepared essentially as previously described (Crosthwaite et al., 1995), except that a single phenol extraction was performed. RNA was run on 1.2% agarose–formaldehyde gels (otherwise as in Merrow et al., 1997), and blotted onto nylon (HybondN, Amersham Corp.). Riboprobes were used to probe the blots for frq, wc-1 and rRNA. The frq riboprobe was described elsewhere (Crosthwaite et al., 1995). The wc-1 riboprobe was generated from pGEM4wc-1 using T7 RNA polymerase with the linearized plasmid. The riboprobe for rRNA was generated from pEVEII with T3 polymerase, without linearizing. The al-1 blots were probed and quantified as described (Linden and Macino, 1997). In general, blots were exposed to phosphorimager screens for quantitative analysis. Where films were used for quantification, the GelAnalysis program (T.Roenneberg, University of Munich) was utilized.
Protein analyses
Protein was extracted from mycelia by previously described methods (Garceau et al., 1997). In some cases, the tissue was disrupted with the Ribolyzer (Hybaid-AGS, Germany). A 200 µg aliquot per lane was loaded onto 7.5% SDS–polyacrylamide gels for FRQ or 5% gels for WC-1. FRQ blots were probed with the monoclonal antibody mFRQ3G11. Anti-FRQ monoclonal antibodies were produced from C57Bl/6×Balb/c F1 mice immunized with a total of 120 µg of recombinant myelin basic protein (MBP)–FRQ protein in Freund’s incomplete adjuvant. Hybridomas were produced using standard techniques and the antibodies were characterized by reactivity against recombinant FRQ in enzyme-linked immunosorbent assay (ELISA) and then on western blots with recombinant deletion proteins and cell extracts from frq10. The isotype of mFRQ3G11 is IgG1. The antibodies for WC-1 and WC-2 used in Figures 1, 2 and 6A have been described previously (Talora et al., 1999). Co-immunoprecipitations were performed as described (Talora et al., 1999). The specificity of the rabbit anti-WC-1 used in co-immunoprecipitations (Figure 6B) was confirmed with the Δwc-1 strain.
Data analysis
All mathematical fits were carried out by an iterative, least square method. Adaptation profiles were fitted with an equation combining a Gauss distribution (G), a saturated curve (S) and an exponential decay (D):
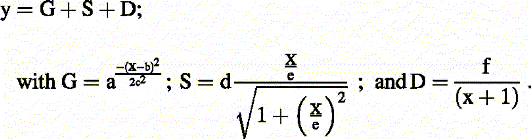
All rhythmic time courses were fitted with a cosine function with one exception: due to the non-sinusoidal shape of the time series, WC-1 (Figure 1D) was fitted by eye. The S-curves in Figure 3D were fitted with the following equation:
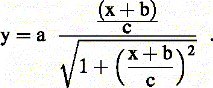
Acknowledgments
Acknowledgements
We would like to thank Ina Contag for her help in producing monoclonal antibodies, Vera Schiewe and Astrid Bauer for excellent technical support, and Dr D.Bell-Pedersen for bd frq10 his3 strains. This work was supported by a DFG grant to T.R. and the Meyer-Struckmann-Stiftung to M.M. and T.R.; MURST Cofin 99 and Cenci Bolognetti to G.M.; and SFB 455, Project B3 of the DFG to J.J.
References
- Aronson B.D., Johnson,K.A. and Dunlap,J.C. (1994a) The circadian clock locus frequency: a single ORF defines period length and temperature compensation. Proc. Natl Acad. Sci. USA, 91, 7683–7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson B.D., Johnson,K.A., Loros,J.J. and Dunlap,J.C. (1994b) Negative feedback defining a circadian clock: autoregulation of the clock gene frequency. Science, 263, 1578–1584. [DOI] [PubMed] [Google Scholar]
- Arpaia G., Loros,J.J., Dunlap,J.C., Morelli,G. and Macino,G. (1993) The interplay of light and the circadian clock. Plant Physiol., 102, 1299–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpaia G., Loros,J.J., Dunlap,J.C., Morelli,G. and Macino,G. (1995) Light induction of the clock-controlled gene ccg-1 is not transduced through the circadian clock in Neurospora crassa. Mol. Gen. Genet., 247, 157–163. [DOI] [PubMed] [Google Scholar]
- Arpaia G., Cerri,F., Baima,S. and Macino,G. (1999) Protein kinase C may be a novel component of the blue light transduction pathway in Neurospora crassa. Mol. Gen. Genet., 262, 314–322. [DOI] [PubMed] [Google Scholar]
- Bae K., Lee,C., Sidote,D., Chuang,K.Y. and Edery,I. (1998) Circadian regulation of a Drosophila homolog of the mammalian Clock gene: PER and TIM function as positive regulators. Mol. Cell. Biol., 18, 6142–6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballario P., Vittorioso,P., Magrelli,A., Talora,C., Cabibbo,A. and Macino,G. (1996) White collar-1, a central regulator of blue light responses in Neurospora, is a zinc finger protein. EMBO J., 15, 1650–1657. [PMC free article] [PubMed] [Google Scholar]
- Beersma D.G., Daan,S. and Hut,R.A. (1999) Accuracy of circadian entrainment under fluctuating light conditions: contributions of phase and period responses. J. Biol. Rhythms, 14, 320–329. [DOI] [PubMed] [Google Scholar]
- Bognar L.K., Adam,A.H., Thain,S.C., Nagy,F. and Millar,A.J. (1999) The circadian clock controls the expression pattern of the circadian input photoreceptor, phytochrome B. Proc. Natl Acad. Sci. USA, 96, 14652–14657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceriani M.F., Darlington,T.K., Staknis,D., Mas,P., Petti,A.A., Weitz,C.J. and Kay,S.A. (1999) Light-dependent sequestration of TIMELESS by CRYPTOCHROME. Science, 285, 553–556. [DOI] [PubMed] [Google Scholar]
- Chang B. and Nakashima,H. (1997) Effects of light–dark cycles on the circadian conidiation rhythm in Neurospora crassa. J. Plant Res., 110, 449–453. [Google Scholar]
- Claus H. and Rau,W. (1956) Über die Blütenbildung von Hyoscyamus niger und Arabidopsis in 72-Stunden-Zyklen. Z. Bot., 44, 437–454. [Google Scholar]
- Crosthwaite S.K., Loros,J.J. and Dunlap,J.C. (1995) Light-induced resetting of a circadian clock is mediated by a rapid increase in frequency transcript. Cell, 81, 1003–1012. [DOI] [PubMed] [Google Scholar]
- Crosthwaite S.K., Dunlap,J.C. and Loros,J.J. (1997) Neurospora wc-1 and wc-2: transcription, photoresponses and the origin of circadian rhythmicity. Science, 276, 763–769. [DOI] [PubMed] [Google Scholar]
- De Fabo E.C., Harding,R.W. and Shropshire,W.,Jr (1976) Action spectrum between 260 and 800 nanometers for the photoinduction of carotenoid biosynthesis in Neurospora crassa. Plant Physiol., 57, 440–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degli-Innocenti F. and Russo,V.E. (1984) Isolation of new white collar mutants of Neurospora crassa and studies on their behavior in the blue light-induced formation of protoperithecia. J. Bacteriol., 159, 757–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap J.C. (1999) Molecular bases for circadian clocks. Cell, 96, 271–290. [DOI] [PubMed] [Google Scholar]
- Emery P., So,W.V., Kaneko,M., Hall,J.C. and Rosbash,M. (1998) CRY, a Drosophila clock and light-regulated cryptochrome, is a major contributor to circadian rhythm resetting and photosensitivity. Cell, 95, 669–679. [DOI] [PubMed] [Google Scholar]
- Feldman J.F. and Hoyle,M.N. (1973) Isolation of circadian clock mutants of Neurospora crassa. Genetics, 75, 605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garceau N.Y., Liu,Y., Loros,J.J. and Dunlap,J. (1997) Alternative initiation of translation and time specific phosphorylation yield multiple forms of essential clock protein FREQUENCY. Cell, 89, 469–476. [DOI] [PubMed] [Google Scholar]
- Glossop N.R.G., Lyons,L.C. and Hardin,P.E. (1999) Interlocked feedback loops within the Drosophila circadian oscillator. Science, 286, 766–778. [DOI] [PubMed] [Google Scholar]
- Harding R.W. and Turner,R.V. (1981) Photoregulation of the carotenoid biosynthetic pathway in albino and white collar mutants of Neurospora crassa. Plant Physiol., 68, 745–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huala E., Oeller,P.W., Liscum,E., Han,I.-S., Larsen,E. and Briggs,W.R. (1997) Arabidopsis NPH1: a protein kinase with a putative redox-sensing domain. Science, 278, 2120–2123. [DOI] [PubMed] [Google Scholar]
- Iwasaki H., Williams,S.B., Kitayama,Y., Ishiura,M., Golden,S.S. and Kondo,T. (2000) A KaiC-interacting sensory hisidine kinase, SasA, necessary to sustain robust circadian oscillation in cyanobacteria. Cell, 101, 223–233. [DOI] [PubMed] [Google Scholar]
- Krishnan B., Dryer,S.E. and Hardin,P.E. (1999) Circadian rhythms in olfactory responses of Drosophila melanogaster. Nature, 400, 375–378. [DOI] [PubMed] [Google Scholar]
- Lakin-Thomas P.L. and Brody,S. (2000) Circadian rhythms in Neurospora crassa: lipid deficiencies restore robust rhythmicity to null frequency and white-collar mutants. Proc. Natl Acad. Sci. USA, 97, 256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C., Bae,K. and Edery,I. (1998) The Drosophila CLOCK protein undergoes daily rhythms in abundance, phosphorylation and interactions with PER–TIM complex. Neuron, 21, 857–867. [DOI] [PubMed] [Google Scholar]
- Lee K., Loros,J.J. and Dunlap,J.C. (2000) Interconnected feedback loops in the Neurospora circadian system. Science, 289, 107–110. [DOI] [PubMed] [Google Scholar]
- Linden H. and Macino,G. (1997) White collar 2, a partner in blue-light signal transduction controlling expression of light-regulated genes in Neurospora crassa. EMBO J., 16, 98–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden H., Rodriguez-Franco,M. and Macino,G. (1997) Mutants of Neurospora crassa defective in regulation of blue light perception. Mol. Gen. Genet., 254, 111–118. [DOI] [PubMed] [Google Scholar]
- Linden H., Ballario,P., Arpaia,G. and Macino,G. (1999) Seeing the light: news in Neurospora blue light signal transduction. Adv. Genet., 41, 35–54. [DOI] [PubMed] [Google Scholar]
- Loros J.J., Richman,A. and Feldman,J.F. (1986) A recessive circadian clock mutation at the frq locus of Neurospora crassa. Genetics, 114, 1095–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Garcia J.F., Huq,E. and Quail,P.H. (2000) Direct targeting of light signals to a promoter element-bound transcription factor. Science, 288, 859–863. [DOI] [PubMed] [Google Scholar]
- Merrow M., Garceau,N. and Dunlap,J. (1997) Dissection of a circadian oscillation into discrete domains. Proc. Natl Acad. Sci. USA, 94, 3877–3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrow M., Brunner,M. and Roenneberg,T. (1999) Assignment of circadian function for the Neurospora clock gene frequency. Nature, 399, 584–586. [DOI] [PubMed] [Google Scholar]
- Millar A.J., Straumer,M., Chorry,J., Chua,N.-H. and Kay,S.A. (1995) The regulation of circadian period by phototransduction pathways in Arabidopsis. Science, 267, 1163–1166. [DOI] [PubMed] [Google Scholar]
- Pittendrigh C.S. (1993) Temporal organization: reflections of a Darwinian clock-watcher. Annu. Rev. Physiol., 55, 17–54. [DOI] [PubMed] [Google Scholar]
- Roenneberg T. and Deng,T.-S. (1997) Photobiology of the Gonyaulax circadian system: I. Different phase response curves for red and blue light. Planta, 202, 494–501. [Google Scholar]
- Roenneberg T. and Foster,R.G. (1997) Twilight times—light and the circadian system. Photochem. Photobiol., 66, 549–561. [DOI] [PubMed] [Google Scholar]
- Roenneberg T. and Merrow,M. (1998) Molecular circadian oscillators—an alternative hypothesis. J. Biol. Rhythms, 13, 167–179. [DOI] [PubMed] [Google Scholar]
- Roenneberg T. and Merrow,M. (1999) Circadian clocks and metabolism. J. Biol. Rhythms, 14, 17–27. [DOI] [PubMed] [Google Scholar]
- Roenneberg T. and Merrow,M. (2000) Circadian light input: omnes viae Romam ducunt. Curr. Biol., 10, R742–R745. [DOI] [PubMed] [Google Scholar]
- Roenneberg T. and Merrow,M. (2001) The role of feedbacks in circadian systems. In Hiroshige,T. and Honma,K. (eds), The Brain That Keeps Time. Hokkaido University Press, Sapporo, in press.
- Roenneberg T. and Taylor,W. (1994) Light induced phase responses in Gonyaulax are drastically altered by creatine. J. Biol. Rhythms, 9, 1–12. [DOI] [PubMed] [Google Scholar]
- Roenneberg T. and Taylor,W. (2000) Automated recordings of bioluminescence with special reference to the analysis of circadian rhythms. Methods Enzymol., 305, 104–119. [DOI] [PubMed] [Google Scholar]
- Russo V.E. (1988) Blue light induces circadian rhythms in the bd mutant of Neurospora: double mutants bd,wc-1 and bd,wc-2 are blind. J. Photochem. Photobiol. B, 2, 59–65. [DOI] [PubMed] [Google Scholar]
- Schmidhauser T.J., Lauter,F.R., Russo,V.E. and Yanofsky,C. (1990) Cloning, sequence and photoregulation of al-1, a carotenoid biosynthetic gene of Neurospora crassa. Mol. Cell. Biol., 10, 5064–5070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selker E.U. and Garrett,P.W. (1988) DNA sequence duplications trigger gene inactivation in Neurospora crassa. Proc. Natl Acad. Sci. USA, 85, 6870–6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearman L.P. et al. (2000) Interacting molecular loops in the mammalian circadian clock. Science, 288, 1013–1019. [DOI] [PubMed] [Google Scholar]
- Shigeyoshi Y. et al. (1997) Light-induced resetting of a mammalian clock is associated with rapid induction of the mPer1 transcript. Cell, 91, 1043–1053. [DOI] [PubMed] [Google Scholar]
- Somers D.E., Devlin,P.F. and Kay,S.A. (1998) Phytochromes and cryptochromes in the entrainment of the Arabidopsis circadian clock. Science, 282, 1488–1490. [DOI] [PubMed] [Google Scholar]
- Stanewsky R., Kaneko,M., Emery,P., Beretta,B., Wagner-Smith,K., Kay,S.A., Rosbash,M. and Hall,J.C. (1998) The cryb mutation identifies cryptochrome as a circadian photoreceptor in Drosophila. Cell, 95, 681–692. [DOI] [PubMed] [Google Scholar]
- Talora C., Franchi,L., Linden,H., Ballario,P. and Macino,G. (1999) Role of a white collar-1–white collar-2 complex in blue-light signal transduction. EMBO J., 18, 4961–4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel H.J. (1964) Distribution of lysine pathways among fungi: evolutionary implications. Am. Nat., 98, 435–446. [Google Scholar]


