Abstract
Systemic lupus erythematosus is an autoimmune disease in which most patients express Abs that bind double-stranded DNA. Recent work has shown that a subset of lupus Abs can crossreact with the NR2A and NR2B subunits of the NMDA receptor. This receptor is expressed in neurons throughout the brain but is at highest density within cells of the hippocampus, amygdala, and hypothalamus. The neurons in the CNS are normally protected from brain-reactive Abs by the blood–brain barrier (BBB); however, a breach in the barrier's integrity exposes neurons to potentially pathogenic Abs. Previously, we have shown that mice that are immunized with a peptide mimetope of DNA produce lupus-like Abs that crossreact with DNA and the NMDA receptor. Moreover, after abrogation of the BBB by treatment with lipopolysaccharide, the immunized mice display hippocampal neuron damage with ensuing memory impairment. Given that rises in epinephrine can increase cerebral blood flow and can cause leaks in the BBB, we decided to investigate whether epinephrine could act as a permissive agent for Ab-mediated neurotoxicity. Here, we show that peptide-immunized mice, given epinephrine to open the BBB, lose neurons in the lateral amygdala and develop a behavioral disorder characterized by a deficient response to fear-conditioning paradigms. Thus, the agent used to open the BBB determines which brain region is made vulnerable to neurotoxic Abs, and Abs that penetrate brain tissue can cause changes not only in cognitive competence, but also in emotional behavior.
Keywords: amygdala, anti-NMDA receptor antibody, anti-DNA antibody, fear conditioning, systemic lupus erythematosus
Many disturbances in behavior, including paraneoplastic syndromes, movement disorders, schizophrenia, and autism, as well as other neuropsychiatric syndromes, have been associated with the presence of serum Abs with specificity for some, often unidentified, brain antigen (1–6). Although data suggesting associations among disease states, brain-reactive Abs, and altered neuronal function continue to accumulate, there is no clear understanding of the mechanisms that permit serum Abs to gain access to brain tissue and then to mediate a change in behavior. Systemic lupus erythematosus (SLE) is an autoimmune disease with protean clinical manifestations, including CNS manifestations (7, 8). In 1999, an international consortium identified 19 different symptom complexes that comprise neuropsychiatric lupus (9). These symptom complexes likely result from many different pathogenic mechanisms, but autoAbs clearly can contribute to lupus brain disease. It has been demonstrated, for example, that antiphospholipid Abs cause a hypercoagulable state and are responsible for strokes in lupus patients (10–12). Several studies have explored the potential CNS pathogenicity of other lupus autoAbs, but there have not yet been clear associations of particular symptom complexes with particular autospecificities.
The most common autoAb present in the serum of patients with SLE is Ab to double-stranded DNA (dsDNA). These Abs are known to deposit in renal glomeruli, thereby contributing to lupus nephritis, as well as in skin and serosal membranes (13–15). We have previously demonstrated that a subset of these Abs cross-reacts with a 5-aa consensus sequence (D/E W D/E Y S/G, or DWEYS for short) present in the NR2A and NR2B subunits of the NMDA receptor (NMDAR) (16, 17). These anti-dsDNA, anti-NR2 cross-reactive Abs can cause neuronal death by excitotoxicity and apoptosis, suggesting that they might play a role in clinical neuropsychiatric dysfunction. We further demonstrated that these Abs can be found in the cerebrospinal fluid of lupus patients and, at the concentrations present in the fluid, can mediate neuronal apoptosis in the mouse CNS. Notably, at a 1:100 dilution of this concentration, the Abs are toxic in cultured human fetal neurons (16).
We are able to examine the in vivo effect of these Abs in the circulation because BALB/c mice immunized with a multimeric form of the DWEYS pentapeptide (MAP) develop high titers of anti-dsDNA, anti-NR2 Abs (17). These Abs deposit in renal glomeruli and cause proteinuria. Immunized mice have no evidence of brain injury, however, until there is a breach in the blood–brain barrier (BBB) (17). When mice expressing anti-dsDNA, anti-NR2 Abs are given bacterial LPS to mimic a bacterial infection, there is an influx of IgG into the brain with preferential binding of anti-dsDNA, anti-NR2 Abs in the hippocampus. By 1 week after LPS administration, there is a significant loss of hippocampal neurons, which causes memory impairment on behavioral tasks. Neuronal death is apoptotic, with no inflammatory cell infiltrate and no detectable complement deposition at the site of Ab binding. Furthermore, neuronal death can be prevented by systemic administration of memantine, an NMDAR antagonist (17). In this study, we show that epinephrine, another agent known to open the BBB (18), preferentially leads to apoptosis of neurons of the lateral amygdala, resulting in a behavioral disorder.
Results
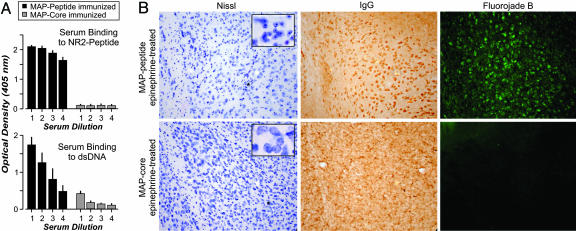
Mice with High Serum Titers of Anti-dsDNA, Anti-NR2 Abs Display Neuronal Damage in the Amygdala When Given Epinephrine. There are several known insults that can open the BBB (19). For example, the rise in epinephrine induced by stress is known to increase cerebral blood flow and to cause leaks in the BBB (18, 20, 21). Because stress is such a prevalent occurrence, we elected to ask whether epinephrine could mediate sufficient abrogation of the BBB to expose neurons to Ab-mediated neurotoxicity. Mice immunized with MAP developed high titers of anti-dsDNA, anti-NR2 Ab, whereas mice immunized with MAP-core (polylysine backbone without peptide) did not (Fig. 1A). We have previously shown that these autoAbs are exclusively IgG1 (22). MAP- and MAP-core-immunized mice that were given epinephrine did not show any evidence of damage to hippocampal neurons (results not shown). When MAP-immunized mice (n = 15) were given epinephrine (two doses of 100 nM, 24 h apart), there was clear evidence of damage to amygdalar neurons (Fig. 1B Upper). Many cells were shrunken and displayed nuclei containing chromatin clumps (Fig. 1B Upper Left). Furthermore, the cells had IgG bound to them (Fig. 1B Upper Center) and displayed reactivity with Fluorojade-B, a marker of neuronal stress (Fig. 1B Upper Right). The stress effect was robust: it was observed in every amygdala section we examined in all 15 animals. There was no evidence of activation of microglial cells or astrocytes (results not shown). Mice immunized with MAP-core and given epinephrine (n = 11) displayed no neuronal damage in the amygdala (Fig. 1B Lower). In summary, both anti-NR2 Ab and an epinephrine-induced breach in the BBB were necessary for regional damage within the amygdala; neither Ab nor epinephrine alone mediated this injury. Furthermore, the insult was noninflammatory.
Fig. 1.
Epinephrine treatment allows selective penetration of anti-dsDNA, anti-NR2 autoAb into the amygdala. (A) Mice immunized with MAP reveal high titer of anti-peptide, anti-dsDNA Ab in the serum. Mice immunized with control MAP-core (polylysine backbone) do not. The graphs show titration of serum binding to NR2-peptide (Upper) or dsDNA (Lower). Each bar shows the mean ± SEM (n = 5 mice). Serum dilutions for each bar: 1, 500; 2, 1,000; 3, 2,000; and 4, 4,000. (B) Immunized mice with anti-dsDNA, anti-NR2 Abs show Nissl-stained amygdalar neurons that are shrunken and possess clumped nuclei (Upper). (Magnification: ×100; Inset, ×800.) These neurons also show IgG binding and are positive for Fluorojade-B, a marker of neurodegeneration. Mice immunized with MAP-core show normal amygdalar neurons with diffuse, nonneuronal IgG binding and show no evidence of neurodegeneration by Fluorojade-B (Lower). (Magnification: ×100; Inset, ×800.)
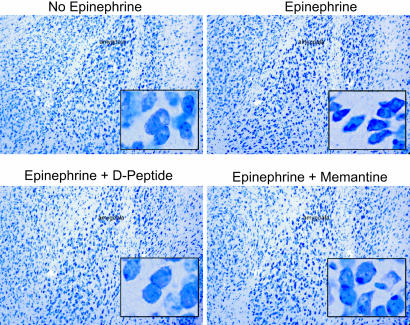
Both Memantine and D-peptide Protect MAP-Immunized, Epinephrine-Exposed Mice from Neuronal Damage. To confirm that the neuronal damage in the amygdala was mediated by activation of the NMDAR, MAP-immunized mice were treated with memantine, an NMDAR antagonist, before and during epinephrine exposure (23). Memantine does not inhibit neuronal binding by anti-DNA, anti-NR2 cross-reactive Abs (results not shown). Because we are interested in therapeutic options to protect the CNS from Ab-mediated injury in SLE, we decided to test whether administration of the D-isoform of the consensus peptide (D-peptide) could also lead to neuronal sparing. The D-peptide is resistant to proteolytic cleavage and is capable of binding to anti-dsDNA, anti-NR2 Abs (13). We hypothesized, therefore, that it would prevent Abs from binding the NMDAR.
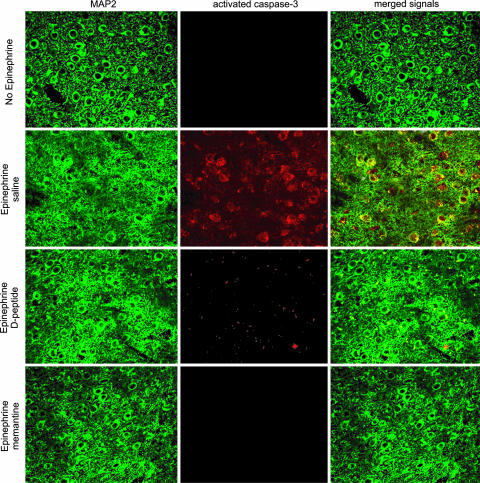
MAP-immunized mice, carrying specific Ab and not treated with epinephrine (n = 8), showed no damage in the amygdala (Fig. 2 Upper Left). Mice that were given epinephrine (n = 15) displayed evidence of apoptotic neurons in the lateral amygdala (Fig. 2 Upper Right). Mice given epinephrine and receiving memantine (n = 6) showed no evidence of neuronal damage (Fig. 2 Lower Right), a result that confirmed that the damage was mediated by NMDAR signaling. Mice given D-peptide before epinephrine exposure (n = 3) were spared neuronal damage as effectively as mice given memantine (Fig. 2 Lower Left). We confirmed this observation by staining for activated caspase-3 in the brains of immunized mice to identify cells undergoing apoptotic death. MAP2 staining was used to identify neurons. We found that only brains from mice given epinephrine and saline showed evidence of caspase-3-positive apoptotic neurons present in the lateral amygdala (Fig. 3). Immunized mice without epinephrine exposure and immunized mice exposed to epinephrine but given either memantine or D-peptide displayed no neuronal death.
Fig. 2.
D-peptide or memantine treatment before epinephrine exposure spares amygdalar neurons in immunized mice. Anti-NR2 Abs do not destroy neurons without an epinephrine-mediated breach of the BBB. Normal neurons in the lateral amygdala of an untreated mouse (BALB/c) immunized with MAP are shown. (Magnification: ×200; Inset, ×400.) Epinephrine treatment of an animal immunized with MAP demonstrates shrunken and pycnotic amygdalar neurons. The sections are comparable, although the amygdala of an animal immunized with MAP and treated with epinephrine and D-peptide or memantine demonstrates normal neurons.
Fig. 3.
D-peptide or memantine treatment before epinephrine exposure prevents activation of caspase-3 in the amygdala of MAP-immunized mice. Neurons are identified by anti-MAP2 immunoreactivity (FITC-IgG, green). Activated caspase-3 (Rhodamine Red-X IgG, red) is apparent only in neurons from mice immunized with anti-peptide Abs and treated with epinephrine. Colocalization of MAP-2 and activated caspase-3 yields a merged yellow signal. (Magnification: ×640.)
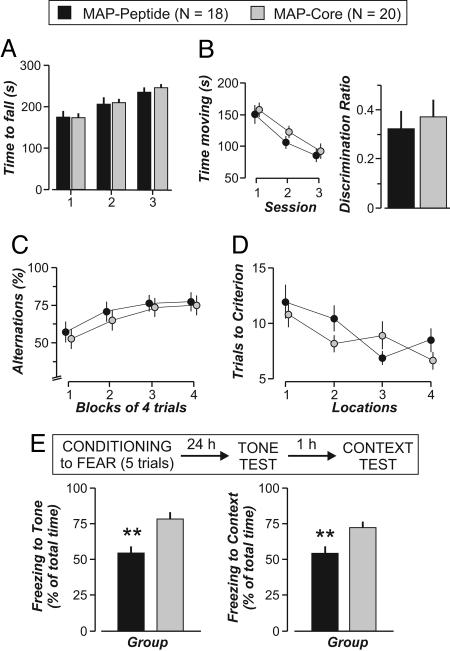
Mice with Ab-Mediated Neuronal Damage in the Amygdala Display an Emotional Disturbance. To study whether the damage to neurons in the amygdala produced detectable alterations in behavior, mice immunized with MAP (n = 18) or MAP-core (n = 20) were given epinephrine, and after several weeks, all mice were subjected to an extensive battery of behavioral tests (17). Both groups performed similarly in a variety of tests that examined basic neurological reflexes. Both groups displayed normal feeding, no difference in average body weight, and normal grooming (results not shown). There was no difference between groups in tests of muscle strength or balance, and both groups behaved equally in the two-object task that tests for recognition memory (Fig. 4 A and B). Importantly, both groups performed similarly on two tests that depend on the integrity of the hippocampus. The T-maze task for spatial working memory and the training-to-criterion test for memory flexibility, which are highly sensitive to hippocampal dysfunction, showed no difference between groups (Fig. 4 C and D).
Fig. 4.
Immunized mice that produce anti-dsDNA, anti-NR2 Abs and are treated with epinephrine show disruption in associative fear conditioning. (A) Normal motor balance in mice immunized with MAP (n = 18) and treated with epinephrine compared with MAP-core mice (n = 20). ANOVA reveals no difference between groups (F = 0.03, df = 1/31, P = 0.85). (B) Recognition memory, assessed with the two-object test, is similar between groups (discrimination ratios, t = 0.46, P = 0.65, t test). Additionally, the groups show comparable habituation to the chamber in which the two-object test will proceed. (C and D) Mice behave similarly in tests that depend on the integrity of the hippocampus: the T-maze test for spatial working memory (C)(F = 1.39, df = 1/31, P = 0.25, ANOVA on alternations) and the training-to-criterion test (D)(F = 0.92, df = 1/36, P = 0.35, ANOVA on locations). (E) Fear conditioning, assessed by fear of an auditory conditional stimulus (tone) and of the context in which the mice are conditioned, shows that the MAP group has a severely impaired fear response (freezing). The time spent freezing during the tone test is significantly lower (t = 4.31, P < 0.0005, t test), as in the context test (t = 3.1, P < 0.001, t test). All values represent the mean ± SEM.
We hypothesized that the mice immunized with MAP and given epinephrine might show deficits in Pavlovian fear conditioning because this well characterized paradigm is known to depend on the integrity of the lateral and the central nuclei of the amygdala (24, 25). For this paradigm, an animal learns to associate a tone (conditional stimulus) with an electric foot shock (unconditional stimulus), and subsequently, it expresses the fear response (freezing) when presented with the tone alone. Moreover, the animal learns to associate the context in which the shock was delivered with the fearful experience, and the animal subsequently freezes when placed in the conditioning context. We found that although MAP-immunized mice (n = 18) given epinephrine displayed no memory impairment, they displayed a significantly diminished fear response when compared with mice immunized with MAP-core and given epinephrine (n = 20) (Fig. 4E). This finding was observed when the mice were assessed for response to a tone stimulus (P < 0.0005, t test) or a contextual stimulus (P < 0.001, t test). Thus, mice with anti-dsDNA, anti-NR2 Ab and an epinephrine-induced breach of the BBB displayed no impairment in hippocampal function, a result consistent with there being no evidence of hippocampal damage, but displayed a selective impairment in a behavioral response known to require an intact amygdala.
Discussion
Patients with SLE demonstrate progressive cognitive impairment that, in most studies, is not correlated with disease activity or medication. The consensus report on neuropsychiatric lupus also notes that SLE patients experience changes in affect, although these are less well defined (9). We have previously shown that Abs cross-reactive with dsDNA and NMDARs are present in patients with SLE (26). When these Abs are present in the serum of mice, there is no tissue damage in the brain because the Abs do not have access to brain tissue. Ab-mediated neuronal damage can occur only after an insult that abrogates that BBB. When LPS is used to break the BBB, Ab-mediated neuronal death occurs in the hippocampus, resulting in impaired memory function (17).
Multiple agents are known to compromise the integrity of the BBB. Reports of CNS toxicity in soldiers given choline esterase inhibitors during the first Gulf War led to studies in rats showing that increased epinephrine will open the BBB (27, 28). Epinephrine causes a general increase in cerebral blood flow, with a 30% increase in the amygdala, compared with a 15% increase in the hippocampus (21). It has been hypothesized that the epinephrine-induced breakdown in the BBB is most manifest in regions where blood flow is greatest and results from a mechanically induced change in vascular permeability (19, 29). It is probable, therefore, that epinephrine causes selective leaks of the BBB in the amygdala. A region-specific abrogation of the barrier might also occur if receptors for epinephrine are differentially expressed throughout the cerebral vasculature, with more epinephrine receptors present on vessels in the amygdala (30).
These observations led us to ask whether epinephrine might also permit cross-reactive anti-dsDNA, anti-NMDAR Abs to enter brain tissue, cause neuronal damage, and lead to a previously uncharacterized behavioral abnormality. Here, we demonstrate that mice with lupus-like Abs that bind NMDARs develop damaged neurons in the amygdala after administration of epinephrine. We believe that the selective injury to the amygdala results from the large regional increase in blood flow, resulting in the change in vascular permeability and Ab extravasation, coupled with the high-density expression of NMDARs containing NR2A and NR2B subunits on neurons in the amygdala. However, it is possible that neuronal apoptosis in vivo requires both neurotoxic Ab and a pharmacological sensitization of neurons to Ab-mediated damage. If epinephrine selectively sensitizes neurons in the amygdala, exposure to Ab and epinephrine would lead to selective damage in this brain region. We do not favor this hypothesis because we have shown that Ab directly injected into the hippocampus, cortex, or amygdala will cause local damage with no need for additional sensitization of neurons for injury (unpublished results).
Neuronal damage in the amygdala leads to a change in affect in a paradigm known to require an intact amygdala. A recently published report shows that blocking AMPA receptor signaling in the lateral amygdala abrogates fear conditioning in experimental mice. Incapacitating as few as 10–20% of the amygdalar neurons resulted in a significant deficit (31). This observation is in agreement with our study in which the focal loss of neurons, secondary to Ab-mediated receptor activation, resulted in a remarkably similar deficit in behavioral testing. It has also recently been shown that activation of NR2B containing NMDARs down-regulates AMPA receptors (32). Because AMPA receptors are known to mediate a fear conditioning response, receptor down-regulation may also contribute to the behavioral deficit in our studies. Detecting and quantifying these symptoms in lupus patients is likely to be more difficult than measuring change in memory and cognition. However, there are recent data from functional magnetic resonance imaging (fMRI) showing that individuals self-described as anxious display activation of the basal lateral amygdala on subliminal exposure to a fearful stimulus, whereas less anxious individuals fail to respond to the stimulus (33). This paradigm may permit an evaluation of the function of the amygdala in SLE patients. It will be of interest to determine by fMRI whether lupus patients fail to respond to subliminal fear stimuli. This failure might suggest a selective loss of neurons in the basal lateral amygdala similar to the loss observed in our murine model.
We have now demonstrated that anti-NMDAR Abs with antigenic specificity similar to those present in the serum of lupus patients can cause selective neuronal damage either in the hippocampus (17) or the amygdala, depending on the nature of the insult that abrogates the BBB. Memantine protected neurons whether LPS or epinephrine breached the BBB, confirming that the neuronal damage is mediated by NMDAR signaling. Because memantine does not block Ab binding, inhibition of cell death implies that neuronal apoptosis is triggered by receptor activation. Although memantine has proven to be an effective therapy in Alzheimer's disease, studies in rodents have shown that memantine can diminish learning (23, 34). Because it is likely that there will be long-term consequences to continuous administration of memantine in humans, we are most excited by the observation that D-peptide can mediate neuronal sparing. This finding provides a therapeutic approach that has obvious advantages over receptor antagonism, which may induce untoward side effects. The D-peptide blocks anti-NR2 Ab binding and does not interfere with receptor function. D-peptides have compelling therapeutic attributes: they can be given orally because they are resistant to protease digestion; they have a half-life of ≈6 h; and they can be engineered to cross the BBB (13, 35–37). D-peptides may, therefore, be a potential therapeutic for many Ab-mediated conditions.
Overall, these studies demonstrate that autoAbs can alter behavior without causing inflammation in the CNS. There are many studies that identify associations between serum Abs and emotional disorders, but none has provided a mechanism whereby the Ab might change emotional responses. Our data provide a model to suggest that Ab penetration of the BBB might cause emotional dysfunction not only in SLE, but also in other clinical situations. It is interesting to speculate, for example, that Abs may contribute to symptoms in multiple sclerosis not just by initiating a complement or Fc receptor-dependent inflammatory response, but also by altering neuronal metabolism and circuitry. Indeed, anti-DNA Abs have been found in the brains of patients with multiple sclerosis (38), and these Abs crossreact with the consensus peptide (unpublished results).
In conjunction with our previous study of an LPS-induced abrogation of the BBB, this study leads to three conclusions. First, Abs can cause a behavioral disorder. Second, the same serum response can cause either a cognitive or an emotional disorder. Third, regional specificity for Ab-mediated neuronal damage depends on the agent used to open the BBB. These conclusions have broad implications. A better understanding of the mechanisms by which the BBB is abrogated and whether these mechanisms also alter neuronal sensitivity to Ab, as well as an understanding of the antigenic specificity of brain-reactive Abs present in many disease states, may prove that the immune system is responsible for many acquired alterations in cognition and behavior. Most specifically, it will be important to determine whether stress-induced alterations in behavior can be Ab-mediated.
Materials and Methods
Animals, Immunization Protocol, ELISA, and Epinephrine Treatment. We used 6- to 8-week-old BALB/c female mice from The Jackson Laboratory for all experiments. The immunization protocols have been published (17). Briefly, mice were immunized by i.p. injection of 100 μg of antigen, MAP, or MAP-core backbone (AnaSpec, San Jose, CA) in 100 μl of saline in complete Freund's adjuvant followed by two or three boosts (2-week intervals) in incomplete Freund's adjuvant. ELISAs were performed after each immunization and required coating the antigens onto Costar plates in 0.1 M NaHCO3 (pH 8.6) overnight at 4°C for peptide antigens (15 μg/ml) or dry coated overnight at 37°C for dsDNA from calf thymus (100 μg/ml). Blocking was performed with 1% BSA/PBS for 1 h at 37°C, followed by incubation with serum at indicated dilutions in 0.2% BSA/PBS for 1 h at 37° C. After washing, the secondary Ab (goat anti-mouse IgG-Ab) was added for 1 h at 37°C or overnight at 4°C at a 1:1,000 dilution in 0.2% BSA/PBS. The assays were developed after a PBS/0.05% Tween 20 wash at room temperature using p-nitrophenyl phosphate disodium salt tablets (Sigma).
For epinephrine treatment, animals that had been immunized with either MAP or MAP-core received an i.p. injection of 100 nM epinephrine (Sigma) in lactated Ringer's solution. Epinephrine treatment was given twice, 48 h apart at 4–10 weeks after the last immunization. Histology and behavioral assays were performed at different time points after the second epinephrine treatment. For histology, the animals were killed 48 h after the second epinephrine injection. As in past protocols (17), some of the mice were given memantine hydrochloride (Sigma) at 5 mg/kg in Ringer's solution. Memantine (100 μl) was delivered intravenously just before i.p. epinephrine administration and 24 h after each epinephrine injection. In addition, some animals were treated intravenously with 200 μg of a D-isoform of the consensus DWEYS pentapeptide in a 100 μl of Ringer's solution that was delivered in an identical manner to memantine.
Immunohistology. These methods are described in refs. 16 and 17. To assess IgG deposition, amygdala sections were incubated in horse anti-mouse IgG at a 1:200 dilution in 0.1 M PBS (pH 7.4) for 1 h (Vector Laboratories). Sections were incubated in avidin–biotin horseradish peroxidase complex at a 1:100 dilution for 1 h. Mouse IgG was visualized by 0.5 mg/ml diaminobenzidine in the presence of 0.03% H2O2.
For Fluorojade-B staining, tissue sections were mounted on gelatinized slides and allowed to dry at room temperature. Slides were immersed sequentially in 100% ethanol for 3 min, 70% ethanol for 1 min, dH2O for 1 min, 0.06% KMnO4 (diluted in dH2O) for 15 min, dH2O for 1 min, and 0.001% Fluorojade-B aqueous staining solution containing 0.1% acetic acid for 30 min (HistoChem, Jefferson, AR) and rinsed three times in dH2O for 1 min each.
To assess colocalization of caspase-3 in neurons, tissue was rinsed twice in 0.1 M PBS, and nonspecific binding sites were blocked with 1% BSA. Sections were incubated in MAP2 (mouse Ab clone Ab20, used at 1:200; Chemicon International, Temecula, CA) overnight at 4°C, followed by a mixture of donkey anti-rabbit Rhodamine Red-X IgG and goat anti-mouse FITC-conjugated IgG for 45 min at room temperature (each at 1:200 in 0.1 M PB; Jackson ImmunoResearch). Preparations were rinsed three times in 0.1 M PB for 45 min, mounted on gelatin-coated slides, air-dried, and coverslipped with Cytoseal (Stephens Scientific, Riverdale, NJ). Final preparations were evaluated by confocal microscopy (LSM 510; Zeiss) using software from Zeiss.
Behavioral Assessment. We described detailed methods for our behavioral paradigms, except fear conditioning, in ref. 17. Briefly, all mice underwent the following sequence of tests: behavioral screen, rotarod test, two-object task, T-maze task, and training-to-criterion task. The mice were run by an experimenter who was “blind” with respect to their group assignments. The behavioral screen measured 38 autonomic responses and neurological reflexes. The rotarod test measured muscle strength and balance by placing each mouse in the rotating drum (ENV-576M; Med Associates, Georgia, VT), which was accelerated from 4 to 40 rpm over 5 min; the time at which each mouse fell from the drum was recorded for three trials. The two-object task measured recognition memory in four steps: (i) familiarization, in which a mouse was placed in a square chamber (625 cm2) for three sessions (5 min each); (ii) sampling, in which the mouse was exposed to two identical objects within the chamber for 5 min; (iii) delay, in which the mouse waited in the home cage for 10 min; and (iv) choice, in which the mouse was presented with two objects, one of them being identical to those in the sample phase (familiar object) and the other being different (novel object). Recognition memory was scored with a discrimination ratio (time exploring the new object minus time exploring the familiar object over total exploration time). The T-maze task measured spatial working memory by scoring the alternations made by a mouse over 16 trials in the apparatus (each arm 30 cm long × 10 cm wide × 29 cm high); for each trial, the mouse navigated from the back of the start arm and chose between the other arms by entering with its whole body. The training-to-criterion task examined memory flexibility by placing the mice in the water maze (circular pool, 160 cm in diameter, filled with opaque water at 20°C), facing the side walls, and allowing them to swim until they found the hidden platform (top surface, 0.5 cm below water level). The maximum trial duration was 90 sec. Mice were required to find the platform in five consecutive locations in the water maze. Each mouse was trained for up to eight trials per day to a criterion of three successive trials in which the mouse found the platform in <20 sec before being transferred to the next location on the next day (maximum number of trials per location was 32).
The fear-conditioning task exploited well known paradigms (24, 25). Our implementation used a conditioning chamber (clear Plexiglas, dim light, metal grid floor; model ENV10; Med Associates, Georgia, VT) and a testing chamber (dark plastic, brightly lit, black floor washed with peppermint soap). Video cameras were mounted on top of the chambers for videotaping. The procedure was as follows (39): on the day before conditioning (day 1), mice were habituated to both chambers for 10–15 min in a counterbalanced manner to control for order effects. On the day of conditioning (day 2), each mouse was acclimated to the conditioning chamber (3 min) and then given five pairings of a conditional stimulus (tone, 20 sec, 5 kHz, 75 dB) that coterminated with an unconditional stimulus (foot shock, 0.5 sec, 0.5 mA). The intertrial interval was 90–120 sec. On the day of testing (day 3), the freezing responses to the conditional stimulus were measured in the testing chamber with five test tones (20 sec, 5 kHz, 75 dB, 100-sec interval), starting from the first tone and lasting until 100 sec after the fifth tone. The freezing response was expressed as the percent of the total time (500 sec) that the animal remained frozen. After 1 h, the mice were placed in the conditioning chamber and were allowed to explore for 5 min (to give them time to recognize the context), after which the duration of freezing was scored for an additional 5 min. Again, the freezing response was expressed as the percent of the total time (300 sec) that the animal remained frozen. An observer who was blinded to the previous treatment of the mice measured freezing manually.
Acknowledgments
We thank Randy D'Amico, Stephen Frattini, and Niharika Samtani for technical assistance. This work was supported by grants from the National Institutes of Health and the Burke Medical Research Institute.
Conflict of interest statement: No conflicts declared.
Abbreviations: SLE, systemic lupus erythematosus; NMDAR, NMDA receptor; MAP, multiantigenic peptide; BBB, blood–brain barrier.
References
- 1.Darnell, R. B. & Posner, J. B. (2003) N. Engl. J. Med. 349, 1543–1554. [DOI] [PubMed] [Google Scholar]
- 2.Edwards, M. J., Trikouli, E., Martino, D., Bozi, M., Dale, R. C., Church, A. J., Schrag, A., Lees, A. J., Quinn, N. P., Giovannoni, G. & Bhatia, K. P. (2004) Neurology 63, 156–158. [DOI] [PubMed] [Google Scholar]
- 3.Fatemi, S. H. (2005) Mol. Psychiatry 10, 251–257. [DOI] [PubMed] [Google Scholar]
- 4.Kirvan, C. A., Swedo, S. E., Heuser, J. S. & Cunningham, M. W. (2003) Nat. Med. 9, 914–920. [DOI] [PubMed] [Google Scholar]
- 5.Padmos, R. C., Bekris, L., Knijff, E. M., Tiemeier, H., Kupka, R. W., Cohen, D., Nolen, W. A., Lernmark, A. & Drexhage, H. A. (2004) Biol. Psychiatry 56, 476–482. [DOI] [PubMed] [Google Scholar]
- 6.Snider, L. A. & Swedo, S. E. (2004) Mol. Psychiatry 9, 900–907. [DOI] [PubMed] [Google Scholar]
- 7.Diamond, B. & Volpe, B. T. (2003) Arthritis Rheum. 48, 2710–2712. [DOI] [PubMed] [Google Scholar]
- 8.Gladman, D. & Urowitz, M. (2002) in DuBois' Lupus Erythematosus, eds. Wallace, D. J. & Hahn, B. H. (Lippincott Williams & Wilkins, Philadelphia), pp. 1255–1273.
- 9.American College of Rheumatology (1999) Arthritis Rheum. 42, 599–608. [DOI] [PubMed] [Google Scholar]
- 10.Roman, M. J., Shanker, B. A., Davis, A., Lockshin, M. D., Sammaritano, L., Simantov, R., Crow, M. K., Schwartz, J. E., Paget, S. A., Devereux, R. B. & Salmon, J. E. (2003) N. Engl. J. Med. 349, 2399–2406. [DOI] [PubMed] [Google Scholar]
- 11.Ruiz-Irastorza, G., Khamashta, M. A. & Hughes, G. R. (2002) Autoimmun. Rev. 1, 43–48. [DOI] [PubMed] [Google Scholar]
- 12.Sastre-Garriga, J. & Montalban, X. (2003) Lupus 12, 877–882. [DOI] [PubMed] [Google Scholar]
- 13.Gaynor, B., Putterman, C., Valadon, P., Spatz, L., Scharff, M. D. & Diamond, B. (1997) Proc. Natl. Acad. Sci. USA 94, 1955–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hahn, B. H. & Tsao, B. (2002) in Dubois' Lupus Erythematosus, eds. Wallace, D. J. & Hahn, B. H. (Lippincott Williams & Wilkins, Philadelphia), pp. 95–114.
- 15.Katz, J. B., Limpanasithikul, W. & Diamond, B. (1994) J. Exp. Med. 180, 925–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeGiorgio, L. A., Konstantinov, K. N., Lee, S. C., Hardin, J. A., Volpe, B. T. & Diamond, B. (2001) Nat. Med. 7, 1189–1193. [DOI] [PubMed] [Google Scholar]
- 17.Kowal, C., DeGiorgio, L. A., Nakaoka, T., Hetherington, H., Huerta, P. T., Diamond, B. & Volpe, B. T. (2004) Immunity 21, 179–188. [DOI] [PubMed] [Google Scholar]
- 18.Johansson, B. B. (1989) in Implications of the Blood–Brain Barrier and Its Manipulation, ed. Neuwelt, E. A. (Plenum, New York), pp. 389–410.
- 19.Mayhan, W. G. (2001) Microcirculation 8, 89–104. [DOI] [PubMed] [Google Scholar]
- 20.Kuang, F., Wang, B. R., Zhang, P., Fei, L. L., Jia, Y., Duan, X. L., Wang, X., Xu, Z., Li, G. L., Jiao, X. Y. & Ju, G. (2004) Int. J. Neurosci. 114, 575–591. [DOI] [PubMed] [Google Scholar]
- 21.Tuor, U. I., McKenzie, E. & Tomanek, B. (2002) Magn. Reson. Imaging 20, 707–712. [DOI] [PubMed] [Google Scholar]
- 22.Putterman, C. & Diamond, B. (1998) J. Exp. Med. 188, 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reisberg, B., Doody, R., Stoffler, A., Schmitt, F., Ferris, S. & Mobius, H. J. (2003) N. Engl. J. Med. 348, 1333–1341. [DOI] [PubMed] [Google Scholar]
- 24.Fanselow, M. S. & Poulos, A. M. (2005) Annu. Rev. Psychol. 56, 207–234. [DOI] [PubMed] [Google Scholar]
- 25.LeDoux, J. E. (2000) Annu. Rev. Neurosci. 23, 155–184. [DOI] [PubMed] [Google Scholar]
- 26.Sharma, A., Isenberg, D. & Diamond, B. (2003) Rheumatology (Oxford) 42, 453–463. [DOI] [PubMed] [Google Scholar]
- 27.Abdel-Rahman, A., Shetty, A. K. & Abou-Donia, M. B. (2002) Neurobiol. Dis. 10, 306–326. [DOI] [PubMed] [Google Scholar]
- 28.Friedman, A., Kaufer, D., Shemer, J., Hendler, I., Soreq, H. & Tur-Kaspa, I. (1996) Nat. Med. 2, 1382–1385. [DOI] [PubMed] [Google Scholar]
- 29.Abbott, N. J. (2004) Neurochem. Int. 45, 545–552. [DOI] [PubMed] [Google Scholar]
- 30.Ballabh, P., Braun, A. & Nedergaard, M. (2004) Neurobiol. Dis. 16, 1–13. [DOI] [PubMed] [Google Scholar]
- 31.Rumpel, S., LeDoux, J., Zador, A. & Malinow, R. (2005) Science 308, 83–88. [DOI] [PubMed] [Google Scholar]
- 32.Kim, M. J., Dunah, A. W., Wang, Y. T. & Sheng, M. (2005) Neuron 46, 745–760. [DOI] [PubMed] [Google Scholar]
- 33.Etkin, A., Klemenhagen, K. C., Dudman, J. T., Rogan, M. T., Hen, R., Kandel, E. R. & Hirsch, J. (2004) Neuron 44, 1043–1055. [DOI] [PubMed] [Google Scholar]
- 34.Willmore, C. B., LaVecchia, K. L. & Wiley, J. L. (2001) Neuropharmacology 41, 916–927. [DOI] [PubMed] [Google Scholar]
- 35.Adessi, C. & Soto, C. (2002) Curr. Med. Chem. 9, 963–978. [DOI] [PubMed] [Google Scholar]
- 36.Egleton, R. D. & Davis, T. P. (1997) Peptides 18, 1431–1439. [DOI] [PubMed] [Google Scholar]
- 37.Egleton, R. D. & Davis, T. P. (2005) NeuroRx 2, 44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williamson, R. A., Burgoon, M. P., Owens, G. P., Ghausi, O., Leclerc, E., Firme, L., Carlson, S., Corboy, J., Parren, P. W., Sanna, P. P., et al. (2001) Proc. Natl. Acad. Sci. USA 98, 1793–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodrigues, S. M., Shafe, G. E. & LeDoux, J. E. (2001) J. Neurosci. 21, 6889–6896. [DOI] [PMC free article] [PubMed] [Google Scholar]