Abstract
The ATR (ATM- and rad3-related)-mediated checkpoint pathway has a crucial role in regulating the cellular responses to DNA damage and DNA-replication stress. ATRIP (ATR-interacting protein), the regulatory partner of ATR, binds directly to replication protein A (RPA)-coated ssDNA and enables the ATR–ATRIP complex to recognize this DNA damage-induced structure. Here, we show that ATRIP associates with RPA–ssDNA through multiple interactions. Two major RPA–ssDNA-interacting domains of ATRIP were mapped to the regions flanking the conserved coiled-coil domain. In contrast to a recent article, we found that ATRIP mutants lacking the N terminus retained the ability to bind to RPA–ssDNA, suggesting that the multiple interactions between ATRIP and RPA–ssDNA may function redundantly in the recruitment of ATR–ATRIP. Unexpectedly, one internal region of ATRIP exhibited affinity to ssDNA, suggesting that ATRIP may interact with ssDNA in the ATRIP–RPA–ssDNA complex. Also, the N terminus of ATRIP associated with RPA–ssDNA in two distinct ways, indicating a dynamic and regulated association between ATRIP and RPA–ssDNA.
Keywords: ATR, cell cycle, checkpoint, DNA damage, genomic stability
DNA damage and other forms of intrinsic or extrinsic genotoxic stresses constantly challenge the integrity of the genome in all living cells. In response to such stresses, a complex signaling network called the checkpoint is evoked in cells to protect genomic stability by regulating and coordinating various cellular processes or to eliminate nonrepairable cells by triggering apoptosis (1, 2). ATM (ataxia-telangiectasia-mutated) and ATR (ATM- and rad3-related), two phosphatidylinositol 3-kinase-like protein kinases, have critical roles in initiating the checkpoint signals. Whereas ATM responds primarily to double-stranded DNA (dsDNA) breaks, ATR is activated by a broad spectrum of DNA damage, especially the DNA damage interfering with DNA replication (3). How ATM and ATR are activated by different types of DNA damage has yet to be determined.
In human cells, the ATR kinase exists as a complex with ATRIP (ATR-interacting protein) (4). Mec1 and rad3, the budding and fission yeast homologues of ATR, also form similar complexes with Ddc2 (also known as Lcd1 or Pie1) and rad26, respectively (5–8). The phenotypes of cells lacking ATRIP, Ddc2, or rad26 closely resemble the phenotypes of cells lacking ATR, Mec1, or rad3, respectively, strongly suggesting that ATR–ATRIP, Mec1–Ddc2, and rad3–rad26 function as complexes (4–8). ATRIP and its homologues have been suggested to have several regulatory roles in the respective kinase complexes. In human cells, depletion of ATRIP by using specific small interfering RNA (siRNA) led to destabilization of ATR, indicating that ATRIP stabilizes ATR in the complex (4). Mec1 isolated from the cells lacking Ddc2 failed to phosphorylate substrates in vitro, implicating Ddc2 in the regulation of Mec1 kinase activity (9). Also, Mec1 cannot localize to sites of DNA damage in the absence of Ddc2, revealing an essential damage-targeting function of Ddc2 in the kinase complex (10–13).
The broad DNA-damage specificity of ATR activation implies that ATR is unlikely to be activated directly by many distinct forms of DNA damage. Instead, it is more probable that ATR is activated by certain DNA and/or protein structures that are commonly induced by different types of DNA damage. Replication protein A (RPA)-coated ssDNA is a DNA–protein structure generated at stalled replication forks, dsDNA breaks, and sites of many types of DNA repair (such as nucleotide excision repair, mismatch repair, and long-patch base-excision repair). RPA is required for the efficient activation of ATR and its homologues in humans, Xenopus, and yeast (11, 14–16). In yeast, RPA is essential for Ddc2 to localize to dsDNA breaks (11–13). In Xenopus egg extracts, RPA is needed for ATR to associate with chromatin and the synthetic DNA structures activating ATR (15–17). We and other researchers (11, 17) have shown that purified ATRIP and Ddc2 associate directly with RPA–ssDNA complexes. Also, the binding of ATRIP to RPA–ssDNA confers DNA-structure specificity to the ATR–ATRIP complex, allowing it to differentiate ssDNA from undamaged dsDNA (11). Collectively, these findings suggest that the interaction between ATRIP and RPA–ssDNA has a critical role in targeting the ATR–ATRIP kinase complex to sites of DNA damage.
The interaction between ATRIP and RPA–ssDNA may be important for the initial recognition of DNA damage by ATR–ATRIP and subsequent ATR–ATRIP recruitment involved in the amplification of DNA damage signals. Also, it may contribute to the activation of the kinase complex on damaged DNA and may facilitate the kinase to phosphorylate specific substrates. To further understand how the ATRIP–RPA–ssDNA interaction regulates ATR, we sought to identify the region of ATRIP that binds to RPA–ssDNA. We found that ATRIP interacts with RPA–ssDNA through multiple interactions. It was recently reported that an ATRIP mutant lacking the N-terminal 107 aa failed to associate with RPA–ssDNA in vitro but was capable of supporting Chk1 phosphorylation in cells (18). Surprisingly, we found that this ATRIP mutant retains the ability to associate with RPA–ssDNA. Thus, the conclusion that the ATRIP–RPA–ssDNA interaction is dispensable for ATR-dependent checkpoint signaling needs to be reassessed. We further mapped the two major RPA–ssDNA-binding sites of ATRIP to two regions flanking the conserved coiled-coil domain. Our data suggest that the N-terminal region of ATRIP can associate with RPA–ssDNA through different mechanisms. Also, we found that an internal region of ATRIP is able to bind to ssDNA. These findings have revealed unexpected complexity of the ATRIP–RPA–ssDNA interaction, raising the possibility that the multiple interactions between ATRIP and RPA–ssDNA may function redundantly in the recruitment of ATR–ATRIP.
Results
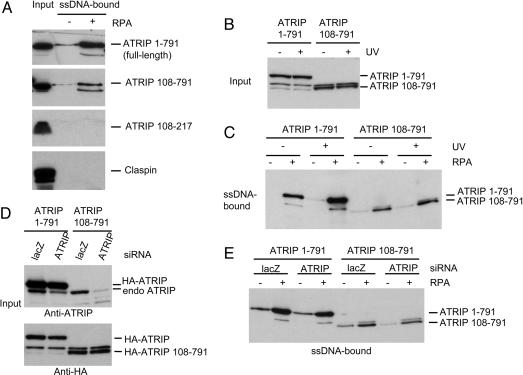
The N Terminus of ATRIP Is Not Essential for the Association of ATRIP with RPA–ssDNA. We have shown (11) that RPA stimulates the binding of purified ATRIP to ssDNA using an in vitro binding assay, demonstrating that ATRIP interacts directly with RPA–ssDNA complexes. To characterize the association of ATRIP with RPA–ssDNA in the presence of other human proteins, we prepared extracts from cells transiently expressing Flag-tagged full-length ATRIP or various fragments of ATRIP and examined the binding of these proteins to biotinylated ssDNA (either uncoated or coated with recombinant human RPA) in extracts. Full-length ATRIP bound to RPA-coated ssDNA much more efficiently than to uncoated ssDNA (Fig. 1A), showing that RPA stimulates the binding of ATRIP to ssDNA in human cell extracts. It was recently reported that the N-terminal 107 aa of ATRIP are required for the association of ATRIP with RPA–ssDNA (18). Surprisingly, we found that the ATRIP mutant lacking this N-terminal region (ATRIP 108–791) was capable of binding to ssDNA in an RPA-stimulated manner (Fig. 1 A), suggesting that, in extracts, ATRIP can associate with RPA–ssDNA independently of its N terminus. The amount of ATRIP 108–791 associated with RPA–ssDNA was lower than that of full-length ATRIP, suggesting that the N terminus of ATRIP contributes to the association with RPA–ssDNA. In contrast to ATRIP 108–791, the conserved coiled-coil domain of ATRIP (ATRIP 108–217) and Claspin (a mediator protein important for the phosphorylation of Chk1 by ATR) did not associate with ssDNA even when it was coated with RPA, demonstrating the specificity of the association of ATRIP 108–791 with RPA–ssDNA.
Fig. 1.
ATRIP 108–791, an ATRIP mutant lacking the N terminus, retains the ability to associate with RPA–ssDNA. (A) Flag-tagged full-length ATRIP (ATRIP 1–791), ATRIP 108–791, ATRIP 108–217, and Claspin were transiently expressed in 293T cells. Biotinylated ssDNA (either uncoated or coated with recombinant human RPA) was incubated with the extracts derived from the transfected cells. The Flag-tagged proteins that were present in input (5%) or associated with ssDNA were detected by Western blotting. (B and C) U2OS-derived cells stably expressing HA–ATRIP or HA–ATRIP 108–791 were treated with 50 J/m2 of UV or left untreated. Extracts were prepared from these cells after 2 h and incubated with ssDNA or RPA–ssDNA. (B) Levels of full-length HA–ATRIP and HA–ATRIP 108–791 in the extracts. (C) HA–ATRIP and HA–ATRIP 108–791 bound to ssDNA or RPA–ssDNA. (D and E) The cells stably expressing HA–ATRIP and HA–ATRIP 108–791 were transfected with control siRNA (LacZ) or ATRIP siRNA as indicated. (Upper) The levels of HA–ATRIP (full-length) and endogenous ATRIP in the extracts were analyzed by using an antibody recognizing the N terminus of ATRIP. (Lower) The levels of HA–ATRIP and HA–ATRIP 108–791 were also examined by using anti-HA antibody. (E) HA–ATRIP and HA–ATRIP 108–791 associated with ssDNA or RPA–ssDNA.
To assess whether full-length ATRIP and ATRIP 108–791 can bind to RPA–ssDNA when expressed at lower levels, we reexamined these interactions by using previously characterized cell lines stably expressing hemagglutinin (HA)-tagged full-length ATRIP or ATRIP 108–791 (18). By using these cell lines, it has been shown that ATRIP 108–791 can support DNA damaged-induced Chk1 phosphorylation in cells (18). As estimated by ref. 18, the levels of HA–ATRIP and HA–ATRIP 108–791 in these cells lines were ≈3- to 6-fold higher than that of endogenous ATRIP. Consistent with Fig. 1 A, full-length HA–ATRIP bound to ssDNA in an RPA-stimulated manner (Fig. 1 B and C). The binding of HA–ATRIP to RPA–ssDNA was enhanced in extracts derived from cells irradiated with UV light, indicating that although ATRIP can associate directly with RPA–ssDNA, this interaction is regulated by additional mechanisms in damaged cells. Like that of HA–ATRIP, the binding of HA–ATRIP 108–791 to ssDNA was stimulated by RPA and was enhanced in extracts derived from UV-treated cells (Fig. 1 B and C). The amounts of HA–ATRIP 108–791 associated with RPA–ssDNA were reduced compared with those of HA–ATRIP. Thus, when expressed at the levels sufficient for Chk1 activation, ATRIP 108–791 retains some of the ability to associate with RPA–ssDNA in a regulated manner.
It was recently reported that ATRIP molecules form oligomers through their coiled-coil domains (19). This finding prompted us to question whether the binding of HA–ATRIP 108–791 to RPA–ssDNA was mediated by the endogenous ATRIP associated with HA–ATRIP 108–791. To remove endogenous ATRIP, cells stably expressing HA–ATRIP or HA–ATRIP 108–791 were transfected with siRNA targeting ATRIP. ATRIP siRNA effectively knocked down the levels of endogenous ATRIP in both cell lines (Fig. 1D). Because the coding sequence of HA–ATRIP had been engineered to be resistant to the siRNA-targeting endogenous ATRIP, its level was reduced only slightly by ATRIP siRNA (Fig. 1D). The expression of HA–ATRIP 108–791, which lacks the target sequence of the ATRIP siRNA, was unaffected by the ATRIP siRNA treatment (Fig. 1D). Like HA–ATRIP, HA–ATRIP 108–791 bound to ssDNA in an RPA-stimulated manner even when endogenous ATRIP was virtually absent in the extracts (Fig. 1E), showing that HA–ATRIP 108–791 has the ability to associate with RPA–ssDNA independently of endogenous ATRIP. It is important to note that although the cells expressing HA–ATRIP 108–791 were able to support Chk1 phosphorylation in the absence of endogenous ATRIP, the binding of HA–ATRIP 108–791 to RPA–ssDNA had not been examined in these cells (18). Our results suggest that HA–ATRIP 108–791 is still capable of recognizing RPA–ssDNA in these cells.
ATRIP Associates Directly with RPA–ssDNA Independently of Its N Terminus. The association of ATRIP 108–791 with RPA–ssDNA in extracts may have resulted from a direct interaction between ATRIP 108–791 and RPA–ssDNA or an indirection interaction mediated by other human proteins. To distinguish these possibilities, we synthesized full-length ATRIP and various ATRIP fragments by using the coupled in vitro transcription and translation system in rabbit reticulocyte lysate and examined how these proteins bound to ssDNA or RPA–ssDNA in the absence of other human proteins. Like that in human cell extracts, the binding of full-length ATRIP to ssDNA in rabbit reticulocyte lysate was clearly stimulated by RPA (Fig. 2A). The binding of ATRIP 108–791 to ssDNA was also stimulated by RPA (Fig. 2 A). Consistent with our experiments using human cell extracts, the amount of ATRIP 108–791 associated with RPA–ssDNA was reduced compared with that of full-length ATRIP. Also, ATRIP 198–791, an ATRIP fragment lacking not only the N terminus but also most of the coiled-coil domain, still associated with ssDNA in an RPA-stimulated manner (Fig. 2 A). These results suggest the presence of additional RPA–ssDNA-interacting domain(s) in ATRIP on the carboxyl side of the coiled-coil domain.
Fig. 2.
ATRIP 108–791 associates directly with RPA–ssDNA. (A) Full-length ATRIP (ATRIP 1–791), ATRIP 108–791, and ATRIP 198–791 were synthesized by using coupled in vitro transcription and translation in rabbit reticulocyte lysate and labeled with [35S]methionine. The reticulocyte lysates containing the in vitro-translated proteins were incubated with ssDNA or RPA–ssDNA. The in vitro-translated proteins present in the input (5%) or associated with ssDNA or RPA–ssDNA were detected by autoradiograph. (B) The binding of the in vitro-translated full-length ATRIP and ATRIP 108–791 to RPA–ssDNA was analyzed in the same buffer as described above (buffer A) and in NETN buffer (buffer B). The proteins that were present in the input (10%) or associated with RPA–ssDNA were detected by autoradiography. (C) Purified GST–ATRIP 108–791 on a Coomassie blue-stained SDS/PAGE. (D) We incubated ≈0.5 μgof purified GST–ATRIP 108–791 and 10 μg of purified GST with ssDNA or RPA–ssDNA, respectively. The proteins that were present in the input (10%) or associated with ssDNA or RPA–ssDNA were detected by using anti-GST antibody.
To determine why ATRIP 108–791 associated with RPA–ssDNA in our binding assays but not in that of ref. 18, we directly compared the bindings of full-length ATRIP and ATRIP 108–791 with RPA–ssDNA under the two assay conditions. The binding buffer of the previous study (buffer B) contains higher concentrations of detergent and salt compared with the buffer used in our experiments (buffer A). As shown in Fig. 2B, the binding of full-length ATRIP to RPA–ssDNA was clearly less efficient in buffer B. The association of ATRIP 108–791 with RPA–ssDNA was readily detected in buffer A but not in buffer B. Thus, some of the RPA-dependent binding of ATRIP to ssDNA is disrupted under the stringent binding conditions of the previous study.
To further assess whether ATRIP 108–791 interacts directly with RPA–ssDNA, we expressed a GST fusion of ATRIP 108–791 in Escherichia coli and purified it by using glutathione beads (Fig. 2C). When purified GST–ATRIP 108–791 was incubated with naked ssDNA, no binding was detected (Fig. 2D). However, when ssDNA was coated with recombinant human RPA purified from E. coli, GST–ATRIP 108–791 bound to ssDNA efficiently (Fig. 2D). In marked contrast, purified GST did not bind to RPA–ssDNA at all even when used at a concentration 20-fold higher than that of GST–ATRIP 108–791. These results strongly suggest that ATRIP 108–791 retains the ability to associate directly with RPA–ssDNA.
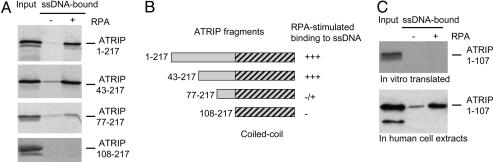
The N Terminus of ATRIP Interacts with RPA–ssDNA by Different Mechanisms. Although the binding of ATRIP 108–791 to ssDNA is clearly stimulated by RPA, the amount of ATRIP 108–791 associated with RPA–ssDNA was lower than that of full-length ATRIP, suggesting that the N terminus of ATRIP contributes to the association of ATRIP with RPA–ssDNA. Consistent with this idea, in vitro-translated ATRIP 1–217 (an ATRIP fragment containing the N terminus and the coiled-coil domain) bound to ssDNA in an RPA-stimulated manner (Fig. 3A). To reveal the region in ATRIP 1–217 that is responsible for the RPA-stimulated binding to ssDNA, we generated a number of N-terminal deletion mutants of ATRIP 1–217 (Fig. 3B). ATRIP 43–217, but not ATRIP 77–217 and ATRIP 108–217, bound to ssDNA more efficiently when it was coated with RPA (Figs. 3 A and B), suggesting that amino acids 43–76 of ATRIP are important for the association of the N terminus of ATRIP with RPA–ssDNA. Because amino acids 1–42 of ATRIP are clearly dispensable for the association with RPA–ssDNA, we concluded that the region 43–108 contains a major RPA–ssDNA-interacting domain, which we termed RPA–ssDNA-interacting domain 1 (RID1) (see Fig. 4C).
Fig. 3.
The association between the N terminus of ATRIP with RPA–ssDNA. (A) ATRIP 1–217, ATRIP 43–217, ATRIP 77–217, and ATRIP 108–217 were in vitro-translated and labeled with [35S]methionine. The in vitro-translated ATRIP fragments were incubated with ssDNA or RPA–ssDNA. The 35S-labeled proteins present in the input (5%) or associated with ssDNA or RPA–ssDNA were detected by autoradiography. (B) Schematic representation of the ATRIP fragments analyzed in A and their RPA-stimulated associations with ssDNA. Shaded region indicates the coiled-coil domain of ATRIP. (C) ATRIP 1–107 was in vitro-translated (Upper) or transiently expressed in 293T cells (Lower). The reticulocyte lysate containing the in vitro-translated ATRIP 1–107 and the extracts derived from the cells transiently expressing Flag-ATRIP 1–107 were incubated with ssDNA or RPA–ssDNA. The ATRIP 1–107 present in the inputs (5%) or associated with ssDNA or RPA–ssDNA was detected by autoradiography and Western blotting.
Fig. 4.
ATRIP associates with RPA–ssDNA through multiple interactions. (A) ATRIP 1–791 (full-length), ATRIP 1–217, ATRIP 108–390, and ATRIP 390–791 were in vitro-translated and labeled with [35S]methionine. The in vitro-translated proteins were incubated with ssDNA or RPA–ssDNA. The 35S-labeled ATRIP fragments present in the input (5%) or associated with ssDNA or RPA–ssDNA were detected by autoradiography. (B) Schematic representation of the ATRIP fragments analyzed in this study and their associations with RPA–ssDNA. Shaded region indicates the coiled-coil domain of ATRIP. (C) Summary of RPA–ssDNA binding, ATRIP foci formation, and Chk1 phosphorylation data of the human ATRIP mutants characterized in this study and previous studies (18, 19, 28). The approximate positions of RPA–ssDNA-interacting domains 1–3 (RID1–3) in ATRIP are indicated. LG is a coiled-coil-defective mutant of ATRIP (28).
We then questioned whether the N terminus of ATRIP was sufficient for association with RPA–ssDNA. Consistent with ref. 18, in extracts derived from cells transiently expressing ATRIP 1–107, this ATRIP fragment bound to ssDNA in an RPA-stimulated manner (Fig. 3C). Surprisingly, however, in vitro-translated ATRIP 1–107 did not bind to RPA–ssDNA efficiently (Fig. 3C). These results suggest that the binding of ATRIP 1–107 to RPA–ssDNA in human cell extracts might be mediated or stabilized by other proteins and/or protein modifications. Alternatively, certain human proteins may be important for the proper folding of ATRIP 1–107. However, in vitro-translated ATRIP 1–217 bound to ssDNA in an RPA-stimulated manner in rabbit reticulocyte lysate (Fig. 3A), indicating that the coiled-coil domain of ATRIP facilitates the binding of the N terminus of ATRIP to RPA–ssDNA. Thus, the N terminus of ATRIP associated with RPA–ssDNA in vitro in two distinct ways; one way requires the presence of other human proteins, and the other way depends on the adjacent coiled-coil domain of ATRIP. The identification of the human protein(s) that enables ATRIP 1–107 to associate with RPA–ssDNA could help to address whether and how these distinct interactions between ATRIP and RPA–ssDNA contribute to the association of ATR–ATRIP with DNA damage in vivo.
ATRIP Associates with RPA–ssDNA Through Multiple Interactions. The data shown in Figs. 1 and 2 suggest the presence of RPA–ssDNA-interacting domain(s) in ATRIP 108–791. To locate these domains, we expressed ATRIP 108–390 and ATRIP 390–791 by using in vitro translation and tested their binding to ssDNA that was coated or uncoated with RPA (Fig. 4A). ATRIP 108–390 bound to RPA–ssDNA efficiently (Figs. 4 A and B), suggesting that this ATRIP fragment contains a domain capable of interacting with RPA–ssDNA. Given that the coiled-coil domain of ATRIP itself (ATRIP 108–217) is not sufficient for binding to RPA–ssDNA (Fig. 1 A), these results revealed the presence of a second major RPA–ssDNA-interacting domain in the region 218–390, which we termed RID2 (Fig. 4C). Interestingly, we have detected a weak but reproducible interaction between ATRIP 108–390 and uncoated ssDNA (Figs. 4A). The fact that only ATRIP 108–390, but not full-length ATRIP nor other ATRIP fragments, can weakly associate with uncoated ssDNA in reticulocyte lysate suggests that this fragment might have the ability to bind to ssDNA but that it does so only under certain conditions, such as when full length ATRIP is bound to RPA-ssDNA. These results raise the possibility that, although full-length ATRIP is unable to bind to ssDNA by itself, its internal region (ATRIP 108–390) has the potential to interact with ssDNA.
Also, we detected association of ATRIP 390–791 with RPA–ssDNA (Fig. 4 A and B). Compared with that of ATRIP 108–390, the association of ATRIP 390–791 with RPA–ssDNA was weak. Nonetheless, ATRIP 390–791 associated with ssDNA in an RPA-stimulated manner, indicating the presence of a weak RPA–ssDNA-interacting domain in the region 390–791. Two nonoverlapping ATR-interacting domains have been mapped to the C terminus of ATRIP (18, 20). To assess whether the rabbit ATR in reticulocyte lysate contributes to the binding of ATRIP 390–791 to RPA–ssDNA, we removed the ATR-interacting domains from ATRIP 390–791. The resultant ATRIP fragments (including 390–765, 390–658, and 390–617) retained the ability to associate with RPA–ssDNA (Fig. 4B and data not shown), suggesting that ATR is dispensable for the interaction between ATRIP 390–791 and RPA–ssDNA. Together, these data suggest the presence of at least two previously uncharacterized RPA–ssDNA-interacting domains in ATRIP outside of its N terminus (RID2 and RID3; Fig. 4C).
Discussion
How the ATR–ATRIP kinase complex is recruited to and regulated at sites of DNA damage is a critical question for understanding the signaling of the ATR-mediated checkpoint. RPA-coated ssDNA plays several important roles in the regulation of the ATR signaling pathway. We and other researchers (11, 17) have shown that ATRIP and Ddc2 directly associate with RPA–ssDNA in vitro. RPA also facilitates the recruitment of Rad17 complexes to ssDNA and stimulates the loading of 9-1-1 complexes to DNA structures with single- and double-strand junctions (21, 22). Also, RPA was shown to interact with other proteins involved in checkpoint signaling, such as the 9-1-1 complex, Mre11, and 53BP1 in humans, and Mec1 in yeast (13, 23–25). These findings suggest that RPA–ssDNA is not only important for localizing the ATR–ATRIP kinase to sites of DNA damage but is also important for assembling and organizing the active signaling complexes on damaged DNA.
Whether a direct interaction between ATRIP and RPA–ssDNA is required for ATR activation is an important issue under investigation. ATRIP and its homologues from humans, Xenopus, and yeast all directly associate with RPA–ssDNA (11, 17). In yeast, rfa1-t11, a specific mutant allele of RPA1, is proficient for DNA replication but partially defective for recruiting Ddc2 to dsDNA breaks and stalled replication forks in cells (11, 26, 27). Importantly, when the purified rfa1-tll mutant complex was tested in vitro, it displayed reduced ability to recruit Ddc2 to ssDNA (11). Thus, a weakened direct interaction between Ddc2 and RPA–ssDNA correlates well with the diminished Ddc2 recruitment in damaged cells. However, a recent study reported that an ATRIP mutant lacking the N-terminal 107 aa (ATRIP 108–791) was unable to bind to RPA–ssDNA in vitro but still supported Chk1 phosphorylation by ATR in cells (18). Based on these observations, it was concluded that ATRIP binding to RPA–ssDNA is dispensable for ATR signaling (18). Although ATRIP 108–791 failed to associate with RPA–ssDNA in one in vitro experiment, it still formed small nuclear foci after DNA damage (18). Whether this ATRIP mutant is completely defective for RPA–ssDNA binding has not been extensively investigated.
In this study, we carefully examined the association of ATRIP 108–791 with RPA–ssDNA and found that the N terminus of ATRIP is not essential for the binding to RPA–ssDNA. ATRIP 108–791 retained the ability to associate with RPA–ssDNA in cell extracts, even in the absence of endogenous ATRIP. Also, purified ATRIP 108–791 directly associated with RPA–ssDNA in vitro. These results show that the abilities of ATRIP 108–791 to bind to RPA–ssDNA and to support Chk1 phosphorylation have not been uncoupled as suggested by the previous study. Thus, the conclusion that ATRIP binding to RPA–ssDNA is dispensable for ATR signaling cannot be drawn. We found that even the full-length ATRIP bound to RPA–ssDNA inefficiently under the stringent conditions used in the previous study. It is likely that the absence of the N terminus of ATRIP weakened the association of ATRIP with RPA–ssDNA, rendering the ATRIP–RPA–ssDNA complex unstable under the conditions used in the previous study. Under our experimental conditions, the binding of ATRIP 108–791 to ssDNA was clearly stimulated by RPA. Like that of full-length ATRIP, the association of ATRIP 108–791 with RPA–ssDNA was enhanced in extracts derived from damaged cells. Also, several other ATRIP fragments and Claspin did not bind to RPA–ssDNA under the same conditions. All of this evidence supports the conclusion that the binding of ATRIP 108–791 to RPA–ssDNA is specific and significant.
In agreement with ref. 18, we found that the N terminus of ATRIP contains a major RPA–ssDNA-interacting domain. Surprisingly, the N terminus of ATRIP can associate with RPA–ssDNA in vitro in two distinct ways, only one of which requires the presence of other human proteins. These results suggest that the interaction between ATRIP and RPA may be dynamic and it can be regulated by other proteins. Our results have also revealed the presence of at least two RPA–ssDNA-interacting domains in ATRIP outside of its N terminus. One major RPA–ssDNA-interacting domain was mapped to amino acids 218–390. Thus, the conserved coiled-coil domain of ATRIP, which is not essential for the binding to RPA–ssDNA, is flanked by two RPA–ssDNA-interacting domains on both sides (Fig. 4C) (19, 28). Interestingly, the region 108–390 also possesses affinity to ssDNA. This finding raises the possibility that in the ATRIP–RPA–ssDNA complex, ATRIP interacts with not only RPA but also ssDNA. Although the interaction between ATRIP and ssDNA is not sufficient to recruit full-length ATRIP to ssDNA, it might help to stabilize the ATRIP–RPA–ssDNA complex, and might have additional roles in regulating the activity and/or function of ATR–ATRIP on DNA.
Many proteins associated with RPA (such as XPA, Dna2, Rad51, and Rad52) are known to contact RPA through multiple interactions (29–32). The multiple interactions between ATRIP and RPA might function redundantly in the recruitment of ATR–ATRIP. Alternatively, these interactions may have distinct roles in ATR activation and signaling. Some of these interactions may be important for the initial recognition of RPA–ssDNA at sites of DNA damage, whereas others may be involved in the maintenance or amplification of DNA damage signals or in the phosphorylation of specific substrates. The fact that ATRIP 108–791 fails to form large nuclear foci in damaged cells indicates that the interaction between the N terminus of ATRIP and RPA–ssDNA cannot be fully substituted by other interactions under certain circumstances (18). In addition to ATRIP, a number of proteins involved in checkpoint signaling also associate with RPA and may potentially compete with ATRIP for the interacting surface on RPA. The multiple interactions of ATRIP with RPA–ssDNA may allow ATR–ATRIP to associate with RPA simultaneously with these proteins and facilitate the interactions between ATR–ATRIP and these proteins. The exact functions of the multiple interactions between ATRIP and RPA–ssDNA have yet to be determined by biochemical and cell-biological investigations.
Materials and Methods
Plasmids and Cells. The U2OS-derived cell lines stably expressing HA–ATRIP and HA–ATRIP 108–791 were kindly provided by David Cortez (Vanderbilt University, Nashville, TN) (18). Various ATRIP fragments were generated by using PCRs and cloned into the pUNI50 vector (33). For transient expression in human cells, the pUNI50-based plasmids were fused with a pCMV-Flag2-derived expression vector by using Cre–lox reactions so that each of the ATRIP fragments was tagged with a Flag epitope and a nuclear localization signal at its N terminus (33). For the expression of GST–ATRIP 108–791 in E. coli, the coding sequence of this ATRIP fragment was cloned directly into the pGEX-2TKcs vector.
siRNA and Transfections. The lacZ siRNA and ATRIP siRNA duplexes were purchased from Dharmacon Research (Layfayette, CO). The target sequence of ATRIP siRNA is AAGGUCCACAGAUUAUUAGAU (18). To knock endogenous ATRIP, cells were transfected twice with ATRIP siRNA duplexes by using Oligofectamine (Invitrogen) according to the manufacturer's instructions. For the expression of various ATRIP fragments in 293T cells, cells were transfected with the respective expression plasmid by using Lipofectamine 2000 (Invitrogen).
Protein Purification. GST and GST–ATRIP 108–791 were expressed in BL21-DE3 cells and purified by using glutathione beads (Amersham Pharmacia). Recombinant human RPA complex was expressed in E. coli and purified essentially as described in ref. 34.
In Vitro Transcription and Translation. Full-length ATRIP and various ATRIP fragments were transcribed and translated in vitro by using the transcription and translation (TNT)-coupled reticulocyte lysate system (Promega). Most of the ATRIP fragments were transcribed efficiently from the T3 promoter on the pUNI50 vector. For the ATRIP fragments that were not transcribed efficiently by T3 polymerase, we fused the pUNI50 plasmids of these fragments to a pET15b-derived vector, which carries a T7 promoter, by using Cre–lox reactions. All of the ATRIP fragments were transcribed efficiently by T7 polymerase.
ATRIP–RPA–ssDNA Binding Assay. A 3′-biotinylated 75-nucleotide DNA oligomer (5′-TTGTAAAACGACGGCCAGTGAATTCATCATCAATAATATACCTTATTTTGGCAGGCGGTGTTAATACTGACCGCC-3′) was used as ssDNA in the binding experiments. The ssDNA was attached to streptavidin-coated beads in buffer containing 10 mM Tris·Cl (pH 7.5) and 100 mM NaCl. To precoat ssDNA with RPA, 5 pmol of ssDNA was incubated with ≈200–400 ng of purified RPA for 30 min in binding buffer A (10 mM Tris·Cl, pH 7.5/100 mM NaCl/10 μg/ml BSA/0.01% Nonidet P-40/10% glycerol). In the binding experiments using human cell extracts, ≈2 × 106 293T cells transiently expressing ATRIP or ATRIP fragments were lysed in 220 μl of lysis buffer (10 mM Tris·Cl, pH 7.5/100 mM NaCl/0.05% Nonidet P-40/10 μg/ml BSA/10% glycerol). Resultant lysate (100 μl) was then added to 500 μl of binding buffer containing either 5 pmol of uncoated ssDNA or RPA-coated ssDNA. In the experiments using in vitro-translated ATRIP and ATRIP fragments, 10 μl of the transcription–translation reactions was added to the binding reactions. In the experiments using purified GST and GST–ATRIP 108–791, ≈10 μg of GST and 500 ng of GST–ATRIP 108–791 were added to the binding reactions, respectively. After a 30-min incubation at room temperature, the beads were retrieved from binding reactions and washed three times with binding buffer A. The binding assay was also performed in NETN buffer (binding buffer B: 50 mM Tris·Cl, pH 7.5/150 mM NaCl/0.5% Nonidet P-40/5 μg/ml aprotinin/5 μg/ml leupeptin/1 mM NaF/20 mM β-glycerol phosphate/1 mM Na3VO4/1 mM DTT/1 mM PMSF). Proteins that were bound to ssDNA or RPA–ssDNA were released from beads and separated by SDS/PAGE before blotting.
Acknowledgments
We thank Drs. Stephen Elledge (Harvard Medical School) and David Cortez for reagents, Drs. Stephen Elledge and Xiaohong H. Yang for critical reading of the manuscript, and Dr. David Cortez for communicating unpublished results. This work was supported by the Richard and Susan Smith New Investigator Award (to L.Z.). Y.N. is partly supported by a postdoctoral fellowship from the Naito Foundation.
Conflict of interest statement: No conflicts declared.
Abbreviations: RPA, replication protein A; HA, hemagglutinin; siRNA, small interfering RNA.
References
- 1.Zhou, B. & Elledge, S. J. (2000) Nature 408, 433–439. [DOI] [PubMed] [Google Scholar]
- 2.Bakkenist, C. & Kastan, M. (2004) Cell 118, 9–17. [DOI] [PubMed] [Google Scholar]
- 3.Abraham, R. (2001) Genes Dev. 15, 2177–2196. [DOI] [PubMed] [Google Scholar]
- 4.Cortez, D., Guntuku, S., Qin, J. & Elledge, S. J. (2001) Science 294, 1713–1716. [DOI] [PubMed] [Google Scholar]
- 5.Edwards, R., Bentley, N. & Carr, A. M. (1999) Nat. Cell Biol. 1, 393–398. [DOI] [PubMed] [Google Scholar]
- 6.Paciotti, V., Clerici, M., Lucchini, G. & Longhese, M. P. (2000) Genes Dev. 15, 2046–2059. [PMC free article] [PubMed] [Google Scholar]
- 7.Rouse, J. & Jackson, S. P. (2000) EMBO J. 19, 5801–5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wakayama, T., Kondo, T., Ando, S., Matsumoto, L. & Sugimoto, K. (2001) Mol. Cell. Biol. 21, 755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takata, H., Kanoh, Y., Gunge, N., Shirahige, K. & Matsuura, A. (2004) Mol. Cell 14, 515–522. [DOI] [PubMed] [Google Scholar]
- 10.Rouse, J. & Jackson, S. P. (2002) Mol. Cell 9, 857–869. [DOI] [PubMed] [Google Scholar]
- 11.Zou, L. & Elledge, S. J. (2003) Science 300, 1542–1548. [DOI] [PubMed] [Google Scholar]
- 12.Lisby, M., Barlow, J., Burgess, R. & Rothstein, R. (2004) Cell 118, 699–713. [DOI] [PubMed] [Google Scholar]
- 13.Nakada, D., Hirano, Y., Tanaka, Y. & Sugimoto, K. (2005) Mol. Biol. Cell 16, 5227–5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee, S., Moore, J., Holmes, A., Umezu, K., Kolodner, R. D. & Haber, J. E. (1998) Cell 94, 399–409. [DOI] [PubMed] [Google Scholar]
- 15.You, Z., Kong, L. & Newport, J. (2002) J. Biol. Chem. 277, 27088–27093. [DOI] [PubMed] [Google Scholar]
- 16.Costanzo, V., Shechter, D., Lupardus, P., Cimprich, K., Gottesman, M. & Gautier, J. (2003) Mol. Cell 11, 203–213. [DOI] [PubMed] [Google Scholar]
- 17.Kumagai, A., Kim, S. & Dunphy, W. G. (2004) J. Biol. Chem. 279, 49599–49608. [DOI] [PubMed] [Google Scholar]
- 18.Ball, H., Myers, J. & Cortez, D. (2005) Mol. Biol. Cell 16, 2372–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ball, H. & Cortez, D. (2005) J. Biol. Chem. 280, 31390–31396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Falck, J., Coates, J. & Jackson, S. P. (2005) Nature 434, 605–611. [DOI] [PubMed] [Google Scholar]
- 21.Zou, L., Liu, D. & Elledge, S. J. (2003) Proc. Natl. Acad. Sci. USA 100, 13827–13832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ellison, V. & Stillman, B. (2003) PLoS Biol. 1, E33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu, X., Shell, S. & Zou, Y. (2005) Oncogene 24, 4728–4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robison, J., Elliott, J., Dixon, K. & Oakley, G. G. (2004) J. Biol. Chem. 279, 34802–34810. [DOI] [PubMed] [Google Scholar]
- 25.Yoo, E., Kim, B., Lee, S., Cho, C., Chung, J. & Lee, C. H. (2005) Oncogene 24, 5423–5430. [DOI] [PubMed] [Google Scholar]
- 26.Lucca, C., Vanoli, F., Cotta-Ramusino, C., Pellicioli, A., Liberi, G., Haber, J. & Foiani, M. (2004) Oncogene 23, 1206–1213. [DOI] [PubMed] [Google Scholar]
- 27.Nakada, D., Hirano, Y. & Sugimoto, K. (2004) Mol. Cell. Biol. 24, 10016–10025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Itakura, E., Sawada, I. & Matsuura, A. (2005) Mol. Biol. Cell 16, 5551–5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li, L., Lu, X., Peterson, C. & Legerski, R. (1995) Mol. Cell. Biol. 15, 5396–5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bae, K., Kim, H., Bae, S., Kang, H., Brill, S. & Seo, Y. S. (2003) Nucleic Acids Res. 31, 3006–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hays, S., Firmenich, A., Massey, P., Banerjee, R. & Berg, P. (1998) Mol. Cell. Biol. 18, 4400–4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stauffer, M. & Chazin, W. J. (2004) J. Biol. Chem. 279, 25638–25645. [DOI] [PubMed] [Google Scholar]
- 33.Liu, Q., Li, M., Leibbam, D., Cortez, D. & Elledge, S. J. (1998) Curr. Biol. 8, 1300–1309. [DOI] [PubMed] [Google Scholar]
- 34.Henricksen, L., Umbricht, C. & Wold, M. S. (1994) J. Biol. Chem. 269, 11121–11132. [PubMed] [Google Scholar]