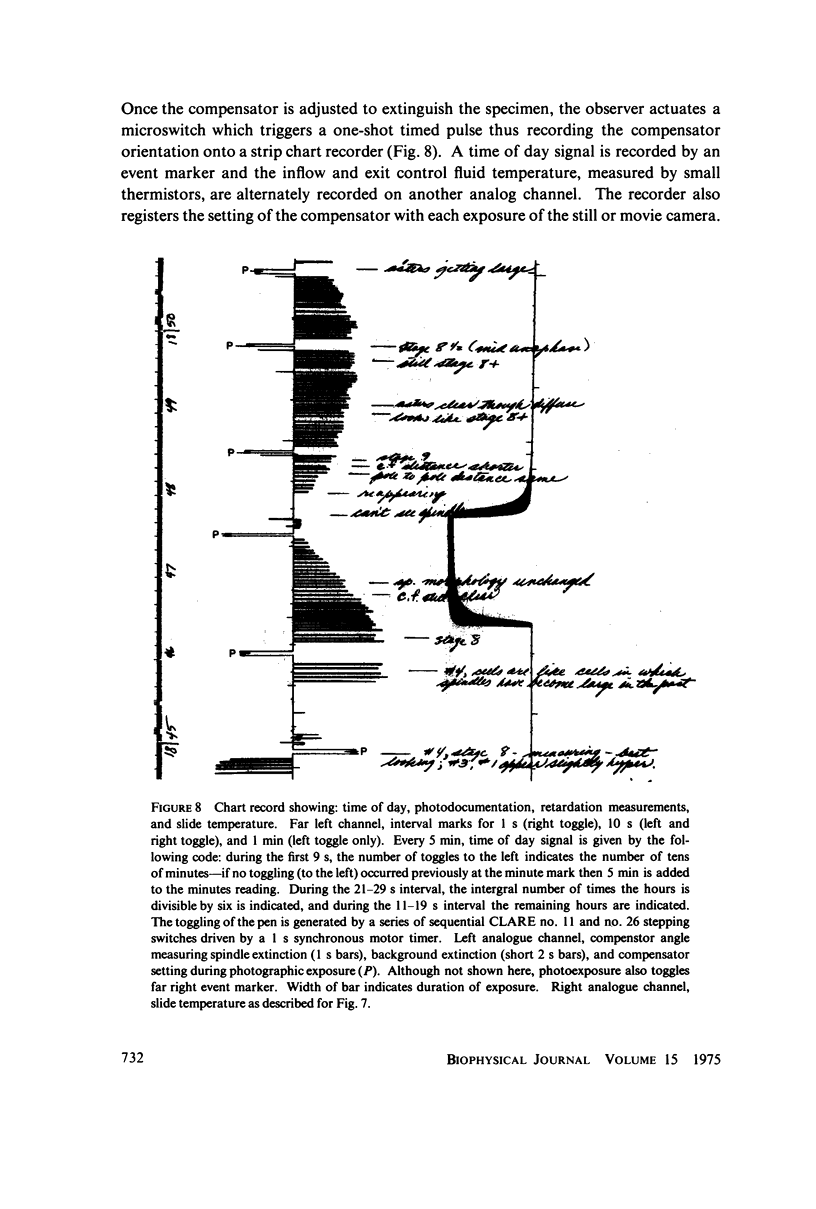
Abstract
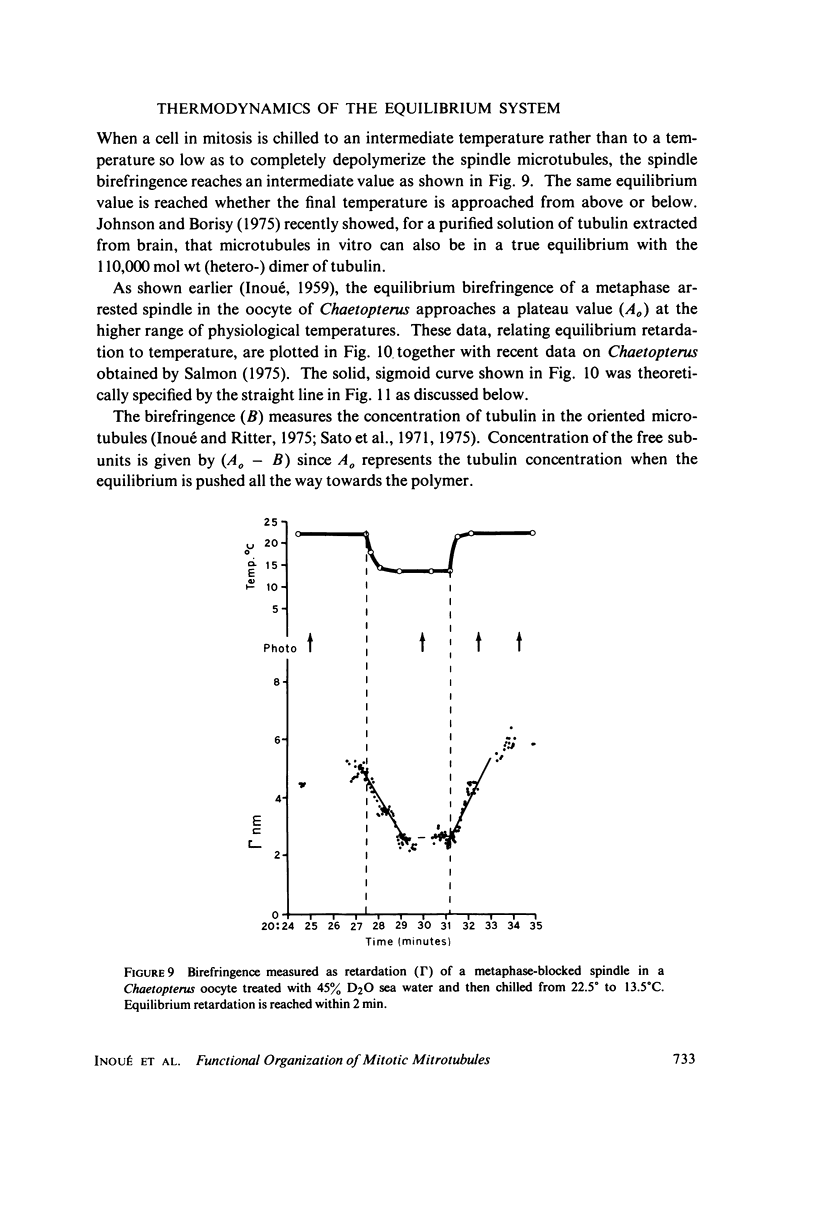
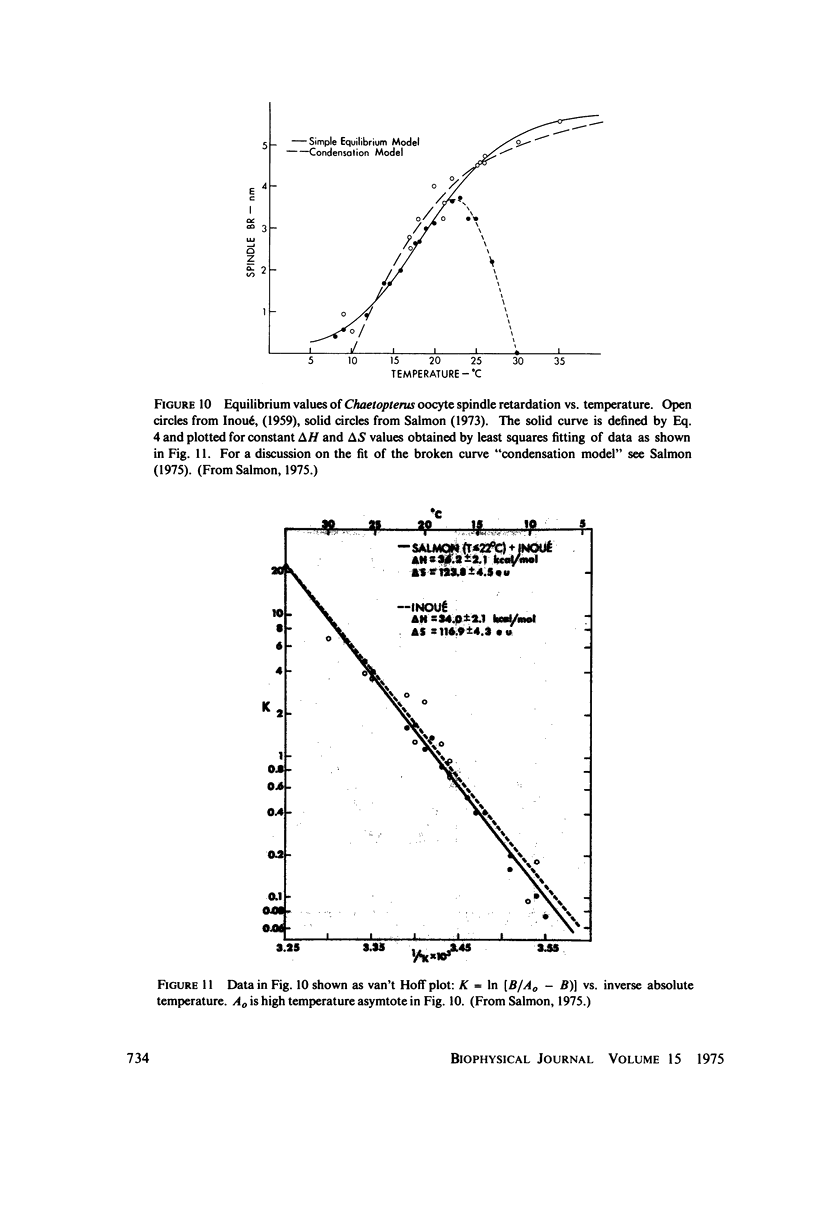
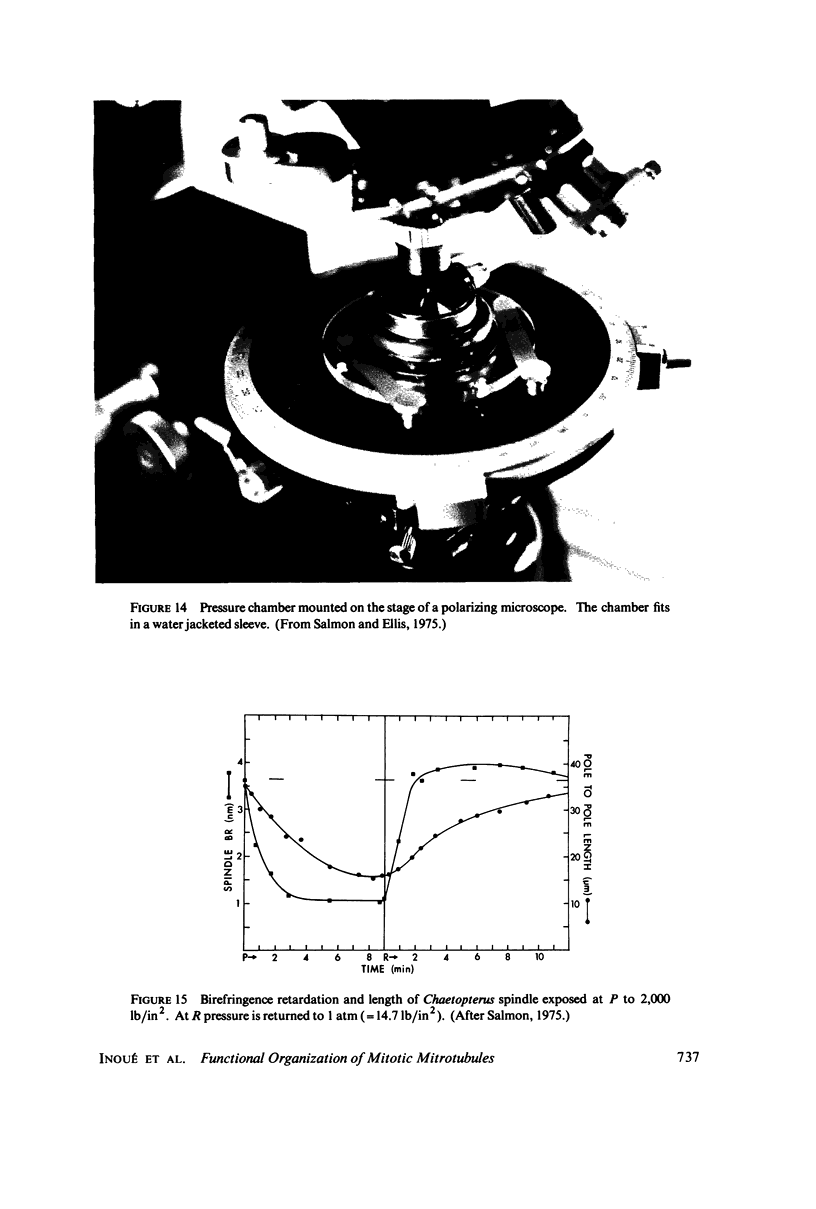
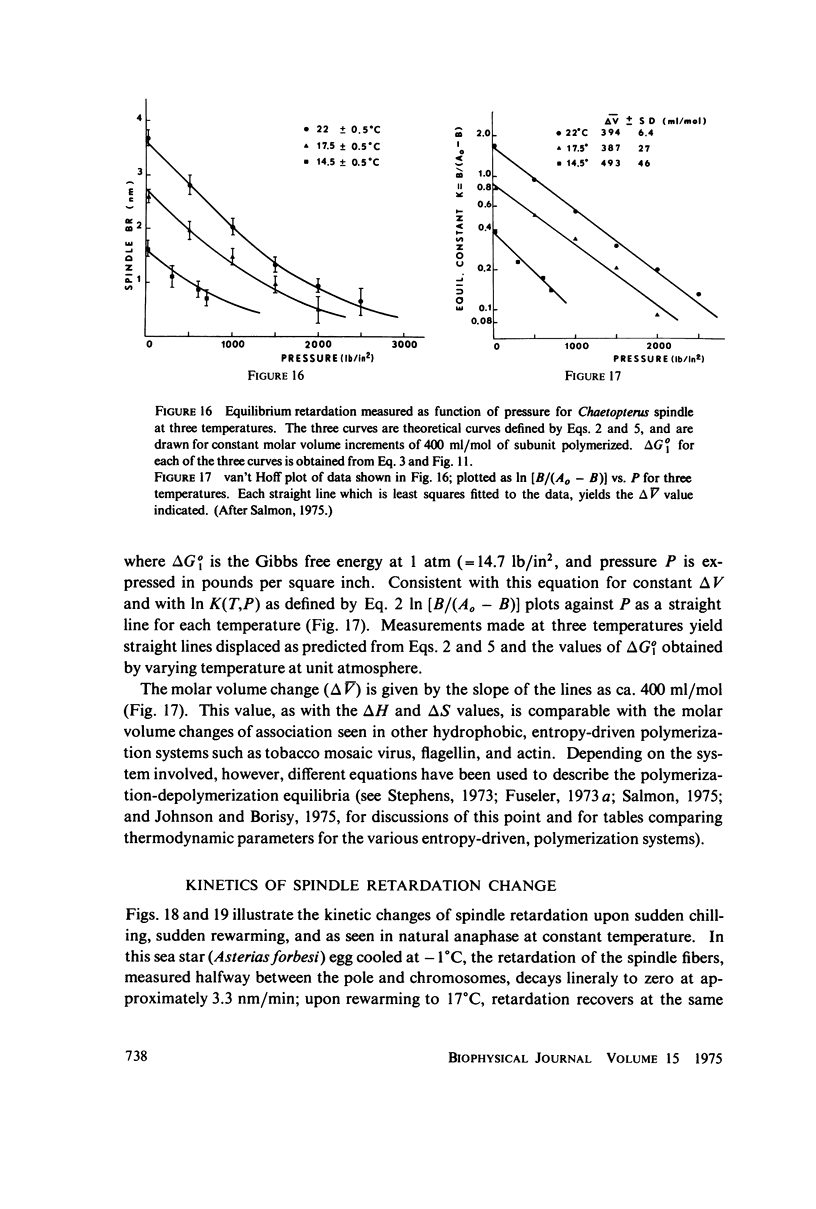
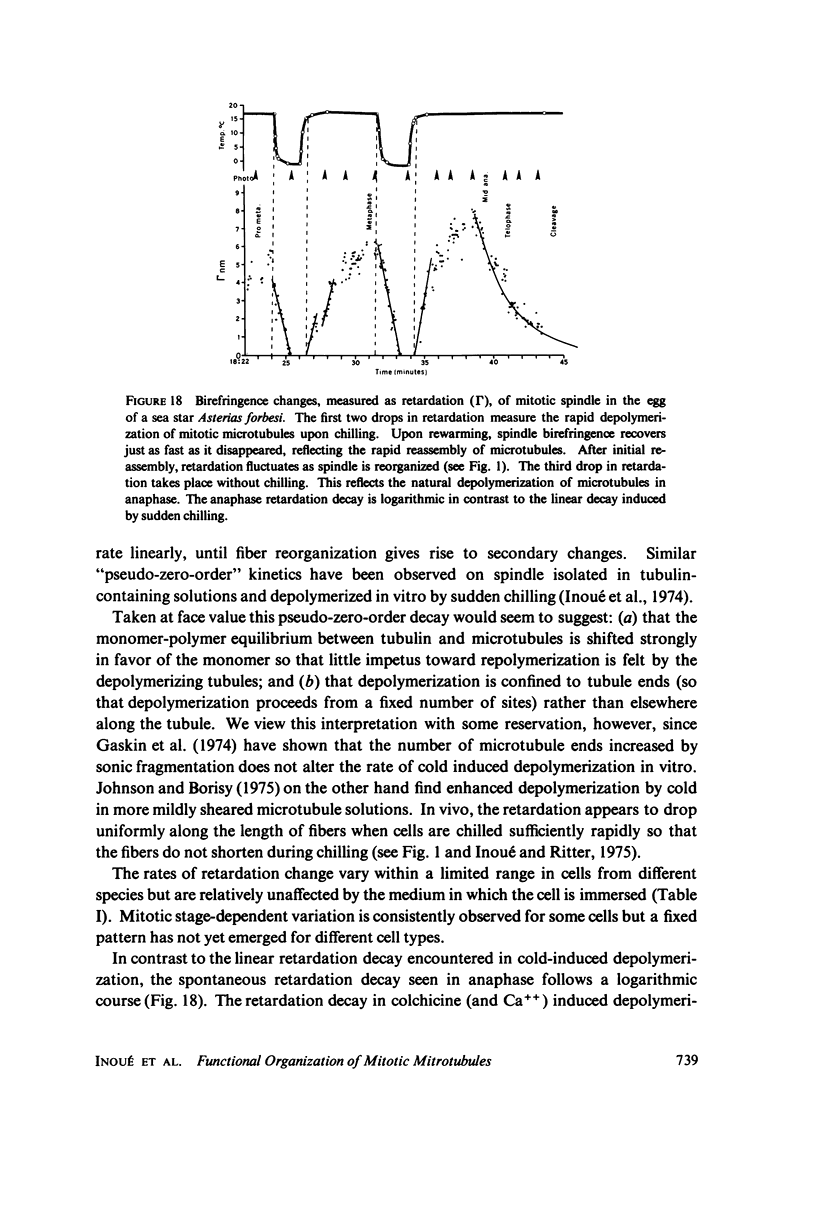
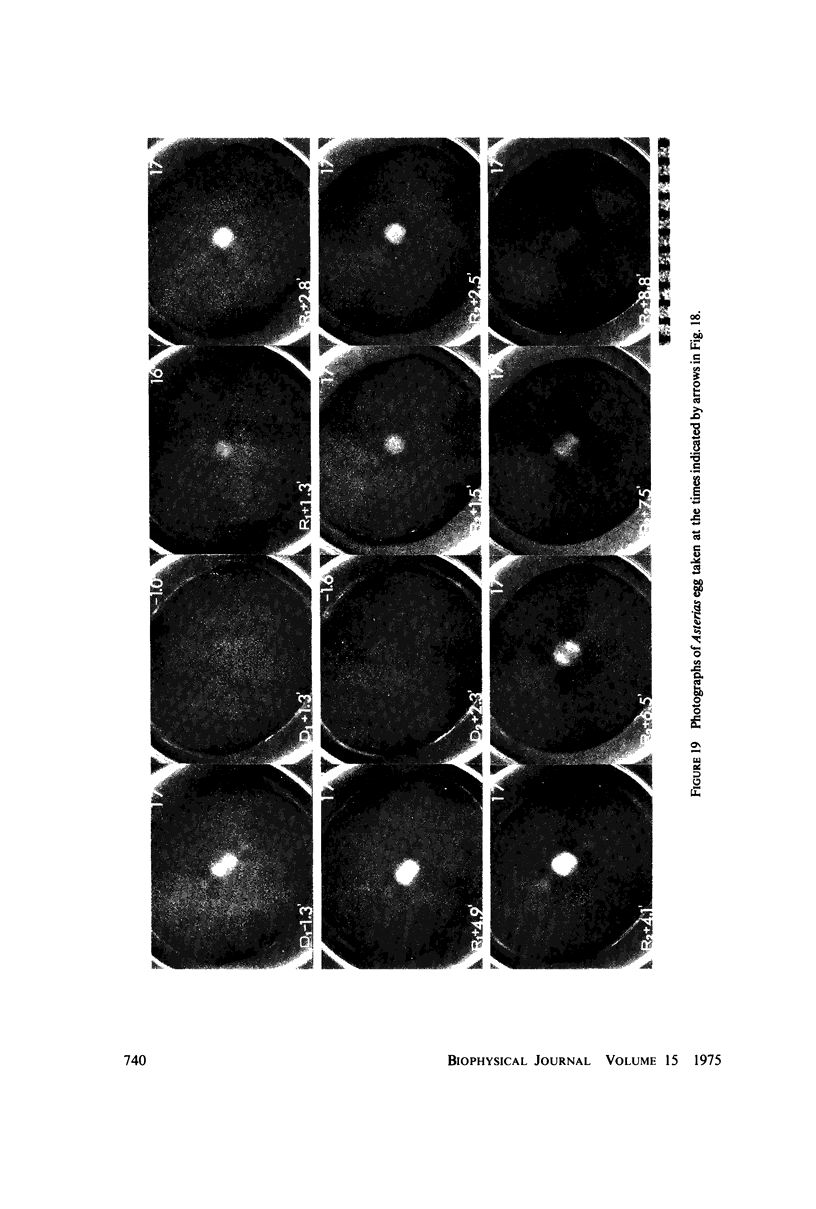
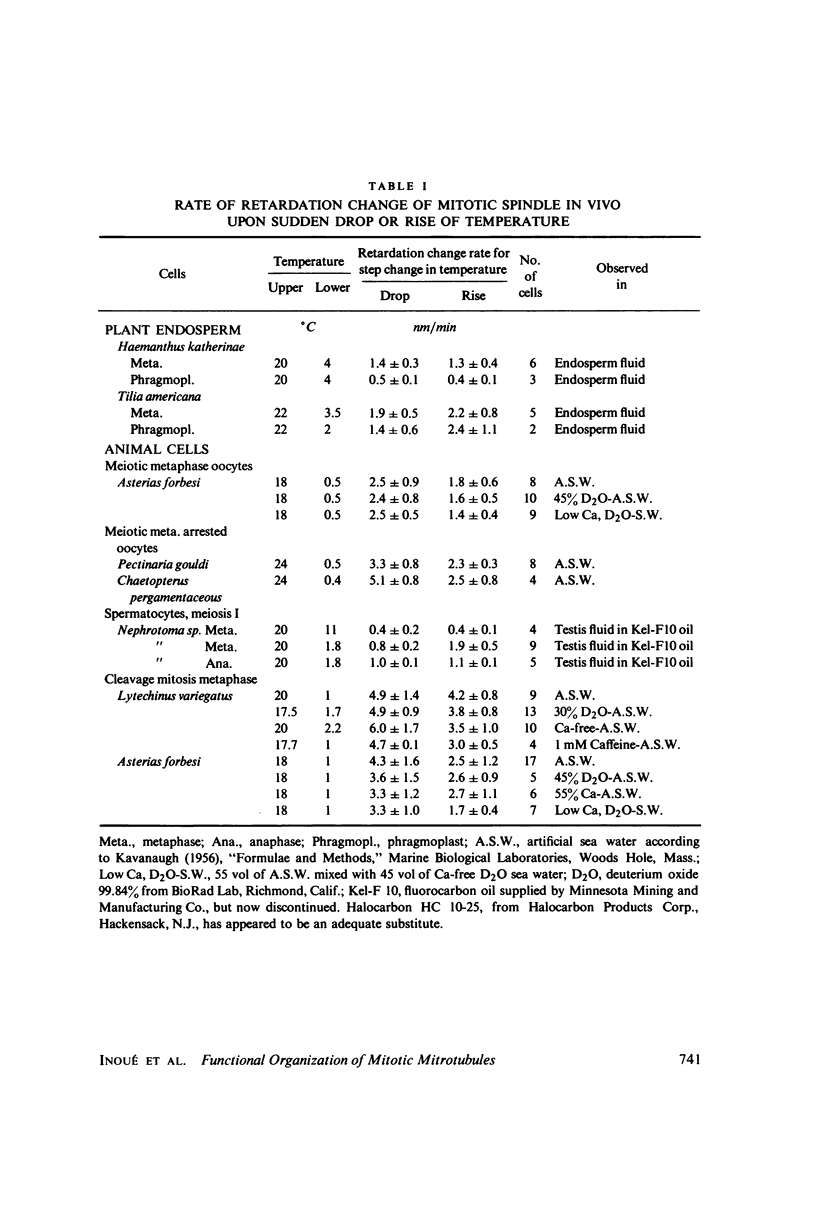
Equilibrium between mitotic microtubules and tubulin is analyzed, using birefringence of mitotic spindle to measure microtubule concentration in vivo. A newly designed temperature-controlled slide and miniature, thermostated hydrostatic pressure chamber permit rapid alteration of temperature and of pressure. Stress birefringence of the windows is minimized, and a system for rapid recording of compensation is incorporated, so that birefringence can be measured to 0.1 nm retardation every few seconds. Both temperature and pressure data yield thermodynamic values (delta H similar to 35 kcal/mol, delta S similar to 120 entropy units [eu], delta V similar to 400 ml/mol of subunit polymerized) consistent with the explanation that polymerization of tubulin is entropy driven and mediated by hydrophobic interactions. Kinetic data suggest pseudo-zero-order polymerization and depolymerization following rapid temperature shifts, and a pseudo-first-order depolymerization during anaphase at constant temperature. The equilibrium properties of the in vivo mitotic microtubules are compared with properties of isolated brain tubules.
Full text
PDF


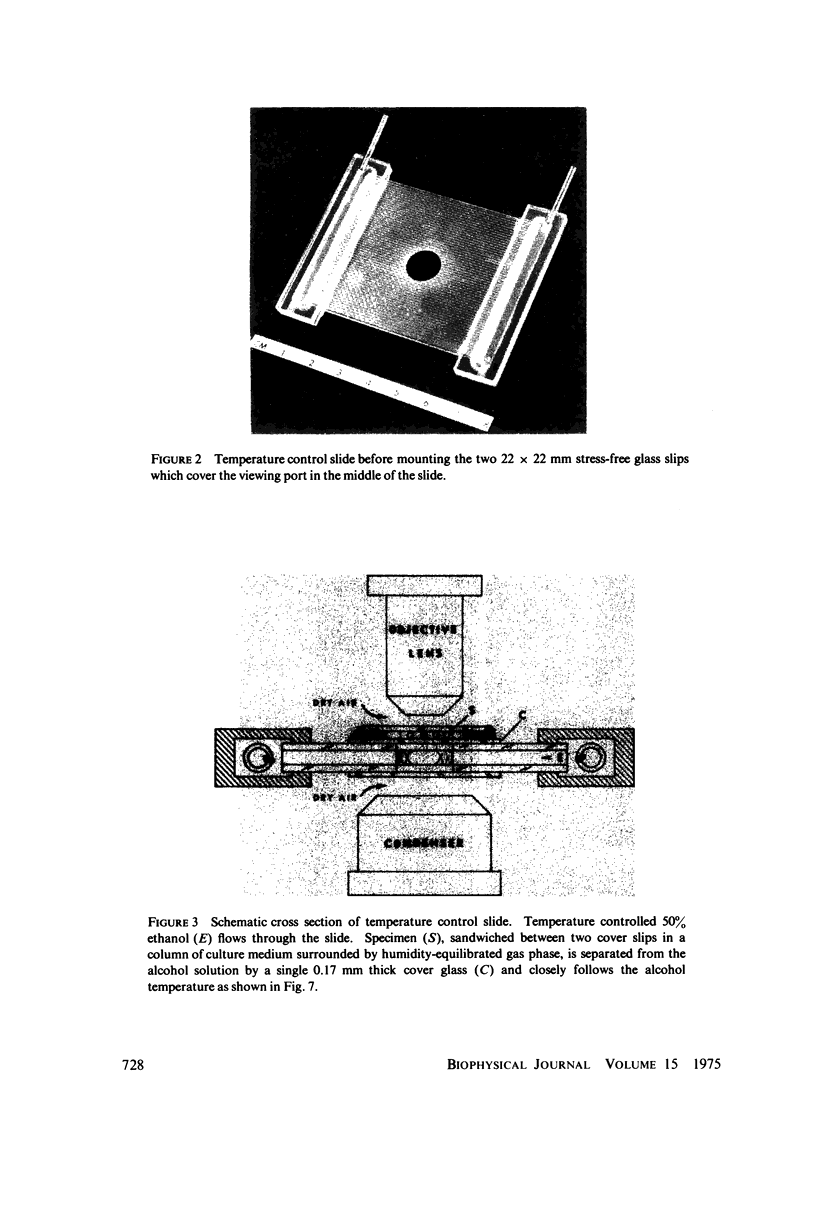
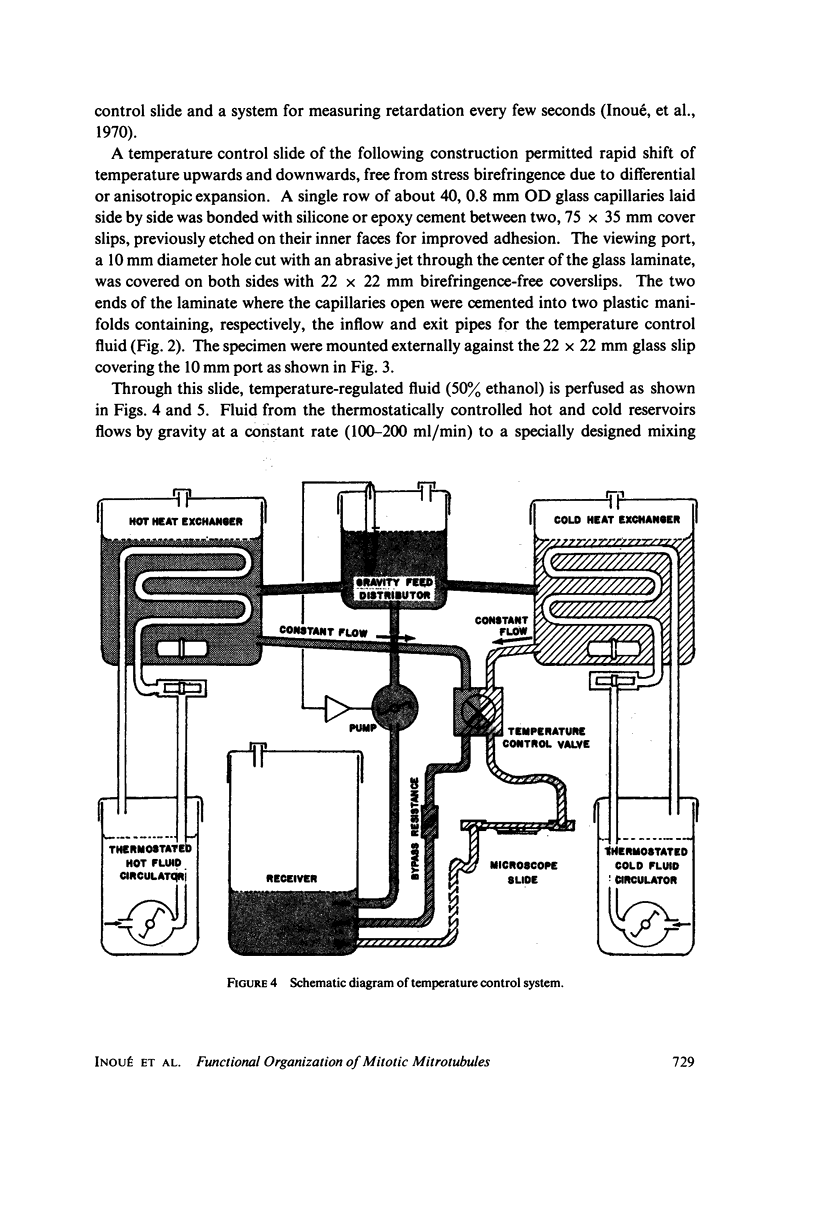

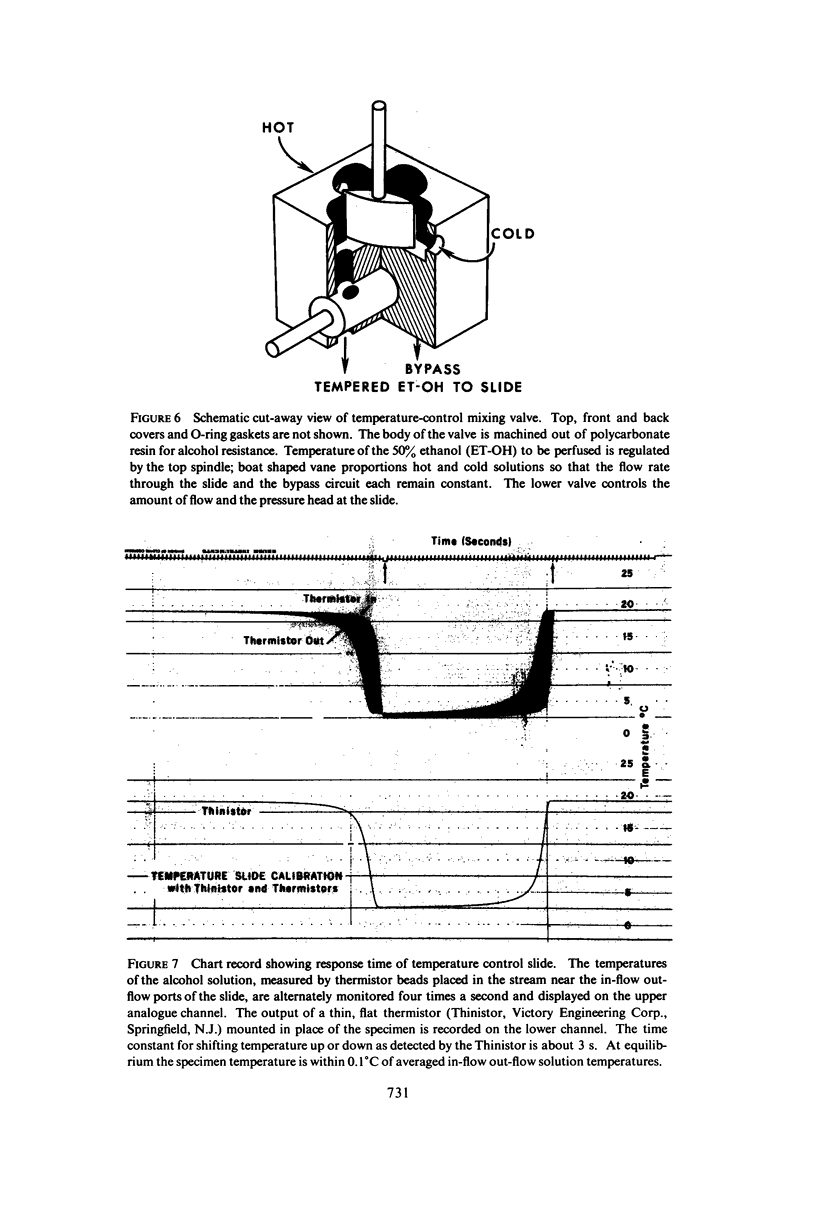





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson J. F. Nuclear membrane fusion in fertilized Lytechinus variegatus eggs. J Cell Biol. 1973 Jul;58(1):126–134. doi: 10.1083/jcb.58.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borisy G. G., Olmsted J. B., Marcum J. M., Allen C. Microtubule assembly in vitro. Fed Proc. 1974 Feb;33(2):167–174. [PubMed] [Google Scholar]
- Bryan J. Biochemical properties of microtubules. Fed Proc. 1974 Feb;33(2):152–157. [PubMed] [Google Scholar]
- Fuseler J. W. Mitosis in Tilia americana endosperm. J Cell Biol. 1975 Jan;64(1):159–171. doi: 10.1083/jcb.64.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskin F., Cantor C. R., Shelanski M. L. Turbidimetric studies of the in vitro assembly and disassembly of porcine neurotubules. J Mol Biol. 1974 Nov 15;89(4):737–755. doi: 10.1016/0022-2836(74)90048-5. [DOI] [PubMed] [Google Scholar]
- Haga T., Abe T., Kurokawa M. Polymerization and depolymerization of microtubules in vitro as studied by flow birefringence. FEBS Lett. 1974 Mar 1;39(3):291–295. doi: 10.1016/0014-5793(74)80133-x. [DOI] [PubMed] [Google Scholar]
- Inoué S., Borisy G. G., Kiehart D. P. Growth and lability of Chaetopterus oocyte mitotic spindles isolated in the presence of porcine brain tubulin. J Cell Biol. 1974 Jul;62(1):175–184. doi: 10.1083/jcb.62.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoué S., Sato H. Cell motility by labile association of molecules. The nature of mitotic spindle fibers and their role in chromosome movement. J Gen Physiol. 1967 Jul;50(6 Suppl):259–292. [PMC free article] [PubMed] [Google Scholar]
- Kresheck G. C., Schneider H., Scheraga H. A. The effect of D2-O on the thermal stability of proteins. Thermodynamic parameters for the transfer of model compounds from H2-O to D2-O. J Phys Chem. 1965 Sep;69(9):3132–3144. doi: 10.1021/j100893a054. [DOI] [PubMed] [Google Scholar]
- Olmsted J. B., Borisy G. G. Characterization of microtubule assembly in porcine brain extracts by viscometry. Biochemistry. 1973 Oct 9;12(21):4282–4289. doi: 10.1021/bi00745a037. [DOI] [PubMed] [Google Scholar]
- Rebhun L. I., Mellon M., Jemiolo D., Nath J., Ivy N. Regulation of size and birefringence of the in vivo mitotic apparatus. J Supramol Struct. 1974;2(2-4):466–485. doi: 10.1002/jss.400020232. [DOI] [PubMed] [Google Scholar]
- Salmon E. D., Ellis G. W. A new miniature hydrostatic pressure chamber for microscopy. Strain-free optical glass windows facilitate phase-contrast and polarized-light microscopy of living cells. Optional fixture permits simultaneous control of pressure and temperature. J Cell Biol. 1975 Jun;65(3):587–602. doi: 10.1083/jcb.65.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. E. A thermodynamic analysis of mitotic spindle equilibrium at active metaphase. J Cell Biol. 1973 Apr;57(1):133–147. doi: 10.1083/jcb.57.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisenberg R. C. Microtubule formation in vitro in solutions containing low calcium concentrations. Science. 1972 Sep 22;177(4054):1104–1105. doi: 10.1126/science.177.4054.1104. [DOI] [PubMed] [Google Scholar]
- Weisenberg R. C., Rosenfeld A. C. In vitro polymerization of microtubules into asters and spindles in homogenates of surf clam eggs. J Cell Biol. 1975 Jan;64(1):146–158. doi: 10.1083/jcb.64.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]