Abstract
Heat shock transcription factors (HSFs) play important roles in the cellular response to physiological stress signals. To examine the control of HSF activity, we undertook a yeast two-hybrid screen for proteins interacting with Drosophila HSF. DROJ1, the fly counterpart of the human heat shock protein HSP40/HDJ1, was identified as the dominant interacting protein (15 independent isolates from 58 candidates). Overexpression of DROJ1 in Drosophila SL2 cells delays the onset of the heat shock response. Moreover, RNA interference involving transfection of SL2 cells with double-stranded droj1 RNA depletes the endogenous level of DROJ1 protein, leading to constitutive activation of endogenous heat shock genes. The induction level, modest when DROJ1 was depleted alone, reached maximal levels when DROJ1 and HSP70/HSC70, or DROJ1 and HSP90, were depleted concurrently. Chaperone co-depletion was also correlated with strong induction of the DNA binding activity of HSF. Our findings support a model in which synergistic interactions between DROJ1 and the HSP70/HSC70 and HSP90 chaperones modulate HSF activity by feedback repression.
Keywords: Droj1/heat shock factor/HSP70/HSP83/transcription
Introduction
Organisms must be able to adapt quickly to survive changing environmental conditions. One of the best studied responses to environmental stress is the heat shock response, which leads to increased expression of a highly conserved group of proteins, called heat shock proteins (HSPs). Since protein instability is the major problem faced by cells after exposure to high temperature, many HSPs are molecular chaperones involved in protein folding and maintenance of protein stability (reviewed in Lindquist, 1986; Lindquist and Craig, 1988; Morimoto et al., 1994; Hartl, 1996; Fink, 1999; Feldman and Frydman, 2000). The induction of HSPs by heat as well as other types of stress is mediated by the heat shock transcription factor (HSF). HSF is a highly conserved protein, present in all eukaryotic organisms studied, from yeast to human. All known HSFs have a modular structure, comprised of a ‘winged’ helix–turn–helix DNA binding domain, a trimerization domain containing hydrophobic heptad repeats or leucine zippers, and a C-terminal transactivation domain (reviewed in Wu, 1995; Nover et al., 1996; Morimoto, 1998; Morano and Thiele, 1999; Santoro, 2000). HSFs of higher eukaryotes also contain a C-terminal leucine zipper that is apparently involved in the repression of HSF trimerization (Rabindran et al., 1993; Zou et al., 1994; Farkas et al., 1998).
While most metazoans contain several hsf genes, only a single hsf gene is present in Drosophila (Jedlicka et al., 1997). Like HSF1, the major heat-inducible HSF, Drosophila HSF, is normally present as a transcriptionally inactive monomer, generally located in the nucleus (Westwood and Wu, 1993; Orosz et al., 1996; Mercier et al., 1999). Upon exposure to heat stress, the HSF monomer is converted to a trimer and binds to its cognate site, the heat shock element (HSE), which is composed of inverted repeats of nGAAn. Under certain circumstances, such as upon salicylate treatment, activation of the transcriptional potency of HSF can be uncoupled from trimerization and DNA binding, suggesting that the activation process is divided in two discrete steps (Hensold et al., 1990; Jurivich et al., 1992; Giardina and Lis, 1995; Bharadwaj et al., 1998). Indeed, in the budding yeast Saccharomyces cerevisiae, HSF is constitutively trimeric and partially bound to DNA, and its activity is primarily regulated at the level of transactivation (Nieto-Sotelo et al., 1990; Sorger, 1990; Jakobsen and Pelham, 1991; Bonner et al., 1992; Chen et al., 1993).
The mechanism of activation of HSF has been intensively studied. In vitro experiments using a purified system have revealed that Drosophila HSF trimerization is affected by the physico-chemical environment, such as pH, redox state and temperature (Zhong et al., 1998, 1999). Other mechanisms have been implicated in the regulation of trimerization and transactivation of HSF, including serine phosphorylation, which accompanies activation (Knauf et al., 1996; Kline and Morimoto, 1997), and the binding by a small protein regulator called HSBP1 (Satyal et al., 1998). It has also been proposed that a common stress signal activating HSF is the relief of repression imposed by the HSPs (Lindquist and Craig, 1988; Craig and Gross, 1991; Cotto and Morimoto, 1999). Such a derepression would occur via the titration or sequestration of HSPs by the stress-induced malfolding or denaturation of native proteins, leading to the activation of heat shock gene transcription and the consequent expression of HSPs. When the level of available HSPs is replenished, HSF becomes inhibited by feedback repression.
Human HSP90, HSP70 and to some extent HSP40 have been implicated in feedback repression of human HSF1 (Abravaya et al., 1992; Mosser et al., 1993; Kim et al., 1995; Ali et al., 1998; Duina et al., 1998; Shi et al., 1998; Zou et al., 1998; Bharadwaj et al., 1999). However, in invertebrates such as Drosophila, studies have generally emphasized a direct physical or chemical modulation of HSF activity under stress conditions, although there is evidence for a role of HSP70 in the regulation of Drosophila HSF (Solomon et al., 1991). In this report, we demonstrate interactions between Drosophila HSF and DROJ1, the Drosophila homolog of the human HSP40/HDJ1. We map the domains of HSF and DROJ1 required for interaction, and evaluate the functional consequences of DROJ1 overexpression or depletion in tissue culture cells. We also compare the effects of co-depletion of DROJ1 with other molecular chaperones. Our results provide evidence for synergistic interactions between DROJ1 and HSP70/HSC70 and HSP90 in feedback regulation of HSF.
Results
Identification of a DROJ1 as an interaction partner of Drosophila HSF
To identify proteins that interact with Drosophila heat shock factor (HSF) we performed a two-hybrid screen using HSF as the bait (G.Marchler, A.Orosz and C.Wu, in preparation). We screened a Drosophila embryonic cDNA library using HSF(1–602), a truncated version of the Drosophila HSF lacking the extreme C-terminal transactivation domain (Wisniewski et al., 1996). Out of the 58 clones analyzed that showed positive interaction with GAL4 DNA-binding domain (GAL4DBD)-HSF(1–602) fusion, DNA sequencing revealed that 15 clones (∼25%) contained overlapping segments of the same cDNA. The cDNA encodes DROJ1, a HSP40 homolog, originally isolated and named by Karen Palter (DDBJ/EMBL/GenBank accession No. U34904). (The interaction screen did not identify other HSPs, such as HSP90 or HSP70.) The junctions of the fusions between DROJ1 and the GAL4AD (activation domain) are indicated in Figure 1. The sequence of full-length droj1 cDNA predicts a protein of 334 residues, with a mol. wt of 36.7 kDa. DROJ1 is highly related to the Escherichia coli chaperone DnaJ (Bardwell et al., 1986; Ohki et al., 1986) and falls into the same subgroup of DnaJ homologs as the human HDJ1 (55% identity) and the S.cerevisiae Sis1 protein (34% identity). (DROJ1 can functionally substitute for Sis1 in vivo; G.Marchler, unpublished observations.) The DROJ1 protein sequence contains all four conserved domains characteristic for the J1-subgroup of DnaJ proteins (Cheetham and Caplan, 1998), including the highly conserved J-box, the FG-region, a J1-specific region, and the least conserved C-terminal domain that plays a role in substrate binding (Goffin and Georgopoulos, 1998). DROJ1 lacks the cysteine repeats that are characteristic of the J2 subgroup.
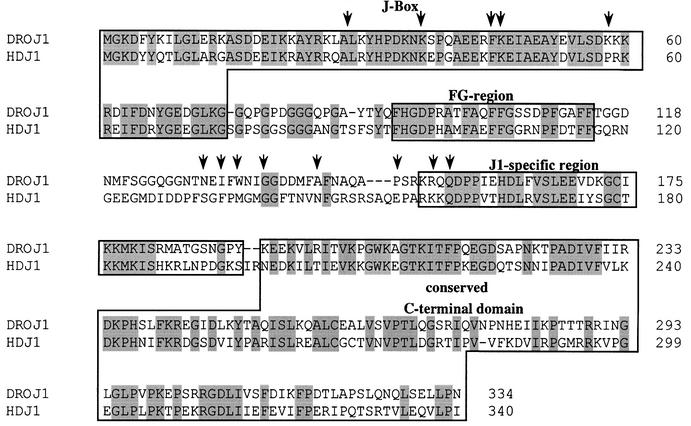
Fig. 1. DROJ1, a DnaJ/HSP40 homolog from Drosophila. Alignment of DROJ1 (DDBJ/EMBL/GenBank accession No. U34904) with its closest human homolog HDJ1 (DDBJ/EMBL/GenBank accession No. 1706473, DNJ1_HUMAN). Identical amino acids are shaded. Boxes indicate conserved protein domains. The arrows represent the starting points of the DROJ1 clones that showed positive interaction.
Analysis of independently isolated DROJ1 fusions revealed that the C-terminal half of the DROJ1 open reading frame was necessary for a productive interaction with HSF. All positive clones contained the J1-specific region and the entire C-terminal domain of the protein (Figure 1). Since the cDNA library we screened was constructed by priming from the poly(A) tail, i.e. from the C-terminal end of the protein, we attempted to refine the C-terminal boundary of the interacting domain by constructing additional C-terminal truncations of GAL4AD-DROJ1(152–334), which contains the smallest interacting segment of DROJ1. As indicated in Figure 2A and C, all three C-terminal truncations [GAL4AD-DROJ1(152– 257), GAL4AD-DROJ1(152–236) and GAL4AD-DROJ1 (152–221)] abolished interaction with HSF. Therefore, most, if not the entire C-terminal half of DROJ1, containing the J1 domain and the C-terminal domain, is necessary to bind to HSF in the two-hybrid assay. It is interesting that the recently published crystal structure of the peptide binding fragment of Sis1, the yeast homolog of DROJ1, shows that the corresponding C-terminal region and the adjacent J1 domain are potentially involved in interactions with peptide substrate and HSP70 proteins (Sha et al., 2000).
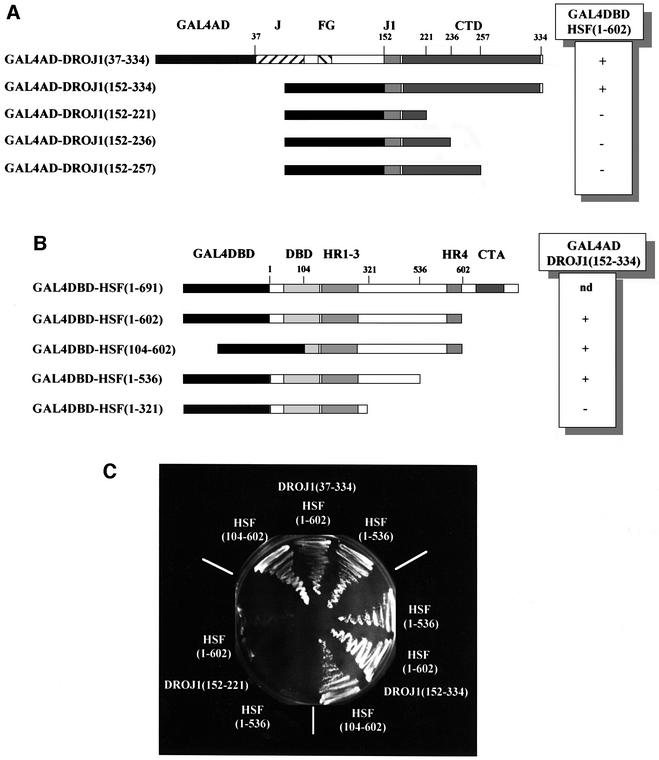
Fig. 2. Mapping of the interaction site between HSF and DROJ1. (A) Interaction between the Drosophila HSF(1–602) fused to the GAL4DBD and DROJ1 segments fused to the GAL4AD. Shaded boxes indicate conserved domains of DROJ1 (J, J-box; FG, FG-rich region; J1, J1-specific domain; CTD, C-terminal domain). Numbers indicate amino acids of DROJ1 at the fusion point or endpoint of the constructs. (+), interaction with partner protein, but not with the GAL4DBD; (–), interactions with neither, as scored by growth on plates containing 20 mM 3-aminotriazole. (B) Interaction between the smallest interacting fusion of DROJ1 [GAL4AD-DROJ1(152–334)] with different segments of Drosophila HSF fused to the GAL4DBD. Shaded boxes indicate conserved domains of HSF (HR, heptad repeat; CTA, C-terminal transactivation domain). Numbers indicate amino acids of HSF at the fusion or endpoint of the constructs. (+), interaction with partner protein, but not with GAL4AD; (–), interactions with neither, as scored by growth on plates containing 20 mM 3-aminotriazole; nd, not determined. (C) Interaction of different pairs of deletion constructs detected by growth on plates containing 20 mM 3-aminotriazole.
To map the domain of HSF responsible for interacting with DROJ1, we introduced N- and C-terminal deletion constructs of HSF in the two-hybrid screen (Figure 2B and C). A construct deleted for most of the DNA binding domain of HSF, GAL4DBD-HSF(104–602), and a deletion of the C-terminal heptad repeat, GAL4DBD-HSF(1–536), had no influence on the interaction with DROJ1(152–334). Similar results for these constructs were obtained when DROJ1(37–334) was employed as the bait (Figure 2C). However, a construct deleting a substantial part of HSF from the c-terminus to residue 321, GAL4DBD-HSF(1–321), failed to interact with DROJ1, as scored by the absence of growth on plates lacking histidine (Figure 2C). Hence, the region between the N- and C-terminal leucine-zippers contains a region of HSF that is critical for the interaction with DROJ. We note that the interactions between DROJ1 and HSF revealed by the two-hybrid assay are likely to be weak, as co-immunoprecipitation studies showed no detectable interactions between the proteins in native cell extracts (G.Marchler, unpublished results).
DROJ1 is a constitutive and inducible HSP present in nucleus and cytoplasm
The closest relative of DROJ1 is human HDJ1, also known as HSP40 (Raabe and Manley, 1991). To examine whether DROJ1 is also a heat shock protein, we shifted Schneider Line 2 (SL2) cells to the heat shock temperature (36°C), and analyzed the transcription of droj1 by RNA dot blots. We found that droj1 is constitutively expressed under non-shock conditions (Figure 3A, 0 min), and that expression is significantly heat-inducible (∼12-fold in 30 min), with kinetics similar to the induction of the classical hsp genes hsp26 and hsp70 (Figure 3A). Similar heat inducibility of droj1 was also observed by Karen Palter (personal communication). droj1 mRNA levels remained constant over a period of 2 h during recovery from heat shock (data not shown). The induction of droj1 mRNA leads to increased expression of DROJ1 protein (∼2-fold over the high constitutive level; data not shown; see also Figure 5A, lanes 1 and 2). Examination of the genomic sequence of droj1 (DDBJ/EMBL/GenBank accession No. AC014815) revealed sequences within 500 bp of the transcription start site that closely match the canonical heat shock element (HSE), providing a basis for the heat shock inducibility.
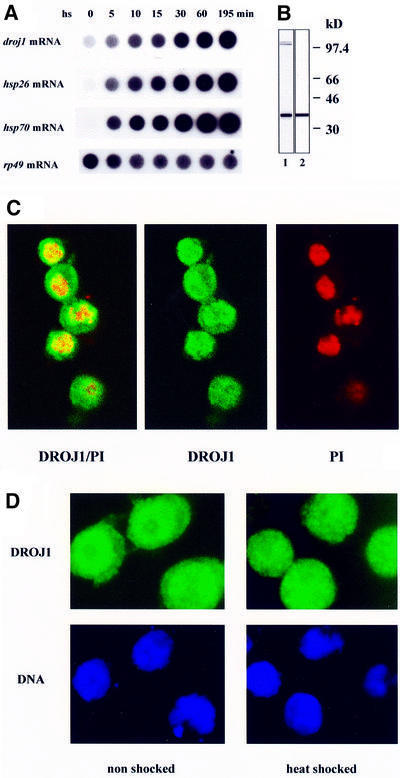
Fig. 3. DROJ1 is a HSP located in nucleus and cytoplasm. (A) RNA dot blot analysis for droj1, hsp26 and hsp70 mRNA. Total RNA isolated from SL2 cells subjected to heat stress (36°C, water bath) for the times indicated. (B) Antibody purification. Antibody produced against the bacterially expressed C-terminal half of DROJ1 (1:1000 dilution, before purification, lane 1) detects a single band of ∼40 kDa after affinity purification (lane 2). Exposure times are adjusted for equal intensity of the 40 kDa band. (C) Immunolocalization of DROJ1 by confocal microsocopy. Non-shocked cells grown at 22°C were fixed and stained for DROJ1 using purified antibody (dilution 1:10) and FITC-labeled secondary antibody (DROJ1), and for DNA using propidium iodide (PI). DROJ1/PI represents the overlay of the confocal images of DROJ1 and PI, to detect colocalization. (D) Localization of DROJ1 in non-shocked and heat-shocked cells. Cells grown at 22°C (non-shocked) and cells heat shocked for 20 min at 36°C were fixed and stained for DROJ1 as in (B). DNA was stained with Hoechst 33258. Heat-shocked cells were exposed for a shorter period than non-shocked cells.
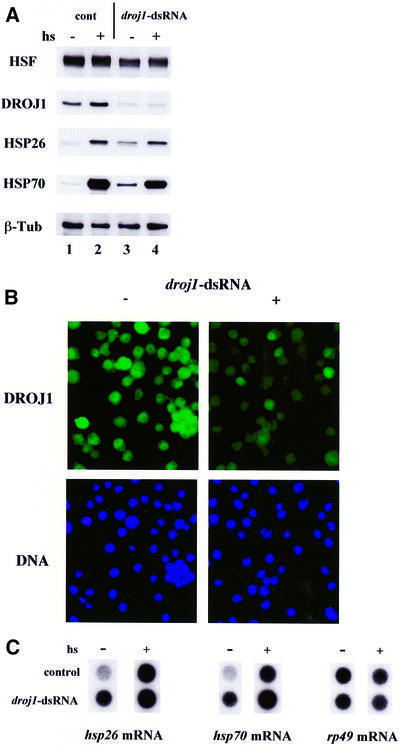
Fig. 5. Depletion of DROJ1 by RNAi. (A) SL2 cells were transfected with (droj1-dsRNA) or without (cont) dsRNA for droj1. Two days after transfection, an aliquot was taken (hs–, lanes 1 and 3) and the rest of the cells were subjected to heat shock (36°C, 20 min) and allowed to recover for 2 h at room temperature (hs+, lanes 2 and 4). Protein extracts were prepared and assayed for HSF, DroJ1, Hsp26, Hsp70 and β-tubulin protein levels by western blotting. (B) SL2 cells (–) and cells transfected with dsRNA against droj1 (+) were fixed 2 days after transfection, and stained for DROJ1 using affinity-purified antibody (dilution 1:10) and FITC-labeled secondary antibody (DROJ1), and for DNA using Hoechst 33258. (C) Dot blot analysis for hsp26 and hsp70 mRNA. Total RNA (10 µg) isolated from cell samples identical to (A). Exposure times of non-shocked (–) and heat-shocked (+) samples are not equivalent. rp49 mRNA represents a control for equal loading.
To detect DROJ1 protein, we produced a polyclonal antibody against a C-terminal segment of DROJ1, excluding the highly conserved J-box, to avoid potential cross-reactivity with other DnaJ homologs. After immunoaffinity purification, the antibody detected a single band of ∼40 kDa in whole-cell extracts prepared from SL2 cells (Figure 3B, lane 2). Using affinity-purified antibody, we analyzed the subcellular localization of DROJ1 in SL2 cells by indirect immunofluorescence. As shown by confocal microscopy, DROJ1 is constitutively localized in both nucleus and cytoplasm of unshocked cells, with higher levels found in the nucleus (Figure 3C). Upon heat shock, DROJ1 protein is found in both nucleus and cytoplasm (Figure 3D) and a slight overall increase in the staining intensity is also observed. A similar distribution of DROJ1 was observed for cells after recovery from heat shock (data not shown). The presence of DROJ1 in the nuclear and cytoplasmic compartments is consistent with a potential function for the protein in modulating the activity of HSF.
Overexpression of DROJ1 delays onset of the heat shock response
To assess the functional relevance of the observed interaction between Drosophila HSF and DROJ1, we investigated the effects of overexpression of DROJ1 in SL2 cells. The droj1 gene was placed under control of the copper-inducible metallothionein promotor using a plasmid containing a selectable hygromycin-resistance marker. We selected for a stable cell line (SL2-Droj1) that showed copper-inducible expression of DROJ1 protein. When compared with untransfected SL2 cells in culture medium lacking copper, SL2-Droj1 cells showed no significant differences in growth and in the constitutive level of DROJ1 protein (data not shown). Introduction of copper sulfate to the medium for 5 h, followed by washing and re-equilibration in copper-free medium, resulted in significant overexpression and retention of high cellular levels of DROJ1 protein (Figure 4A). The washing and re-equilibration step was introduced to ameliorate the minor induction of hsp70 by copper (Bunch et al., 1988; Murata et al., 1999). SL2-Droj1 cells containing overexpressed DROJ1 protein were then heat shocked at 34°C for 5, 15 and 30 min; the levels of DROJ1 and HSF remained roughly unchanged over this time course (Figure 4A). As indicated by RNA dot blot analysis, we found a reproducible delay in the onset of induction of hsp26 as well as hsp70 mRNA in cells overexpressing DROJ1 (Figure 4B). This effect is graphically displayed after quantification of the RNA levels and normalization against the ribosomal protein rp49 mRNA (Figure 4C). Aside from the delay in the onset of induction, overexpression of DROJ1 did not block the full response to heat shock, since hsp26 and hsp70 mRNAs reached at least fully induced levels beyond 30 min of induction (data not shown).
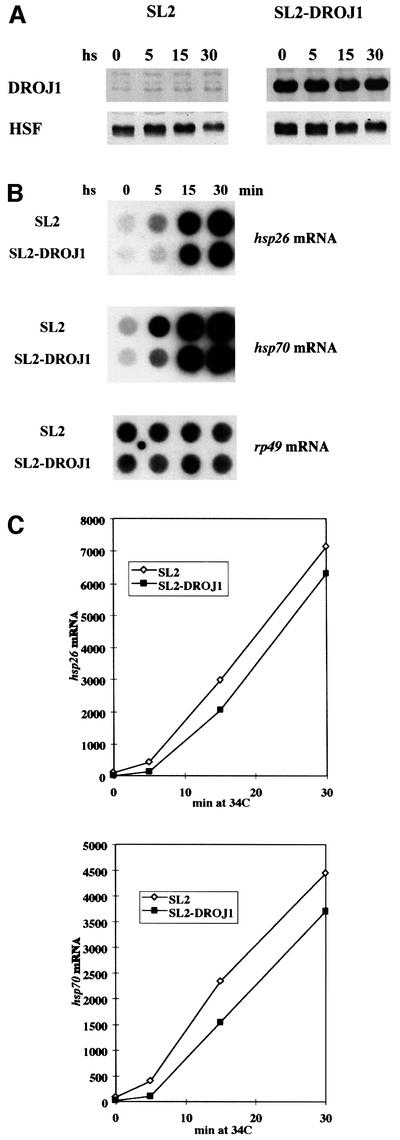
Fig. 4. Heat shock response in SL2 cells overexpressing DROJ1. (A) Wild-type SL2 cells (SL2), and the stable cell line inducibly overexpressing DROJ1 (SL2-DroJ1) were subjected to 34°C heat stress for the indicated times, after induction of DROJ1 expression (0.8 mM CuSO4 for 5 h) and recovery in copper-free medium for 5 h. Protein extracts were prepared and assayed for DROJ1 and HSF protein levels by western blotting. (B) Dot blot analysis for hsp26 and hsp70 mRNA using rp49 mRNA as a loading control. Total RNA (10 µg) was isolated from cells treated as in (A). (C) Quantification of mRNA levels in (B). The experiment was performed three times, data from one experiment are shown.
Depletion of DROJ1 derepresses Hsp synthesis
The development of the RNA interference (RNAi) technique in Caenorhabditis elegans and Drosophila (Fire et al., 1998; Timmons and Fire, 1998; Misquitta and Paterson, 1999), and its recent application to SL2 tissue culture cells (Hammond et al., 2000; Wei et al., 2000), provided an opportunity to study further the functional importance of the two-hybrid interaction. In an SL2 cell population, the RNAi technique was found to deplete proteins expressed from transfected constructs as well as endogenous proteins such as cyclin E and HSF (Hammond et al., 2000; Wei et al., 2000). Since the target of RNAi is mRNA rather than protein, depletion of endogenous proteins by this method requires a waiting period (∼2 days for HSF) that is dependent on protein half-life. To evaluate the effectiveness of DROJ1 depletion, we transfected double-stranded RNA (dsRNA) corresponding to the N-terminal half of the Droj1 message into SL2 cells. As shown by western blotting, the level of DROJ1 was significantly reduced 2 days after transfection relative to the level in cells transfected in the absence of dsRNA (Figure 5A, lanes 3 and 4). The reduction in protein level was confirmed by indirect immunofluorescence (Figure 5B). Cells transfected with droj1 dsRNA showed strongly reduced staining in ∼80–90% of the population compared with controls, indicating that the transfection efficiency was high.
The ability to reduce levels of endogenous DROJ1 significantly in a large fraction of the cell population permitted us to evaluate the effect of DROJ1 depletion on the expression of endogenous heat shock genes. By western blot analysis, we found a modest, but clear increase in Hsp expression under normal conditions in cells depleted for DROJ1 (Figure 5A). This increase was not observed when cells were transfected with a control dsRNA for green fluorescent protein (data not shown). Both depleted and non-depleted cells displayed typical heat shock-induced levels of hsp26 and hsp70. To confirm that the increased expression of HSPs is correlated with increased gene transcription, we analyzed the mRNA levels of hsp26 and hsp70. As shown by RNA dot blots, we found an increase of mRNA levels (2- to 5-fold) in non-shocked cells depleted for DROJ1. There were no significant effects of DROJ1 depletion on the heat shock-induced levels of hsp26 and hsp70 mRNA (Figure 5C).
Synergistic effects of depleting DROJ1 and other chaperones
Previous studies have indicated that HSP40 functions in conjunction with HSP70 family chaperones in protein folding. To examine the possibility that HSP70 family proteins participate with DROJ1/HSP40 in modulating the activity of HSF, we used RNAi to deplete HSP70 proteins from Drosophila SL2 cells. As shown in Figure 6A, transfection of dsRNA against hsp70 depletes the low basal expression of HSP70 and strongly reduces heat shock-induced expression. Because the significant sequence identity between hsp70 and the constitutively expressed hsc70 mRNAs suggested the possibility of cross-interference, we also examined the effects of hsp70 dsRNA transfection on HSC70 protein levels. Using an antibody that recognizes both HSP70 and HSC70 isoforms, we found that on the whole, the HSP70 family proteins displayed a slight, but clear depletion upon transfection with hsp70 dsRNA (Figure 6A).
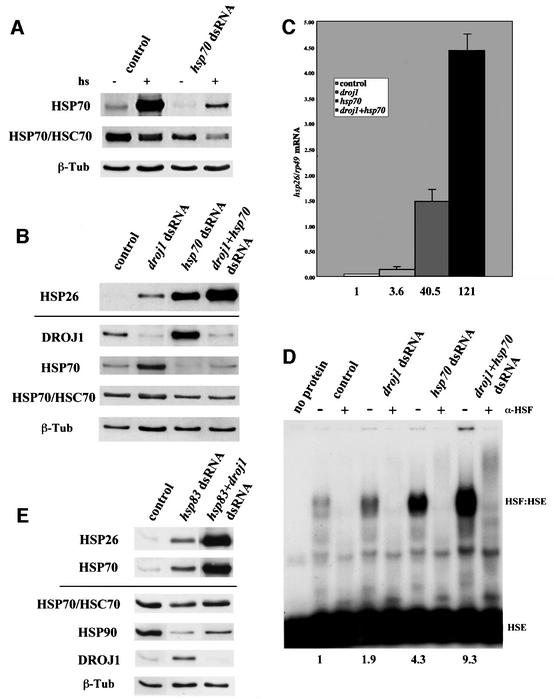
Fig. 6. Effect of depletion of DROJ1, HSP70/HSC70 and HSP90 on heat shock gene expression. (A) SL2 cells were transfected without (control) or with dsRNA for hsp70 (hsp70 dsRNA). Forty-four hours after transfection cells were either harvested immediately (–) or after heat shock (15 min, 36.5°C) and recovery (1.5 h at room temperature) (+). Protein extracts were prepared and assayed for HSP70, Hsp70/hsc70 and β-tubulin protein levels by western blotting. (B) SL2 cells were transfected without (control) or with dsRNA for droj1 (droj1 dsRNA), hsp70 (hsp70 dsRNA) or both (droj1+hsp70 dsRNA). Two days after transfection, cells were harvested. Protein extracts were prepared and assayed for Hsp26, DroJ1, HSP70, Hsp70/hsc70 and β-tubulin protein levels by western blotting. (C) Graphical representation of dot blot analysis for hsp26 mRNA using 10 µg of total RNA isolated from cell samples identical to (A) and normalized for rp49 mRNA. Numbers indicate induction factors relative to control cells. (D) Gel mobility assay for HSF binding activity. Protein extracts from (A), normalized for equal HSF content, were (+) or were not (–) incubated with HSF antibody (α-HSF) prior to the addition of labeled HSE to block HSF:HSE formation. Numbers indicate induction factors of the intensity of the HSF:HSE complex relative to control cells. (E) SL2 cells were transfected without (control) or with dsRNA for hsp83 (hsp83 dsRNA) or both hsp83 and droj1 (hsp83+droj1 dsRNA). Two days after transfection, cells were harvested. Protein extracts were prepared and assayed for Hsp26, HSP70, Hsp70/hsc70, HSP90, DroJ1 and β-tubulin protein levels by western blotting.
This depletion of HSP70/HSC70 leads to increased expression of endogenous HSP26 protein and hsp26 mRNA, as well as increased DNA binding activity of HSF—the increases are greater than those observed by depletion of DROJ1 alone (Figure 6B–D). Moreover, co-depletion of DROJ1 and HSP70/HSC70 reveals even greater induction of the DNA binding activity of HSF, and greater induction of HSP26 protein and mRNA expression (Figure 6B–D). The fold induction of hsp26 mRNA was 4-fold for droj1 depletion, 40-fold for HSP70/HSC70 depletion and 120-fold for the co-depletion, which is strikingly similar to a classic heat shock induction in our hands (Figure 3A). The results indicate that DROJ1 and HSP70/HSC70 act synergistically in modulating the activity of HSF. We further explored interactions between DROJ1 and other chaperone proteins in modulating HSF activity. Transfection with hsp83 (hsp90 family) dsRNA leads to slight induction of HSP26 and HSP70, while co-transfection with dsRNA for droj1 and hsp83 reveals high-level induction of these endogenous heat shock reporters (Figure 6E), and induction of the DNA binding activity of HSF (data not shown). Hence, DROJ1 displays additional synergy with HSP90 in modulating HSF activity, a finding consistent with emerging evidence for functional interactions between these two classes of chaperone protein (Johnson and Craig, 2000; Schnaider et al., 2000).
Discussion
In this paper, we report the isolation of DROJ1 in a two-hybrid screen for proteins interacting with Drosophila HSF. DROJ1 is the closest Drosophila relative of the human molecular chaperone HSP40/HDJ1. The same Drosophila protein (renamed dHDJ1) was also recently identified in a genetic screen for factors modifying polyglutamine toxicity in the Drosophila eye (Kazemi-Esfarjani and Benzer, 2000). We have mapped the domain of HSF interacting in the two-hybrid screen to the region between the N- and C-terminal leucine zippers, previously shown to modulate HSF activity (Orosz et al., 1996). The corresponding interacting domain of DROJ1 maps to the C-terminal half of the protein and is involved in substrate binding (Goffin and Georgopoulos, 1998). In addition, we demonstrate that overexpression of DROJ1 in a Drosophila cell line results in a delay in the onset of the heat shock response, while depletion by means of RNAi leads to a partial derepression of heat shock genes. This derepression is enhanced by co-depletion of DROJ1 with HSP70/HSC70 or HSP90, to an extent that is comparable to a classic heat shock response. These findings indicate that the cellular levels of DROJ1, HSP70/HSC70 and HSP90 can modulate the heat shock response via a negative feedback mechanism (Craig and Gross, 1991; Wu, 1995; Morano et al., 1998; Morimoto, 1998; Cotto and Morimoto, 1999; Morano and Thiele, 1999; Santoro, 2000).
In this autoregulatory model, the heat shock response is controlled under normal conditions via repression of HSF activity by constitutively expressed HSPs. Upon heat stress, the sequestration of HSPs by non-native cellular proteins leads to activation (derepression) of HSF, which in turn, results in elevated Hsp expression. The activity of HSF and the transcription of heat shock genes then attenuate when the available HSPs are replenished. Feedback regulation was originally proposed to account for the failure to attenuate transcription of heat shock genes in Drosophila cells that were induced in the presence of protein synthesis inhibitors (DiDomenico et al., 1982). A specific role for HSPs in feedback control was indicated by a loss of transcriptional attenuation in S.cerevisiae carrying mutations in ssa1 and ssa2, two constitutively expressed hsp70 genes (Boorstein and Craig, 1990). Genetic and biochemical studies in bacteria also indicate autoregulation of the heat shock response (recently reviewed in Polissi et al., 1995; Yura, 1996; Segal and Ron, 1998; Yura and Nakahigashi, 1999; Arsene et al., 2000) In E.coli, the sigma 32 subunit of the RNA polymerase holoenzyme (the regulator analogous to HSF) is unstable under normal conditions. Degradation of sigma 32 is promoted by DnaK, DnaJ (the bacterial counterparts of HSP70 and DROJ1) and GrpE. During heat shock, the concentration of sigma 32 is transiently increased by increasing both translational efficiency and protein stability, owing to sequestration of molecular chaperones. The activity of sigma 32 is also modulated by the levels of molecular chaperones.
In human cells, HSP70 has been implicated in the repression of transcriptional competence of HSF1 (Shi et al., 1998). Human HSP70 has also been shown to bind to the transactivation domain of HSF1 in vitro and, when these chaperones are overexpressed in HeLa cells, they negatively affect activity of a fusion protein carrying the activation domain (Shi et al., 1998). High levels of human HSP70 do not affect the DNA-binding activity of HSF, but have been found to reduce the inducible expression of an endogenous heat shock gene (Shi et al., 1998; Zou et al., 1998; Bharadwaj et al., 1999). HSP90 has also been implicated as a negative regulator of the heat shock response in yeast, Xenopus and human (Ali et al., 1998; Duina et al., 1998; Zou et al., 1998; Bharadwaj et al., 1999). Addition of geldanamycin, a HSP90-specific drug to a HeLa cell extract, or immunodepletion of HSP90 induces trimerization and DNA binding of HSF at an intermediate heat shock temperature (Zou et al., 1998). Immunodepletion of HSP70 (or HSP40) does not influence the DNA-binding activity of HSF, consistent with a role in modulating transcriptional competence instead (Zou et al., 1998; Bharadwaj et al., 1999). In Xenopus oocytes, injection of antibodies against HSP90 enhances and prolongs DNA binding during heat shock (Ali et al., 1998); moreover, immunodepletion of HSP90 and the co-chaperone p23 activates DNA binding, but not transactivation (Zou et al., 1998; Bharadwaj et al., 1999).
Although the role of HSP40 proteins has been somewhat less extensively analyzed, HDJ1, the human counterpart of DROJ1, has been shown to bind to the transactivation domain of HSF1 in vitro (Shi et al., 1998). When overexpressed in cells, HDJ1 reduces the activity of a fusion protein carrying the activator domain (Shi et al., 1998). In addition, studies performed with S.cerevisiae indicate that Sis1, a HSP40 family protein, negatively influences its own expression, although the role of HSF in this process was unclear (T.Zhong et al., 1996). Our identification of DROJ1 in a general screen for proteins interacting with Drosophila HSF provides strong support for a role of HSP40 proteins in modulating the heat shock response. Interestingly, the domain of interaction in Drosophila HSF, mapped between the N- and C-terminal hydrophobic heptad repeats, a region previously implicated in the regulation of trimerization (Orosz et al., 1996), is separate from the C-terminal transactivator, the site of HDJ1 binding for human HSF1. However, since that domain was necessarily removed from the HSF ‘bait’ in the two-hybrid screen, our findings do not exclude a similar interaction for the Drosophila protein as well.
Importantly, we have strengthened the role of DROJ1 in feedback regulation using a new RNAi technique. By transfection with dsRNA, we could deplete DROJ1 from a large percentage of the SL2 cell population, thus allowing detection of constitutive activation of endogenous heat shock genes. However, despite substantial depletion of DROJ1, the level of constitutive activation of heat shock genes was modest, suggesting that the action of other chaperones, such as proposed for HSP70 (Solomon et al., 1991), has a role in regulating HSF activity as well. Indeed, depletion of HSP70/HSC70 induced hsp26 mRNA by 10-fold over the level induced by depletion of DROJ1. However, the greatest induction, comparable to a fully induced heat shock response, was observed when DROJ1 and HSP70/HSC70 were co-depleted in the same cells, suggesting that HSP70/HSC70 and HSP40 cooperate to repress Drosophila HSF. We suggest that by direct interactions with HSF, DROJ1 could assist HSF binding to HSP70 and stimulate changes in HSF conformation driven by the ATP-dependent action of HSP70/HSC70. In this view, depletion of DROJ1 alone would only have a modest effect on HSF activity by decreasing the efficiency of HSP70/HSC70 chaperones, but co-depletion of both chaperones would significantly impair the regulatory process. Interestingly, we also found that co-depletion of DROJ1 and HSP90 (but not HSP90 alone) leads to high-level induction of the heat shock response. This synergy between DROJ1 and HSP90 underscores the function of HSP90 in feedback regulation of HSF, and augments emerging evidence for DROJ1/HSP40 as a common link between the HSP70 and HSP90 chaperone machines. Our present findings should encourage further systematic in vivo analysis of the roles of the individual chaperones and co-chaperones in modulating the activity of HSF, and guide mechanistic investigations of HSF regulation in vitro.
Materials and methods
Two-hybrid system
The yeast two-hybrid system plasmid (pAS1, pACT2) and Drosophila embryonic cDNA library were a gift from Stephen Elledge. The GAL4DBD fusion constructs containing Drosophila HSF(1–602) and HSF(104–602) were provided by Andras Orosz (G.Marchler, A.Orosz and C.Wu, in preparation). GAL4DBD-HSF(1–536) and (1–326) were generated by deletion from the original construct, using appropriate restriction sites. The C-terminal deletions in the GAL4AD-DROJ1 fusions were similarly prepared. DNA was transformed into S.cerevisiae CG1945 (Clontech) using the lithium acetate technique (Schiestl and Gietz, 1989). Out of 5.5 × 106 transformants screened on medium containing glucose under non-inducing conditions, 148 grew on 20 mM 3-aminotriazole (HIS3 reporter) and also showed increased β-galactosidase activity (lacZ reporter). From 58 of these, plasmids were isolated and sequenced, and showed a reproducible phenotye after retransformation.
Antibodies and western blotting
Western blotting was performed as previously described (Rabindran et al., 1994; Orosz et al., 1996) using polyclonal antibodies against Drosophila HSF (1:10 000), Hsp70 (1:50 000; Velazquez et al., 1983), Hsp26 (1:1000; Marin et al., 1993), DROJ1 (1:1000) or monoclonal antibodies against chicken HSP70/HSC70 (1:1000; Stressgen) and E.coli tubulin (E7, 1:100; Chu and Klymkowsky, 1989), both showing cross reactivity with Drosophila, and detected by enhanced chemiluminescence (ECL, Amersham). Typically, 5 µl of whole-cell extract were analyzed by SDS–PAGE.
Affinity purification of the DROJ1 antibody was performed according to Gu et al. (1994) using the His-tagged antigen (DROJ1 residues 130–334).
Gel mobility shift assay
Gel mobility shift assays of whole-cell extracts were performed as previously described (M.Zhong et al., 1996), the only modification being the addition of 1 µl of fetal bovine serum to the reaction mixture. Typically, ∼5 µl of whole-cell extracts (volume is adjusted for equal HSF amount, if necessary) were analyzed, either untreated or pre-incubated with 1 µl of polyclonal antibody against Drosophila HSF (15 min, ice).
Immunostaining of SL2 cells
Cells were fixed and stained according to a previously published protocol (Orosz et al., 1996). For cell staining, a 1:10 dilution of affinity-purified DROJ1 antibody was used. DNA was stained with Hoechst 33342 (1 µg/ml) or after RNase A treatment with propidium iodide (50 µg/ml).
Northern analysis
Total RNA was isolated using TRIAZOL Reagent following the manufacturer’s protocol. Amounts were quantitated by UV-absorption and were dot-blotted on a nylon membrane (Genescreen, NEN) using a vacuum manifold (Schleicher & Schuell) according to standard protocol (Sambrook et al., 1989).
Plasmid construction, protein expression and stable cell line
The cell expression vector pMK-DroJ1 was constructed by cloning a DraI–DraI fragment from pNB40-Droj1 containing the full-length droj1 cDNA (generous gift from Karen Palter) into the EcoRV site of pMK33, a plasmid containing a hygromycin resistance gene, which puts droj1 under the control of the metallothionein promotor. The sequence of the construct was verified by DNA sequencing. Drosophila Schneider Line 2 (SL2) cells were cultured in serum-free HyQ-CCM3 medium (Hyclone) at 22°C in closed flasks. Cells were diluted 1:8 from a dense SL2 culture 24 h prior to transfection with LipoTAXI (Stratagene). One day after transfection, the medium was exchanged against fresh medium containing hygromycin B (final concentration 0.2 mg/ml). In parallel, untransfected control cells were put under the same selection. After ∼3 weeks, transfected cells showed wild-type doubling times and strong overproduction of DROJ1 after induction with 0.7–0.8 mM CuSO4. Heat stress treatments were performed as previously published (Orosz et al., 1996), or as indicated in the text. Whole-cell extracts were prepared by adding 5 vols of the cell pellet of extraction buffer (10 mM Tris–HCl pH 8, 0.1 mM EDTA, 400 mM NaCl, 5% glycerol) with freshly added Complete™ protease inhibitors (Boehringer Mannheim) and repeated freeze-thawing cycles, followed by centrifugation at 40 000 r.p.m. for 20 min, 4°C in a Beckman TL100 centrifuge (TLA 45 rotor). The supernatant was frozen at –80°C.
RNAi
The first 590 bp (EcoRI–BamHI fragment) of the droj1 cDNA, the first 1200 bp (PstI–SalI fragment) of the hsp70 coding region and the last 1300 bp (EcoRV–BamHI fragment) of the hsp83 coding region were subcloned into Bluescript SK. The plasmids were linearized, purified and transcribed in both directions with T7 and T3 polymerase, respectively, using the MEGAscript In vitro Translation kit (Ambion). Equal amounts of sense and antisense RNAs were annealed and the quality of dsRNA was confirmed on an agarose/0.5× TBE gel. One microliter of dsRNA was transfected using Lipofection (Gibco/BRL), as described previously (Orosz et al., 1996). Control cells were transfected without addition of dsRNA.
Supplementary data
Supplementary data to this paper are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We thank R.Tanguay and S.Lindquist for antibody against HSP26 and HSP70, respectively, S.Elledge for the Drosophila two-hybrid library, B.Paterson and W.Qin for assistance with the RNAi experiments, and members of our laboratory, especially Min Zhong, for technical advice and helpful discussions. We are grateful to K.Palter for the full-length droj1 cDNA and M.Arbeitman for the generous gift of DROJ1 protein and the expression vectors. The β-tubulin antibody (E7) was obtained from the Developmental Studies Hybridoma Bank, University of Iowa, Department of Biological Sciences, Iowa City. G.M. was supported by a post-doctoral Erwin Schroedinger Fellowship from the Austrian Science Foundation and by a National Institutes of Health Intramural Post-doctoral Fellowship. This work was supported by the Intramural Research Program of the National Cancer Institute.
References
- Abravaya K., Myers,M.P., Murphy,S.P. and Morimoto,R.I. (1992) The human heat shock protein hsp70 interacts with HSF, the transcription factor that regulates heat shock gene expression. Genes Dev., 6, 1153–1164. [DOI] [PubMed] [Google Scholar]
- Ali A., Bharadwaj,S., O’Carroll,R. and Ovsenek,N. (1998) Hsp90 interacts with and regulates the activity of heat shock factor 1 in Xenpous oocytes. Mol. Cell. Biol., 18, 4949–4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsene F., Tomoyasu,T. and Bukau,B. (2000) The heat shock response of Escherichia coli. Int. J. Food Microbiol., 55, 3–9. [DOI] [PubMed] [Google Scholar]
- Bardwell J.C., Tilly,K., Craig,E., King,J., Zylicz,M. and Georgopoulos,C. (1986) The nucleotide sequence of the Escherichia coli K12 dnaJ+ gene. A gene that encodes a heat shock protein. J. Biol. Chem., 261, 1782–1785. [PubMed] [Google Scholar]
- Bharadwaj S., Hnatov,A., Ali,A. and Ovsenek,N. (1998) Regulation of the DNA-binding and transcriptional activities of heat shock factor 1 is uncoupled in Xenopus oocytes. Biochim. Biophys. Acta, 1402, 79–85. [DOI] [PubMed] [Google Scholar]
- Bharadwaj S., Ali,A. and Ovsenek,N. (1999) Multiple components of the HSP90 chaperone complex function in regulation of heat shock factor 1 in vivo. Mol. Cell. Biol., 19, 8033–8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner J.J., Heyward,S. and Fackenthal,D.L. (1992) Temperature-dependent regulation of a heterologous transcriptional activation domain fused to yeast heat shock transcription factor. Mol. Cell. Biol., 12, 1021–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorstein W.R. and Craig,E.A. (1990) Transcriptional regulation of SSA3, an HSP70 gene from Saccharomyces cerevisiae. Mol. Cell. Biol., 10, 3262–3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunch T.A., Grinblat,Y. and Goldstein,L.S. (1988) Characterization and use of the Drosophila metallothionein promoter in cultured Drosophila melanogaster cells. Nucleic Acids Res., 16, 1043–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham M.E. and Caplan,A.J. (1998) Structure, function and evolution of DnaJ: conservation and adaptation of the chaperone function. Cell Stress Chaperones, 3, 28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Barlev,N.A., Westergaard,O. and Jakobsen,B.K. (1993) Identification of the C-terminal activator domain in yeast heat shock factor: independent control of transient and sustained transcriptional activity. EMBO J., 12, 5007–5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu D.T. and Klymkowsky,M.W. (1989) The appearance of acetylated α-tubulin during early development and cellular differentiation in Xenopus. Dev. Biol., 136, 104–117. [DOI] [PubMed] [Google Scholar]
- Cotto J.J. and Morimoto,R.I. (1999) Stress-induced activation of the heat-shock response: cell and molecular biology of heat-shock factors. Biochem. Soc. Symp., 64, 105–118. [PubMed] [Google Scholar]
- Craig E.A. and Gross,C.A. (1991) Is hsp70 the cellular thermometer? Trends Biochem. Sci., 16, 135–140. [DOI] [PubMed] [Google Scholar]
- DiDomenico B.J., Bugaisky,G.E. and Lindquist,S. (1982) The heat shock response is self-regulated at both the transcriptional and posttranscriptional levels. Cell, 31, 593–603. [DOI] [PubMed] [Google Scholar]
- Duina A.A., Kalton,H.M. and Gaber,R.F. (1998) Requirement for Hsp90 and a CyP-40-type cyclophilin in negative regulation of the heat shock response. J. Biol. Chem., 273, 18974–18978. [DOI] [PubMed] [Google Scholar]
- Farkas T., Kutskova,Y.A. and Zimarino,V. (1998) Intramolecular repression of mouse heat shock factor 1. Mol. Cell. Biol., 18, 906–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman D.E. and Frydman,J. (2000) Protein folding in vivo: the importance of molecular chaperones. Curr. Opin. Struct. Biol., 10, 26–33. [DOI] [PubMed] [Google Scholar]
- Fink A.L. (1999) Chaperone-mediated protein folding. Physiol. Rev., 79, 425–449. [DOI] [PubMed] [Google Scholar]
- Fire A., Xu,S., Montgomery,M.K., Kostas,S.A., Driver,S.E. and Mello,C.C. (1998) Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature, 391, 806–811. [DOI] [PubMed] [Google Scholar]
- Giardina C. and Lis,J.T. (1995) Sodium salicylate and yeast heat shock gene transcription. J. Biol. Chem., 270, 10369–10372. [DOI] [PubMed] [Google Scholar]
- Goffin L. and Georgopoulos,C. (1998) Genetic and biochemical characterization of mutations affecting the carboxy-terminal domain of Escherichia coli molecular chaperone DnaJ. Mol. Microbiol., 30, 329–340. [DOI] [PubMed] [Google Scholar]
- Gu J., Stephenson,C.G. and Iadarola,M.J. (1994) Recombinant proteins attached to a nickel-NTA column: use in affinity purification of antibodies. Biotechniques, 17, 257–262. [PubMed] [Google Scholar]
- Hammond S.M., Bernstein,E., Beach,D. and Hannon,G.J. (2000) An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature, 404, 293–296. [DOI] [PubMed] [Google Scholar]
- Hartl F.U. (1996) Molecular chaperones in cellular protein folding. Nature, 381, 571–579. [DOI] [PubMed] [Google Scholar]
- Hata M. and Ohtsuka,K. (1998) Characterization of HSE sequences in human Hsp40 gene: structural and promoter analysis. Biochim. Biophys. Acta, 1397, 43–55. [DOI] [PubMed] [Google Scholar]
- Hensold J.O., Hunt,S.K., Calderwood,S.K., Housman,D.E. and Kingston,R.E. (1990) DNA binding of heat shock factor to the heat shock element is insufficient for transcriptional activation in murine erythroleukemia cells. Mol. Cell. Biol., 10, 1600–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsen B. and Pelham,H.R. (1991) A conserved heptapeptide restrains the activity of the yeast heat shock transcription factor. EMBO J., 10, 369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedlicka P., Mortin,M.A. and Wu,C. (1997) Multiple functions of Drosophila heat shock transcription factor in vivo. EMBO J., 16, 2452–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J.L. and Craig,E.A. (2000) A role for the Hsp40 Ydj1 in repression of basal steroid receptor activity in yeast. Mol. Cell. Biol., 20, 3027–3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurivich D.A., Sistonen,L., Kroes,R.A. and Morimoto,R.I. (1992) Effect of sodium salicylate on the human heat shock response. Science, 255, 1243–1245. [DOI] [PubMed] [Google Scholar]
- Kazemi-Esfarjani P. and Benzer,S. (2000) Genetic suppression of the polyglutamine toxicity in Drosophila. Science, 287, 1837–1840. [DOI] [PubMed] [Google Scholar]
- Kim D., Ouyang,H. and Li,G.C. (1995) Heat shock protein hsp70 accelerates the recovery of heat-shocked mammalian cells through its modulation of heat shock transcription factor HSF1. Proc. Natl Acad. Sci. USA, 92, 2126–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline M.P. and Morimoto,R.I. (1997) Repression of heat shock factor 1 transcriptional activation domain is modulated by constitutive phosphorylation. Mol. Cell. Biol., 17, 2107–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf U., Newton,E.M., Kyriakis,J. and Kingston,R.E. (1996) Repression of human heat shock factor 1 activity at control temperature by phosphorylation. Genes Dev., 10, 2782–2793. [DOI] [PubMed] [Google Scholar]
- Lindquist S. (1986) The heat-shock response. Annu. Rev. Biochem., 55, 1151–1191. [DOI] [PubMed] [Google Scholar]
- Lindquist S. and Craig,E.A. (1988) The heat-shock proteins. Annu. Rev. Genet., 22, 631–677. [DOI] [PubMed] [Google Scholar]
- Marin R., Valet,J.P. and Tanguay,R.M. (1993) hsp23 and hsp26 exhibit distinct spatial and temporal patterns of constitutive expression in Drosophila adults. Dev. Genet., 14, 69–77. [DOI] [PubMed] [Google Scholar]
- Mercier P.A., Winegarden,N.A. and Westwood,J.T. (1999) Human heat shock factor 1 is predominantly a nuclear protein before and after heat stress. J. Cell Sci., 112, 2765–2774. [DOI] [PubMed] [Google Scholar]
- Misquitta L. and Paterson,B.M. (1999) Targeted disruption of gene function in Drosophila by RNA interference (RNA-i): a role for nautilus in embryonic somatic muscle formation. Proc. Natl Acad. Sci. USA, 96, 1451–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morano K.A. and Thiele,D.J. (1999) Heat shock factor function and regulation in response to cellular stress, growth, and differentiation signals. Gene Expr., 7, 271–282. [PMC free article] [PubMed] [Google Scholar]
- Morano K.A., Liu,P.C. and Thiele,D.J. (1998) Protein chaperones and the heat shock response in Saccharomyces cerevisiae. Curr. Opin. Microbiol., 1, 197–203. [DOI] [PubMed] [Google Scholar]
- Morimoto R.I. (1998) Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev., 12, 3788–3796. [DOI] [PubMed] [Google Scholar]
- Morimoto R.I., Tissieres,A. and Georgopoulos,C. (1994) The Biology of Heat Shock Proteins and Molecular Chaperones. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Mosser D.D., Duchaine,J. and Massie,B. (1993) The DNA-binding activity of the human heat shock transcription factor is regulated in vivo by hsp70. Mol. Cell. Biol., 13, 5427–5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata M., Gong,P., Suzuki,K. and Koizumi,S. (1999) Differential metal response and regulation of human heavy metal-inducible genes. J. Cell. Physiol., 180, 105–113. [DOI] [PubMed] [Google Scholar]
- Nieto-Sotelo J., Wiederrecht,G., Okuda,A. and Parker,C.S. (1990) The yeast heat shock transcription factor contains a transcriptional activation domain whose activity is repressed under nonshock conditions. Cell, 62, 807–817. [DOI] [PubMed] [Google Scholar]
- Nover L., Scharf,K.D., Gagliardi,D., Vergne,P., Czarnecka-Verner,E. and Gurley,W.B. (1996) The Hsf world: classification and properties of plant heat stress transcription factors. Cell Stress Chaperones, 1, 215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohki M., Tamura,F., Nishimura,S. and Uchida,H. (1986) Nucleotide sequence of the Escherichia coli dnaJ gene and purification of the gene product. J. Biol. Chem., 261, 1778–1781. [PubMed] [Google Scholar]
- Orosz A., Wisniewski,J. and Wu,C. (1996) Regulation of Drosophila heat shock factor trimerization: global sequence requirements and independence of nuclear localization. Mol. Cell. Biol., 16, 7018–7030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polissi A., Goffin,L. and Georgopoulos,C. (1995) The Escherichia coli heat shock response and bacteriophage lambda development. FEMS Microbiol. Rev., 17, 159–169. [DOI] [PubMed] [Google Scholar]
- Raabe T. and Manley,J. (1991) A human homologue of the Escherichia coli DnaJ heat-shock protein. Nucleic Acids Res., 19, 6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabindran S.K., Haroun,R.I., Clos,J., Wisniewski,J. and Wu,C. (1993) Regulation of heat shock factor trimer formation: role of the conserved leucine zipper. Science, 259, 230–234. [DOI] [PubMed] [Google Scholar]
- Rabindran S.K., Wisniewski,J., Li,L., Li,G.C. and Wu,C. (1994) Interaction between heat shock factor and hsp70 is insufficient to suppress induction of DNA-binding activity in vivo. Mol. Cell. Biol., 14, 6552–6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Fritsch,E.F. and Maniatis,T. (1989) Molecular Cloning: A Laboratory Manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Santoro M.G. (2000) Heat shock factors and the control of the stress response. Biochem. Pharmacol., 59, 55–63. [DOI] [PubMed] [Google Scholar]
- Satyal S.H., Chen,D., Fox,S.G., Kramer,J.M. and Morimoto,R.I. (1998) Negative regulation of the heat shock transcriptional response by HSBP1. Genes Dev., 12, 1962–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl R.H. and Gietz,R.D. (1989) High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr. Genet., 16, 339–346. [DOI] [PubMed] [Google Scholar]
- Schnaider T., Soti,C., Cheetham,M.E., Miyata,Y., Yahara,I. and Csermely,P. (2000) Interaction of the human DnaJ homologue, HSJ1b with the 90 kDa heat shock protein, Hsp90. Life Sci., 67, 1455–1465. [DOI] [PubMed] [Google Scholar]
- Segal G. and Ron,E.Z. (1998) Regulation of heat-shock response in bacteria. Ann. NY Acad. Sci., 851, 147–151. [DOI] [PubMed] [Google Scholar]
- Sha B., Lee,S. and Cyr,D.M. (2000) The crystal structure of the peptide-binding fragment from the yeast Hsp40 protein Sis1. Struct. Fold. Des., 8, 799–807. [DOI] [PubMed] [Google Scholar]
- Shi Y., Mosser,D.D. and Morimoto,R.I. (1998) Molecular chaperones as HSF1-specific transcriptional repressors. Genes Dev., 12, 654–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon J.M., Rossi,J.M., Golic,K., McGarry,T. and Lindquist,S. (1991) Changes in hsp70 alter thermotolerance and heat-shock regulation in Drosophila. New Biol., 3, 1106–1120. [PubMed] [Google Scholar]
- Sorger P.K. (1990) Yeast heat shock factor contains separable transient and sustained response transcriptional activators. Cell, 62, 793–805. [DOI] [PubMed] [Google Scholar]
- Timmons L. and Fire,A. (1998) Specific interference by ingested dsRNA. Nature, 395, 854. [DOI] [PubMed] [Google Scholar]
- Velazquez J.M., Sonoda,S., Bugaisky,G. and Lindquist,S. (1983) Is the major Drosophila heat shock protein present in cells that have not been heat shocked? J. Cell Biol., 96, 286–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Q., Marchler,G., Edington,K., Karsch-Mizrachi,I. and Paterson,B.M. (2000) Targeted disruption of gene function in cultured insect cells by RNA interference demonstrates a role of nautilus in the mygenic conversion of Schneider cells by daughterless. Dev. Biol., 228, 239–255. [DOI] [PubMed] [Google Scholar]
- Westwood J.T. and Wu,C. (1993) Activation of Drosophila heat shock factor: conformational change associated with monomer-to-trimer transition. Mol. Cell. Biol., 13, 3481–3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski J., Orosz,A., Allada,R. and Wu,C. (1996) The C-terminal region of Drosophila heat shock factor (HSF) contains a constitutively functional transactivation domain. Nucleic Acids Res., 24, 367–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. (1995) Heat shock transcription factors: structure and regulation. Annu. Rev. Cell Dev. Biol., 11, 441–469. [DOI] [PubMed] [Google Scholar]
- Yura T. (1996) Regulation and conservation of the heat-shock transcription factor σ32. Genes Cells, 1, 277–284. [DOI] [PubMed] [Google Scholar]
- Yura T. and Nakahigashi,K. (1999) Regulation of the heat-shock response. Curr. Opin. Microbiol., 2, 153–158. [DOI] [PubMed] [Google Scholar]
- Zhong M., Wisniewski,J., Fritsch,M., Mizuguchi,G., Orosz,A., Jedlicka,P. and Wu,C. (1996) Purification of heat shock transcription factor of Drosophila. Methods Enzymol., 274, 113–119. [DOI] [PubMed] [Google Scholar]
- Zhong M., Orosz,A. and Wu,C. (1998) Direct sensing of heat and oxidation by Drosophila heat shock transcription factor. Mol. Cell, 2, 101–108. [DOI] [PubMed] [Google Scholar]
- Zhong M., Kim,S.-Y. and Wu,C. (1999) Sensitivity of Drosophila heat shock transcription factor to low pH. J. Biol. Chem., 274, 3135–3140. [DOI] [PubMed] [Google Scholar]
- Zhong T., Luke,M.M. and Arndt,K.T. (1996) Transcriptional regulation of the yeast DnaJ homolog SIS1. J. Biol. Chem., 271, 1349–1356. [DOI] [PubMed] [Google Scholar]
- Zou J., Baler,R., Dahl,G. and Voellmy,R. (1994) Activation of the DNA-binding ability of human heat shock transcription factor 1 may involve the transition from an intramolecular to an intermolecular triple-stranded coiled-coil structure. Mol. Cell. Biol., 14, 7557–7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J., Guo,Y., Guettouche,T., Smith,D.F. and Voellmy,R. (1998) Repression of heat shock transcription factor HSF1 activation by Hsp90 (Hsp90 complex) that forms a stress-sensitive complex with HSF1. Cell, 94, 471–480. [DOI] [PubMed] [Google Scholar]


