Abstract
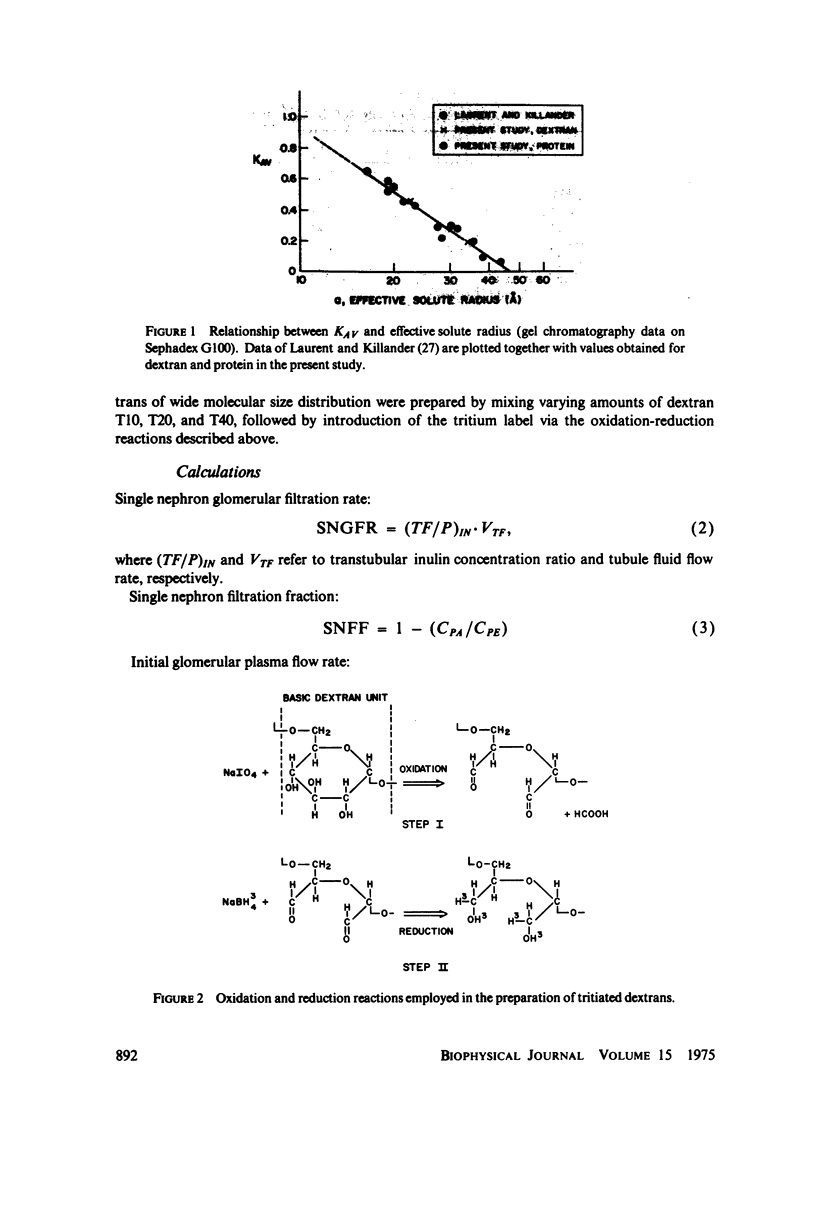
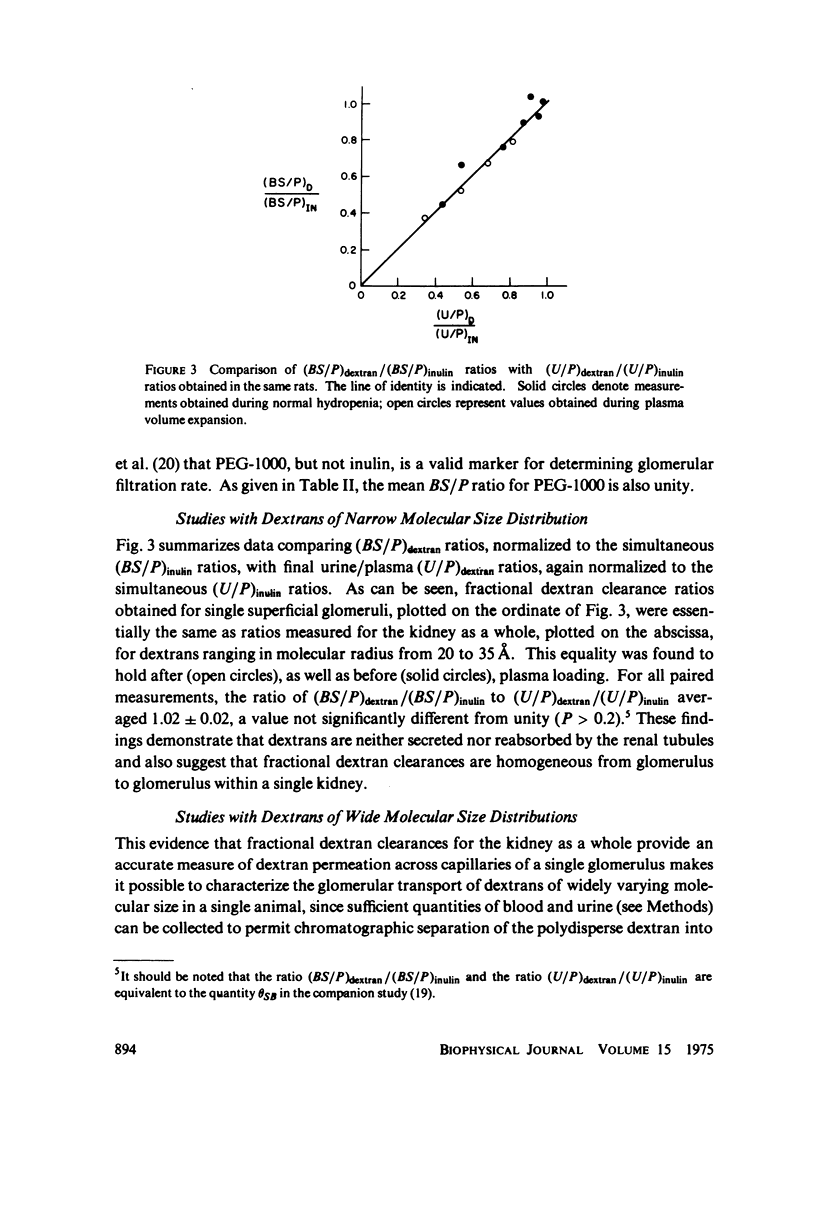
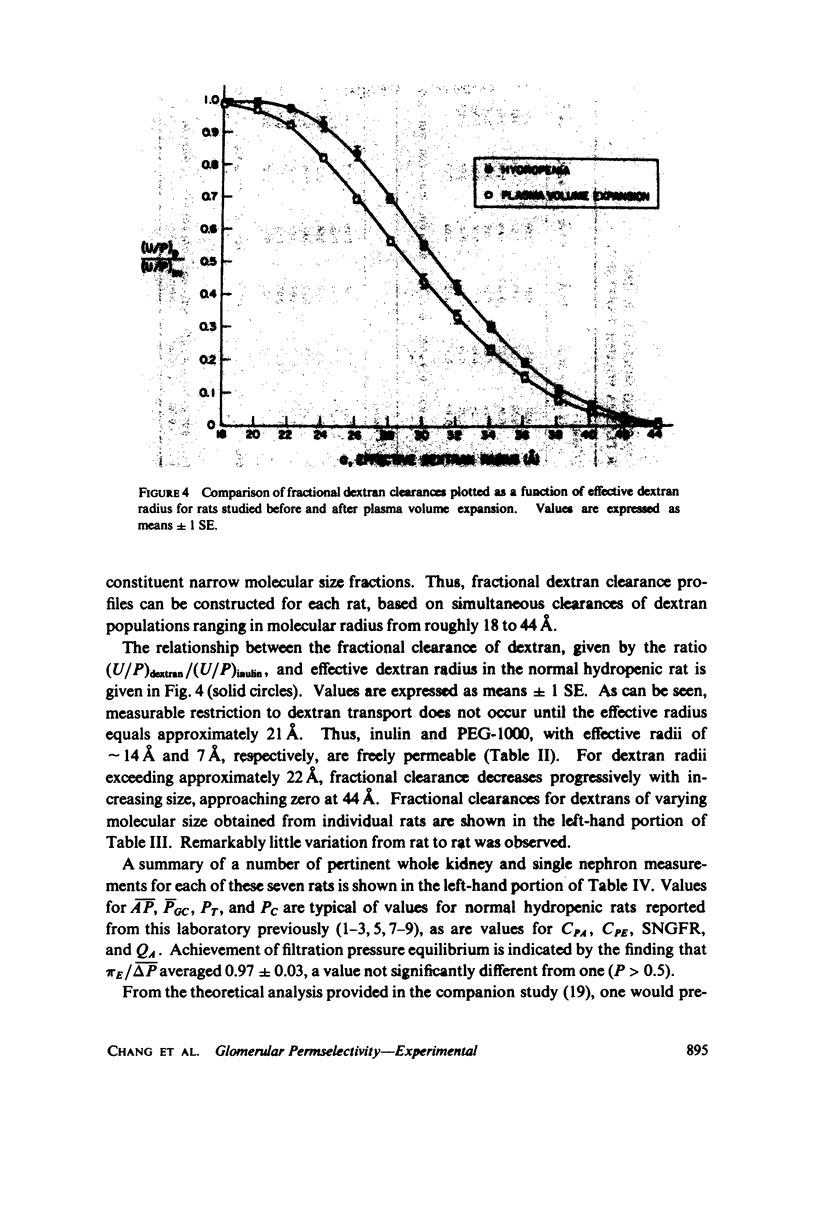
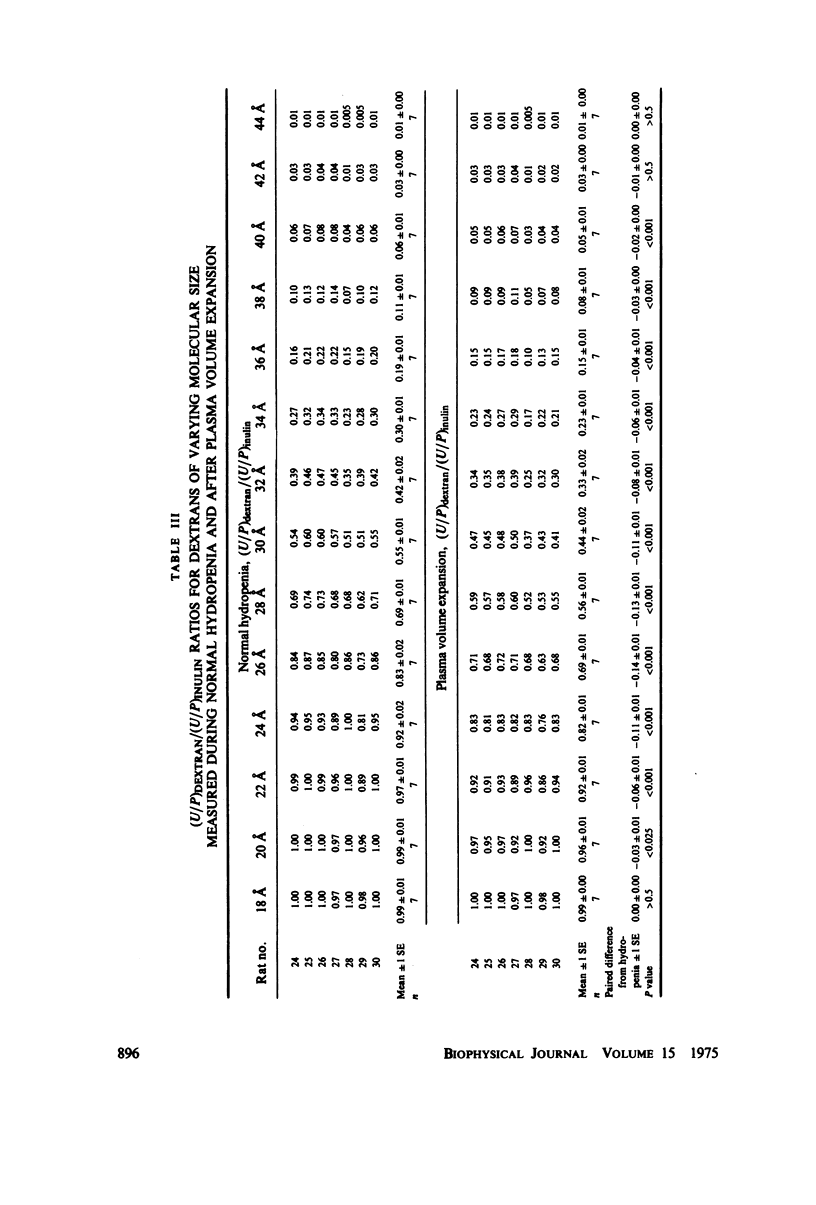
To determine the permselectivity characteristics of the glomerular capillary wall, known molecular size fractions of [3H]dextran, prepared by gel chromatography, were infused into normally hydrated Wistar rats, thus permitting simultaneous measurement of Bowman's space/plasma water (BS/P) and urine/plasma water (U/P) concentration ratios, along with glomerular pressures and flows. Since (BS/P)inulin = 1.01 +/- 0.01 SE(n = 34, radius = approximately 14 A) and since (BS/P)dextran/(BS/P)inulin equaled (U/P)dextran/(U/P)inulin for dextrans ranging in molecular radius from 21 to 35 A, these findings validate that dextrans are neither secreted nor reabsorbed. For dextran radii of 20, 24, 28, 32, 36, 40, and 44 A, (U/P)dextran/(U/P)inulin averaged 0.99, 0.92, 0.69, 0.42, 0.19, 0.06, and 0.01, respectively. In accord with theoretical predictions that these fractional dextran clearances should vary appreciably with changes in glomerular transcapillary pressures and flows, an increase in glomerular plasma flow rate, induced in these same rats by plasma volume expansion, resulted in a highly significant lowering of fractional clearance of all but the smallest and largest dextrans studied. These findings emphasize that fractional solute clearances alone are inadequate to describe the permselective properties of the glomerular capillary wall unless glomerular pressures and flows are also known. This sensitivity of fractional dextran clearance to changes in plasma flow indicates that dextrans are transported across the capillary not only by bulk flow but also to an important extent by diffusion.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arturson G., Groth T., Grotte G. Human glomerular membrane porosity and filtration pressure: dextran clearance data analysed by theoretical models. Clin Sci. 1971 Feb;40(2):137–158. doi: 10.1042/cs0400137. [DOI] [PubMed] [Google Scholar]
- Berglund F., Engberg A., Persson E., Ulfendahl H. Renal clearances of labelled inulin (inulin-carboxyl-14C, inulin-methoxy-3H) and a polyethylene glycol (PEG 1000) in the rat. Acta Physiol Scand. 1969 Aug;76(4):458–462. doi: 10.1111/j.1748-1716.1969.tb04492.x. [DOI] [PubMed] [Google Scholar]
- Brenner B. M., Falchuk K. H., Keimowitz R. I., Berliner R. W. The relationship between peritubular capillary protein concentration and fluid reabsorption by the renal proximal tubule. J Clin Invest. 1969 Aug;48(8):1519–1531. doi: 10.1172/JCI106118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. M., Troy J. L., Daugharty T. M., Deen W. M., Robertson C. R. Dynamics of glomerular ultrafiltration in the rat. II. Plasma-flow dependence of GFR. Am J Physiol. 1972 Nov;223(5):1184–1190. doi: 10.1152/ajplegacy.1972.223.5.1184. [DOI] [PubMed] [Google Scholar]
- Brenner B. M., Troy J. L., Daugharty T. M., MacInnes R. M. Quantitative importance of changes in postglomerular colloid osmotic pressure in mediating glomerulotubular balance in the rat. J Clin Invest. 1973 Jan;52(1):190–197. doi: 10.1172/JCI107164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. M., Troy J. L., Daugharty T. M. Pressures in cortical structures of the rat kidney. Am J Physiol. 1972 Feb;222(2):246–251. doi: 10.1152/ajplegacy.1972.222.2.246. [DOI] [PubMed] [Google Scholar]
- Brenner B. M., Troy J. L., Daugharty T. M. The dynamics of glomerular ultrafiltration in the rat. J Clin Invest. 1971 Aug;50(8):1776–1780. doi: 10.1172/JCI106667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang R. S., Robertson C. R., Deen W. M., Brenner B. M. Permselectivity of the glomerular capillary wall to macromolecules. I. Theoretical considerations. Biophys J. 1975 Sep;15(9):861–886. doi: 10.1016/S0006-3495(75)85862-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugharty T. M., Ueki I. F., Mercer P. F., Brenner B. M. Dynamics of glomerular ultrafiltration in the rat. V. Response to ischemic injury. J Clin Invest. 1974 Jan;53(1):105–116. doi: 10.1172/JCI107527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deen W. M., Maddox D. A., Robertson C. R., Brenner B. M. Dynamics of glomerular ultrafiltration in the rat. VII. Response to reduced renal mass. Am J Physiol. 1974 Sep;227(3):556–562. doi: 10.1152/ajplegacy.1974.227.3.556. [DOI] [PubMed] [Google Scholar]
- Deen W. M., Robertson C. R., Brenner B. M. A model of glomerular ultrafiltration in the rat. Am J Physiol. 1972 Nov;223(5):1178–1183. doi: 10.1152/ajplegacy.1972.223.5.1178. [DOI] [PubMed] [Google Scholar]
- Deen W. M., Robertson C. R., Brenner B. M. Glomerular ultrafiltration. Fed Proc. 1974 Jan;33(1):14–20. [PubMed] [Google Scholar]
- Deen W. M., Troy J. L., Robertson C. R., Brenner B. M. Dynamics of glomerular ultrafiltration in the rat. IV. Determination of the ultrafiltration coefficient. J Clin Invest. 1973 Jun;52(6):1500–1508. doi: 10.1172/JCI107324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUHR J., KACZMARCZYK J., KRUTTGEN C. D. Eine einfache colorimetrische Methode zur Inulinbestimmung für Nieren-Clearance-Untersuchungen bei Stoffwechselgesunden und Diabetikern. Klin Wochenschr. 1955 Aug 1;33(29-30):729–730. doi: 10.1007/BF01473295. [DOI] [PubMed] [Google Scholar]
- Gassée J. P. Effects of acetylcholine on glomerular sieving of macromolecules. Pflugers Arch. 1973 Aug 27;342(3):239–254. doi: 10.1007/BF00591372. [DOI] [PubMed] [Google Scholar]
- Granath K. A., Kvist B. E. Molecular weight distribution analysis by gel chromatography on Sephadex. J Chromatogr. 1967 May;28(1):69–81. doi: 10.1016/s0021-9673(01)85930-6. [DOI] [PubMed] [Google Scholar]
- Harris C. A., Baer P. G., Chirito E., Dirks J. H. Composition of mammalian glomerular filtrate. Am J Physiol. 1974 Oct;227(4):972–976. doi: 10.1152/ajplegacy.1974.227.4.972. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maddox D. A., Bennett C. M., Deen W. M., Glassock R. J., Knutson D., Daugharty T. M., Brenner B. M. Determinants of glomerular filtration in experimental glomerulonephritis in the rat. J Clin Invest. 1975 Feb;55(2):305–318. doi: 10.1172/JCI107934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox D. A., Deen W. M., Brenner B. M. Dynamics of glomerular ultrafiltration. VI. Studies in the primate. Kidney Int. 1974 Apr;5(4):271–278. doi: 10.1038/ki.1974.36. [DOI] [PubMed] [Google Scholar]
- Myers B. D., Deen W. M., Robertson C. R., Brenner B. M. Dynamics of glomerular ultrafiltration in the rat. VIII. Effects of hematocrit. Circ Res. 1975 Mar;36(3):425–435. doi: 10.1161/01.res.36.3.425. [DOI] [PubMed] [Google Scholar]
- PAPPENHEIMER J. R. Passage of molecules through capillary wals. Physiol Rev. 1953 Jul;33(3):387–423. doi: 10.1152/physrev.1953.33.3.387. [DOI] [PubMed] [Google Scholar]
- RENKIN E. M. Filtration, diffusion, and molecular sieving through porous cellulose membranes. J Gen Physiol. 1954 Nov 20;38(2):225–243. [PMC free article] [PubMed] [Google Scholar]
- Robertson C. R., Deen W. M., Troy J. L., Brenner B. M. Dynamics of glomerular ultrafiltration in the rat. 3. Hemodynamics and autoregulation. Am J Physiol. 1972 Nov;223(5):1191–1200. doi: 10.1152/ajplegacy.1972.223.5.1191. [DOI] [PubMed] [Google Scholar]
- Stusnick E., Hurst R. P. Numerical determination of membrane permeability parameters. J Theor Biol. 1972 Nov;37(2):261–271. doi: 10.1016/0022-5193(72)90021-5. [DOI] [PubMed] [Google Scholar]
- Verniory A., Du Bois R., Decoodt P., Gassee J. P., Lambert P. P. Measurement of the permeability of biological membranes. Application to the glomerular wall. J Gen Physiol. 1973 Oct;62(4):489–507. doi: 10.1085/jgp.62.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]


