Abstract
Differential scanning calorimetry studies of dipalmitoyl lecithin show two reversible transitions as the temperature is changed between 20 and 50 degrees C. A pretransition endotherm occurs at 35 degrees C prior to the main chain melting endotherm which occurs at 42 degrees C. X-ray diffraction studies show that below 33 degrees C the chains of the lecithin are fully extended, packed in a hexagonal crystalline lattice but tilted with respect to the plane of the bilayer. Between 35 and 42 degrees C the chains are similarly packed but oriented perpendicular to the bilayer plane. Above 44 degrees C the chains are "melted" or disordered. Monolayer studies of dipalmitoyl lecithin using continuous recording of pressure with molecular area reveal the existence of two solid condensed phases corresponding to these tilted and verticle chain structures. The tilted to perpendicular transition would account for the pretransition endotherm of the lipid; the crystalline to melted change corresponds to the larger transition observed at 42 degrees C.
Full text
PDF

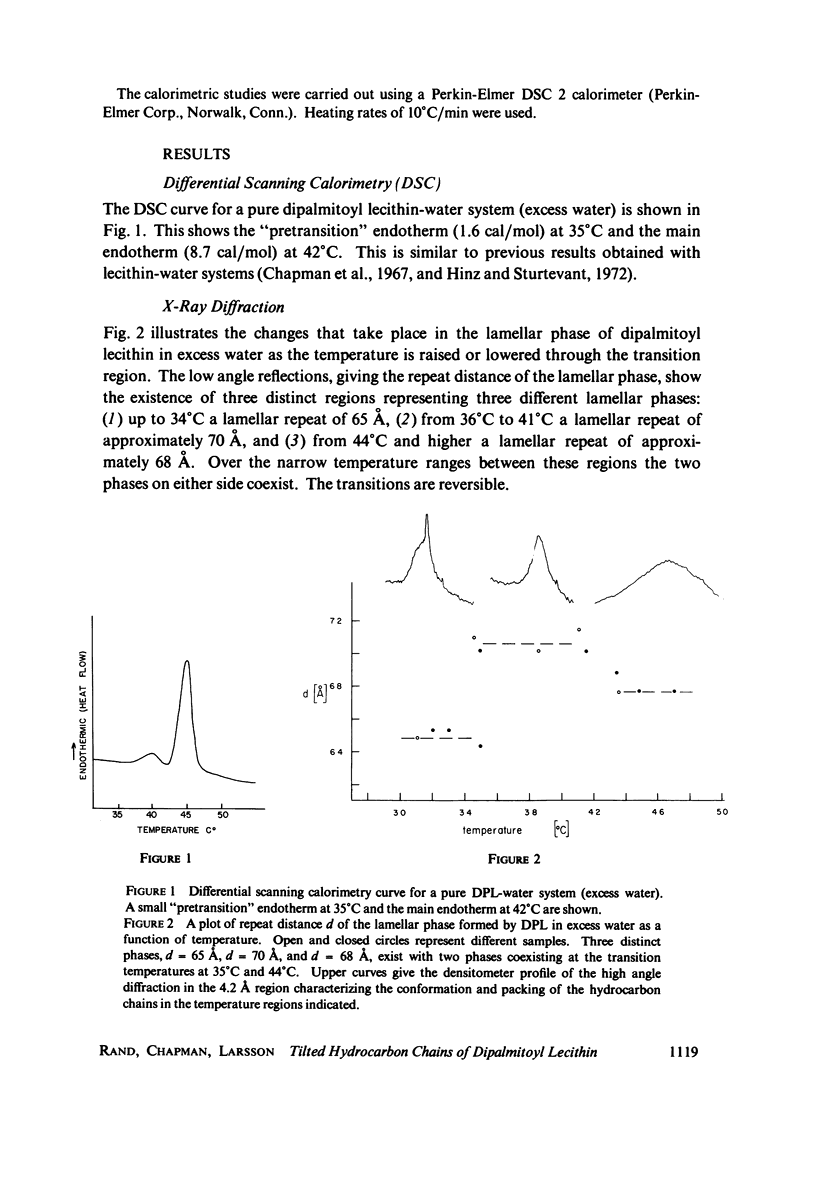
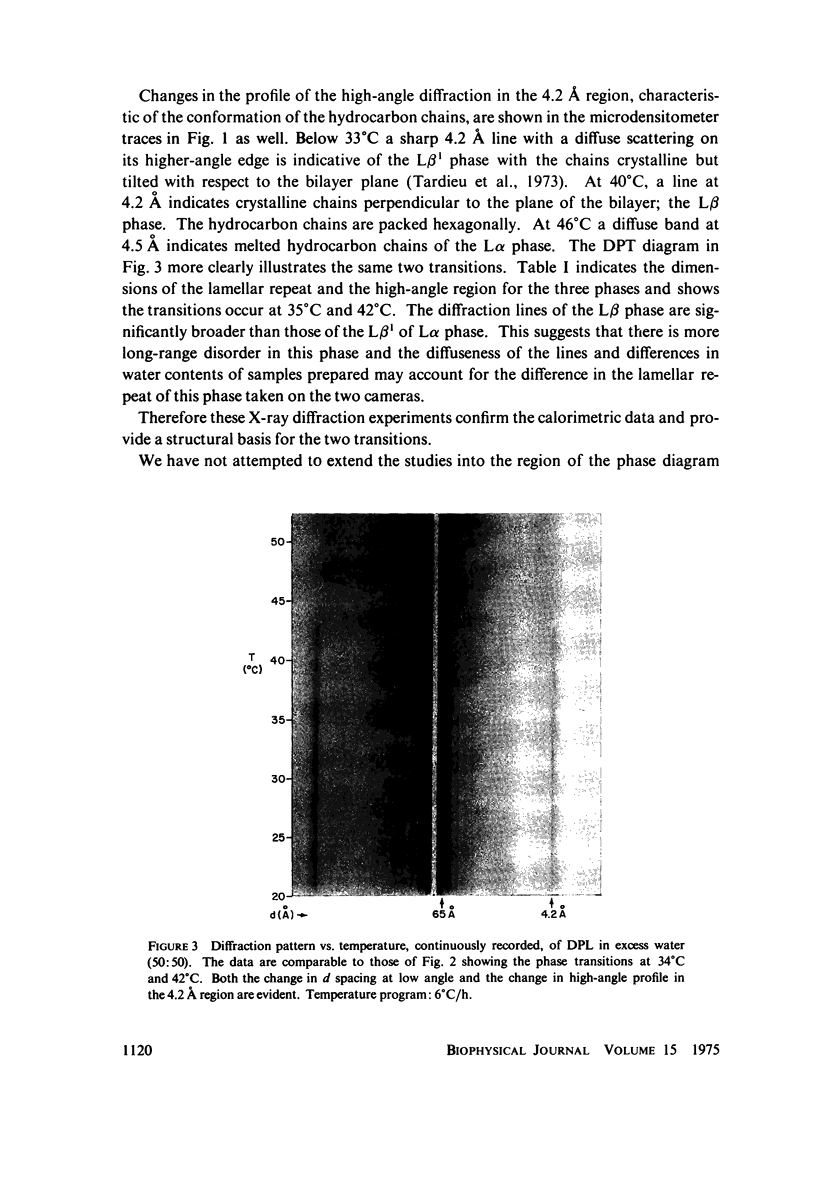

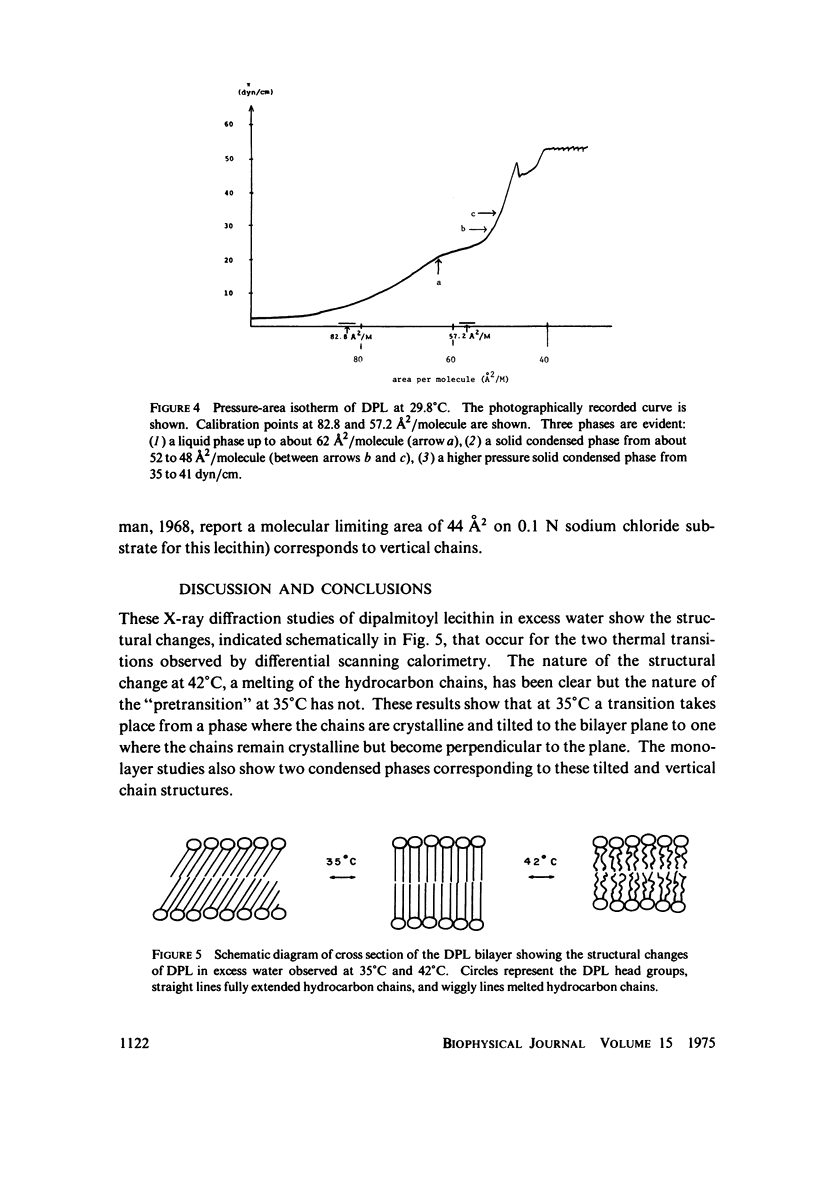


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cater B. R., Chapman D., Hawes S. M., Saville J. Lipid phase transitions and drug interactions. Biochim Biophys Acta. 1974 Aug 21;363(1):54–69. doi: 10.1016/0005-2736(74)90006-6. [DOI] [PubMed] [Google Scholar]
- Chapman D., Chen S. Thermal and NMR spectroscopic studies of lipids and membranes. Chem Phys Lipids. 1972 May;8(4):318–326. doi: 10.1016/0009-3084(72)90061-8. [DOI] [PubMed] [Google Scholar]
- Chapman D., Urbina J. Biomembrane phase transitions. Studies of lipid-water systems using differential scanning calorimetry. J Biol Chem. 1974 Apr 25;249(8):2512–2521. [PubMed] [Google Scholar]
- Darke A., Finer E. G., Flook A. G., Phillips M. C. Nuclear magnetic resonance study of lecithin-cholesterol interactions. J Mol Biol. 1972 Jan 28;63(2):265–279. doi: 10.1016/0022-2836(72)90374-9. [DOI] [PubMed] [Google Scholar]
- Gottlieb M. H., Eanes E. D. Coexistence of rigid crystalline and liquid crystalline phases in lecithin-water mixtures. Biophys J. 1974 May;14(5):335–342. doi: 10.1016/S0006-3495(74)85920-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz H. J., Sturtevant J. M. Calorimetric studies of dilute aqueous suspensions of bilayers formed from synthetic L- -lecithins. J Biol Chem. 1972 Oct 10;247(19):6071–6075. [PubMed] [Google Scholar]
- Ladbrooke B. D., Williams R. M., Chapman D. Studies on lecithin-cholesterol-water interactions by differential scanning calorimetry and X-ray diffraction. Biochim Biophys Acta. 1968 Apr 29;150(3):333–340. doi: 10.1016/0005-2736(68)90132-6. [DOI] [PubMed] [Google Scholar]
- Munden J. W., Swarbrick J. Time-dependent surface behavior of dipalmitoyllecithin and lung alveolar surfactant monolayers. Biochim Biophys Acta. 1973 Jan 26;291(2):344–350. doi: 10.1016/0005-2736(73)90487-2. [DOI] [PubMed] [Google Scholar]
- Phillips M. C., Chapman D. Monolayer characteristics of saturated 1,2,-diacyl phosphatidylcholines (lecithins) and phosphatidylethanolamines at the air-water interface. Biochim Biophys Acta. 1968 Nov 5;163(3):301–313. doi: 10.1016/0005-2736(68)90115-6. [DOI] [PubMed] [Google Scholar]
- Rand R. P., Luzzati V. X-ray diffraction study in water of lipids extracted from human erythrocytes: the position of cholesterol in the lipid lamellae. Biophys J. 1968 Jan;8(1):125–137. doi: 10.1016/S0006-3495(68)86479-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veksli Z., Salsbury N. J., Chapman D. Physical studies of phospholipids. XII. Nuclear magnetic resonance studies of molecular motion in some pure lecithin-water systems. Biochim Biophys Acta. 1969;183(3):434–446. doi: 10.1016/0005-2736(69)90158-8. [DOI] [PubMed] [Google Scholar]



