Abstract
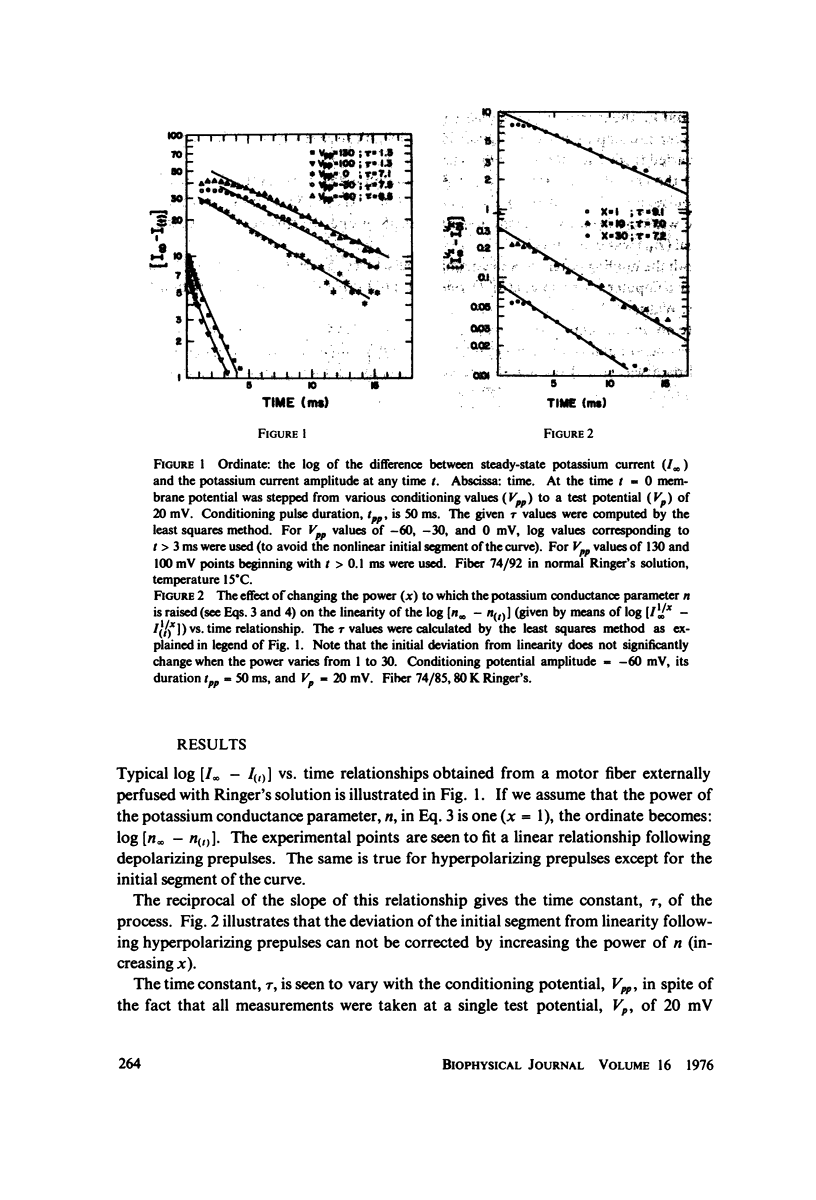
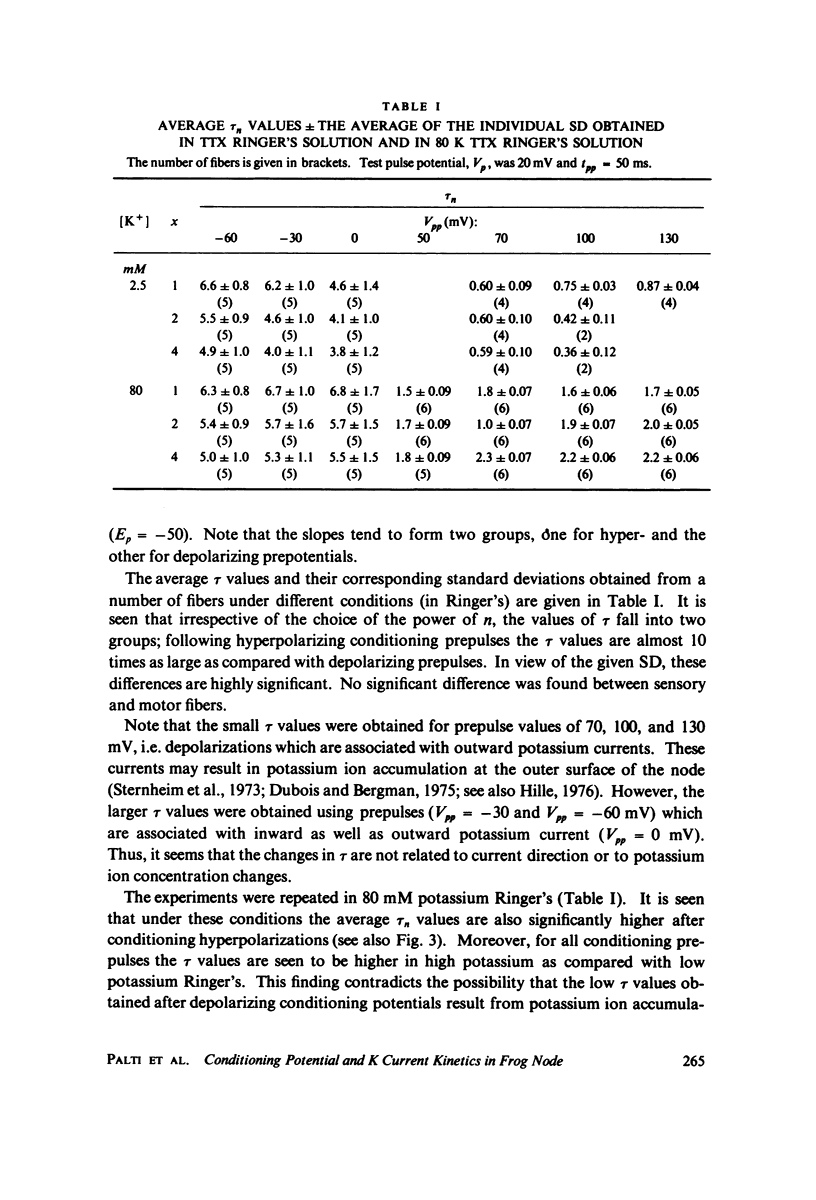
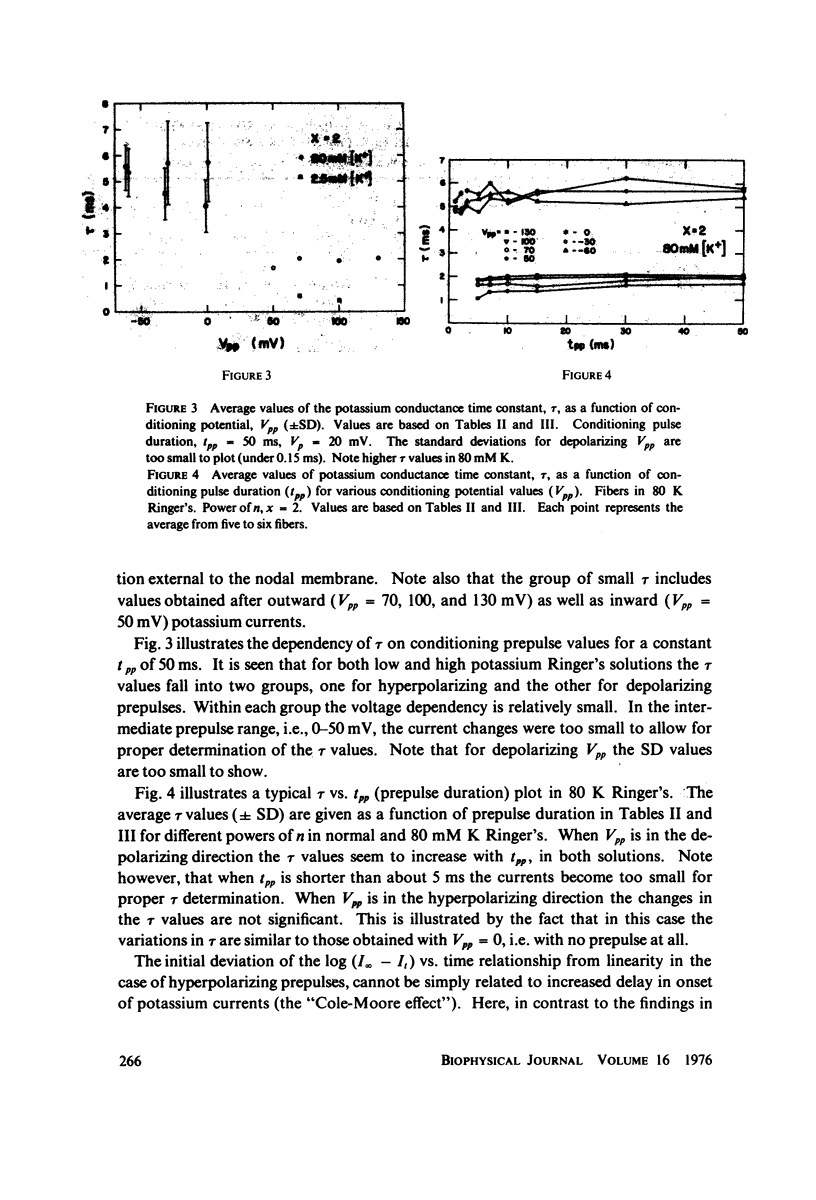
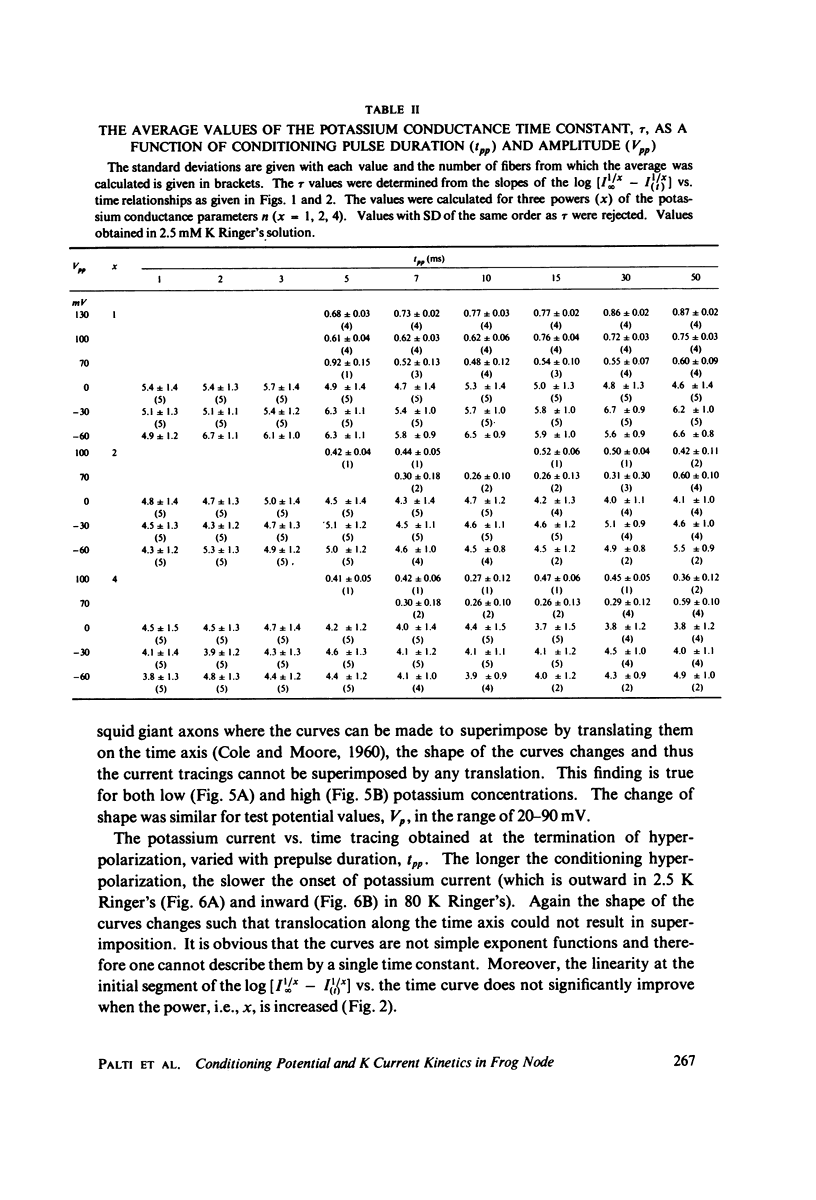
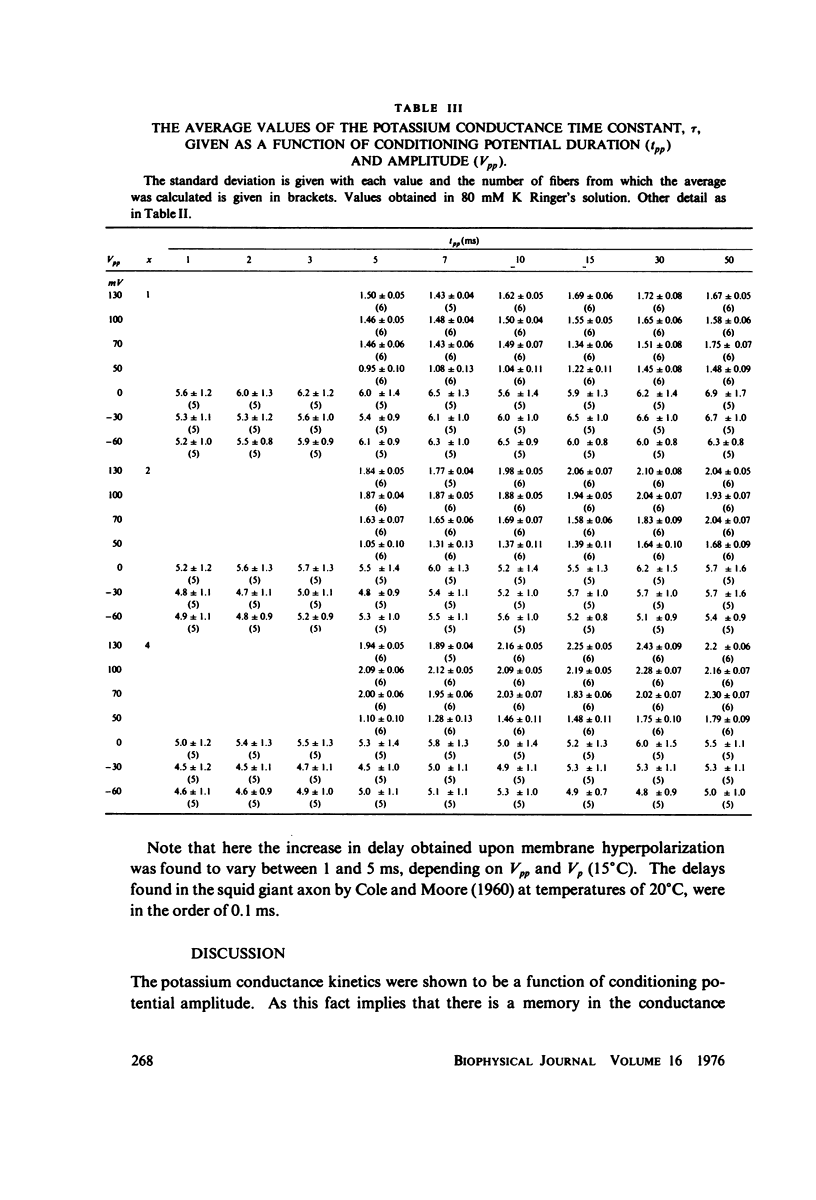
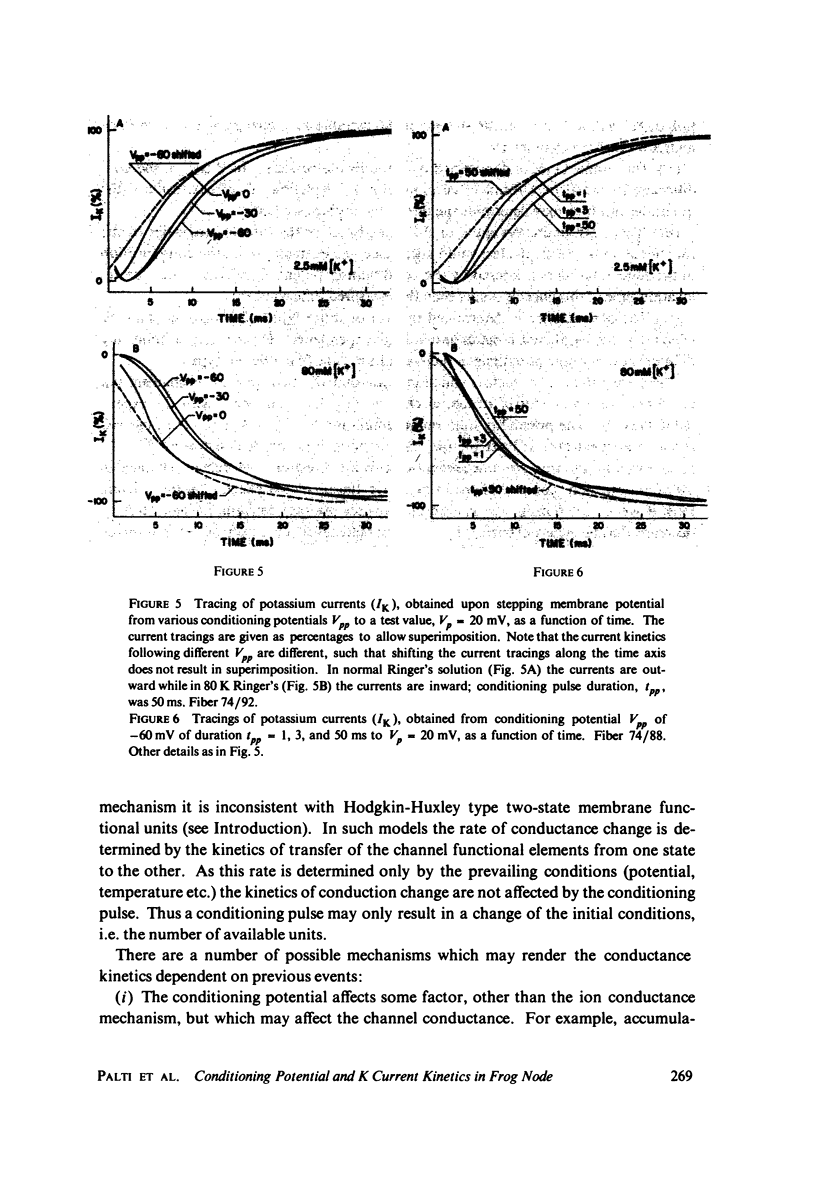
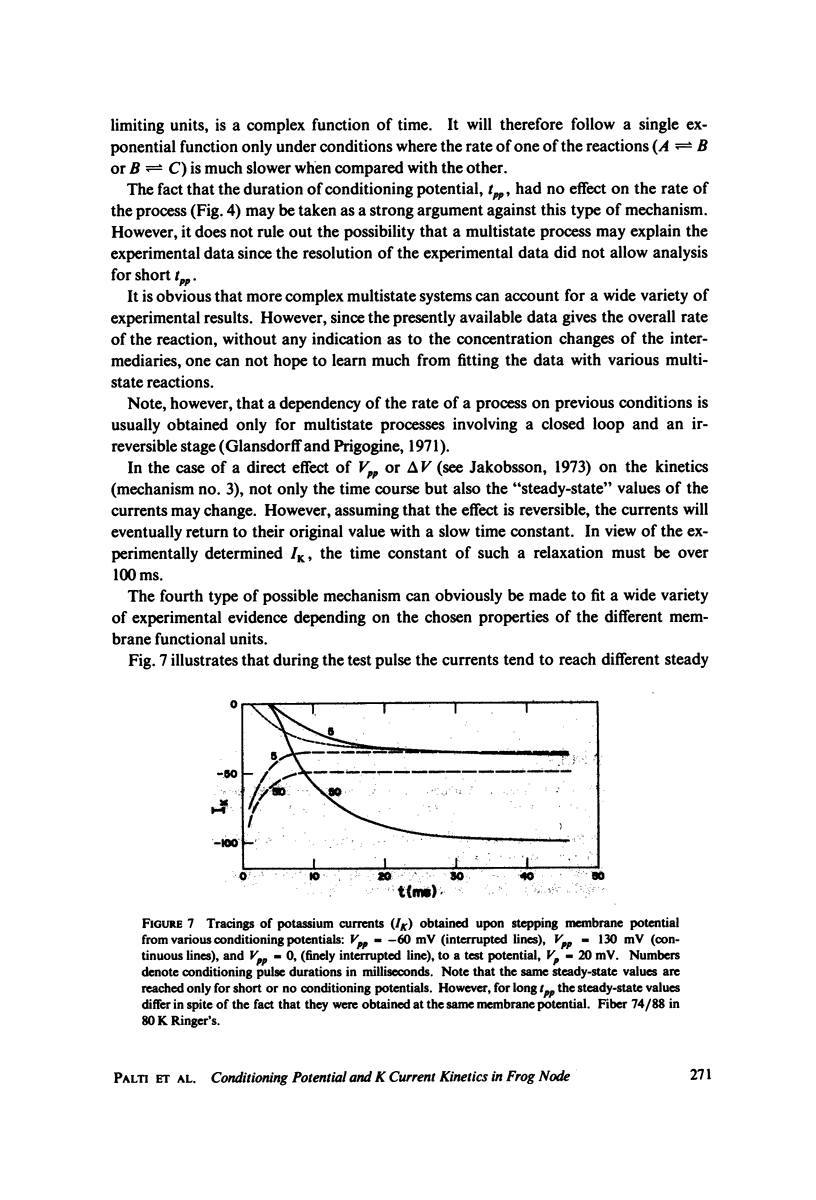
The kinetics of potassium conductance changes were determined in the voltage clamped frog node (Rana esculenta), as a function of conditioning prepotential. The conditioning potential duration varied from 1 to 50 ms and the amplitude between -60 and +130 mV (relative to rest). The conductance kinetics were determined at a single test potential of +20 mV (depolarization) by means of the slope of log [ninfinity - nt] vs. time relationship which defines the time constant of the process (tau). The values of tau, after conditioning hyperpolarizations, were around 5 ms, up to 10 times greater than values obtained following a strong depolarization. The tau vs. pre-potential curve was sigmoid in shape. These differences were only slightly dependent on [K+]0 or conditioning pulse duration. The steady-state current values were also found to be a function of conditioning potential. After conditioning hyperpolarizations, the log [ninfinity - nt] vs. time curve could not be fitted by a single exponent regardless of the power of n chosen. The prepotential dependency of potassium current kinetics is inconsistent with the Hodgkin-Huxley axon model where the conductance parameters are assumed to be in either one of two possible states, and where the rate of transfer from one state to the other follows first order kinetics. In contrast the described kinetics may be consistent with complex multistate potassium "channel" models or membranes consisting of a number of types of channels.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COLE K. S., MOORE J. W. Potassium ion current in the squid giant axon: dynamic characteristic. Biophys J. 1960 Sep;1:1–14. doi: 10.1016/s0006-3495(60)86871-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGE F. A., FRANKENHAEUSER B. Membrane currents in isolated frog nerve fibre under voltage clamp conditions. J Physiol. 1958 Aug 29;143(1):76–90. doi: 10.1113/jphysiol.1958.sp006045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGE F. A., FRANKENHAEUSER B. Sodium currents in the myelinated nerve fibre of Xenopus laevis investigated with the voltage clamp technique. J Physiol. 1959 Oct;148:188–200. doi: 10.1113/jphysiol.1959.sp006281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman L., Schauf C. L. Inactivation of the sodium current in Myxicola giant axons. Evidence for coupling to the activation process. J Gen Physiol. 1972 Jun;59(6):659–675. doi: 10.1085/jgp.59.6.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman L., Schauf C. L. Quantitative description of sodium and potassium currents and computed action potentials in Myxicola giant axons. J Gen Physiol. 1973 Mar;61(3):361–384. doi: 10.1085/jgp.61.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson E. The physical interpretation of mathematical models for sodium permeability changes in excitable membranes. Biophys J. 1973 Nov;13(11):1200–1211. doi: 10.1016/S0006-3495(73)86055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppenhöfer E., Schmidt H. Incomplete sodium inactivation in nodes of Ranvier treated with scorpion venom. Experientia. 1968 Jan 15;24(1):41–42. doi: 10.1007/BF02136780. [DOI] [PubMed] [Google Scholar]
- Moore L. E. Effect of temperature and calcium ions on rate constants of myelinated nerve. Am J Physiol. 1971 Jul;221(1):131–137. doi: 10.1152/ajplegacy.1971.221.1.131. [DOI] [PubMed] [Google Scholar]
- Moore L. E. Membrane currents at large positive internal potentials in single myelinated nerve fibres of Rana pipiens. J Physiol. 1967 Nov;193(2):433–442. doi: 10.1113/jphysiol.1967.sp008368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonner W. A new voltage clamp method for Ranvier nodes. Pflugers Arch. 1969;309(2):176–192. doi: 10.1007/BF00586967. [DOI] [PubMed] [Google Scholar]
- STAMPFLI R. Bau und Funktion isolierter markhaltiger Nervenfasern. Ergeb Physiol. 1952;47:70–165. [PubMed] [Google Scholar]


