Abstract
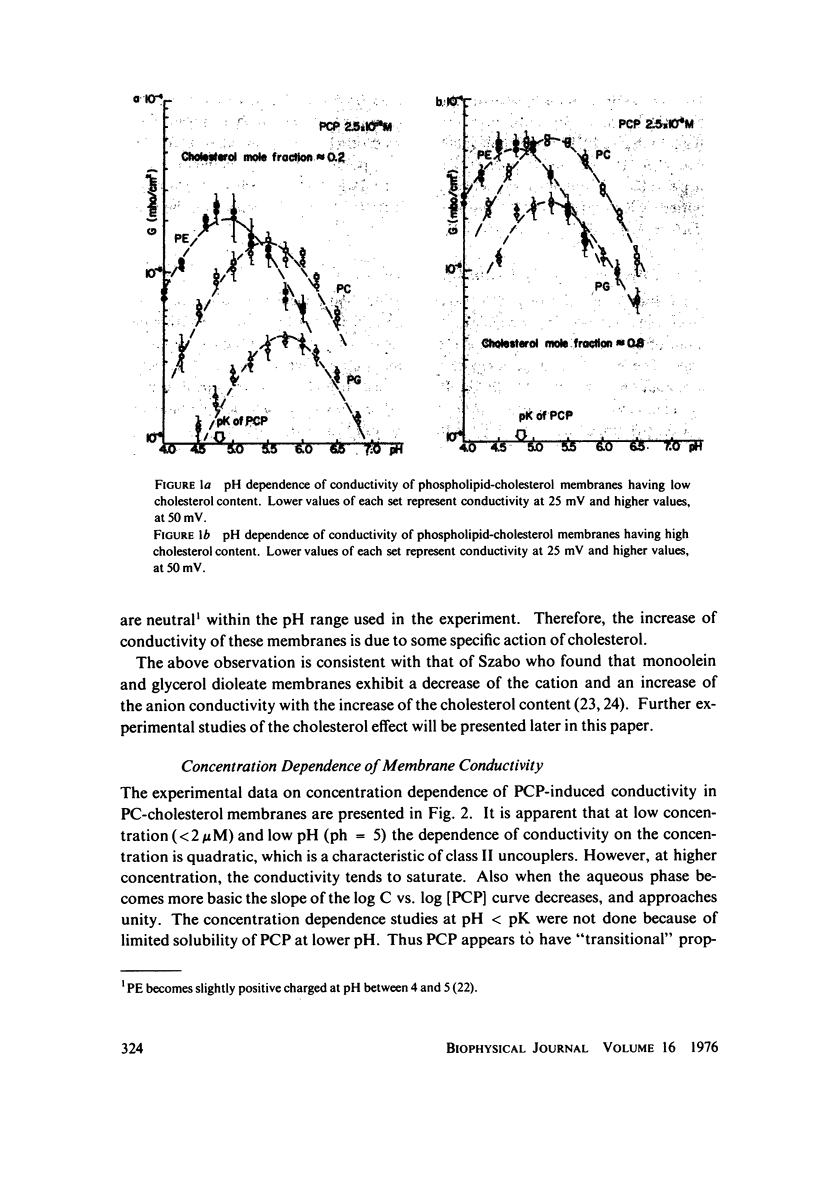
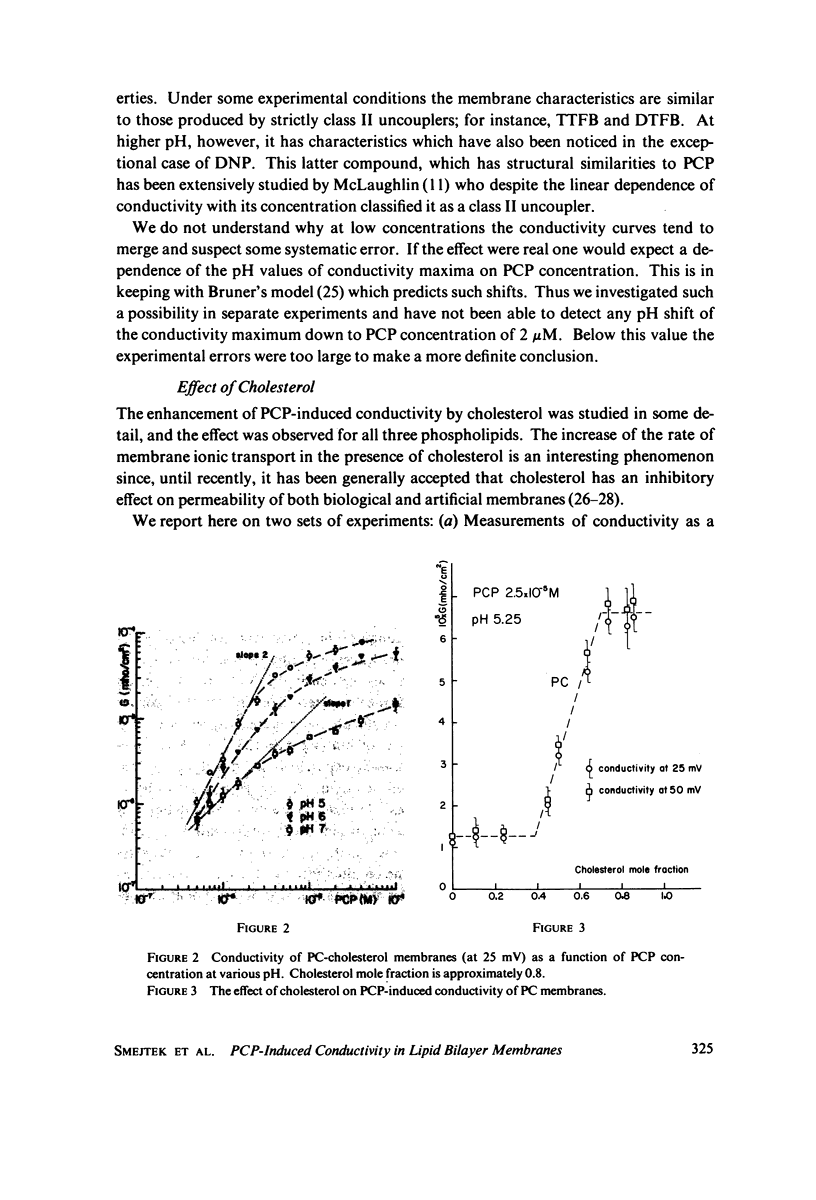
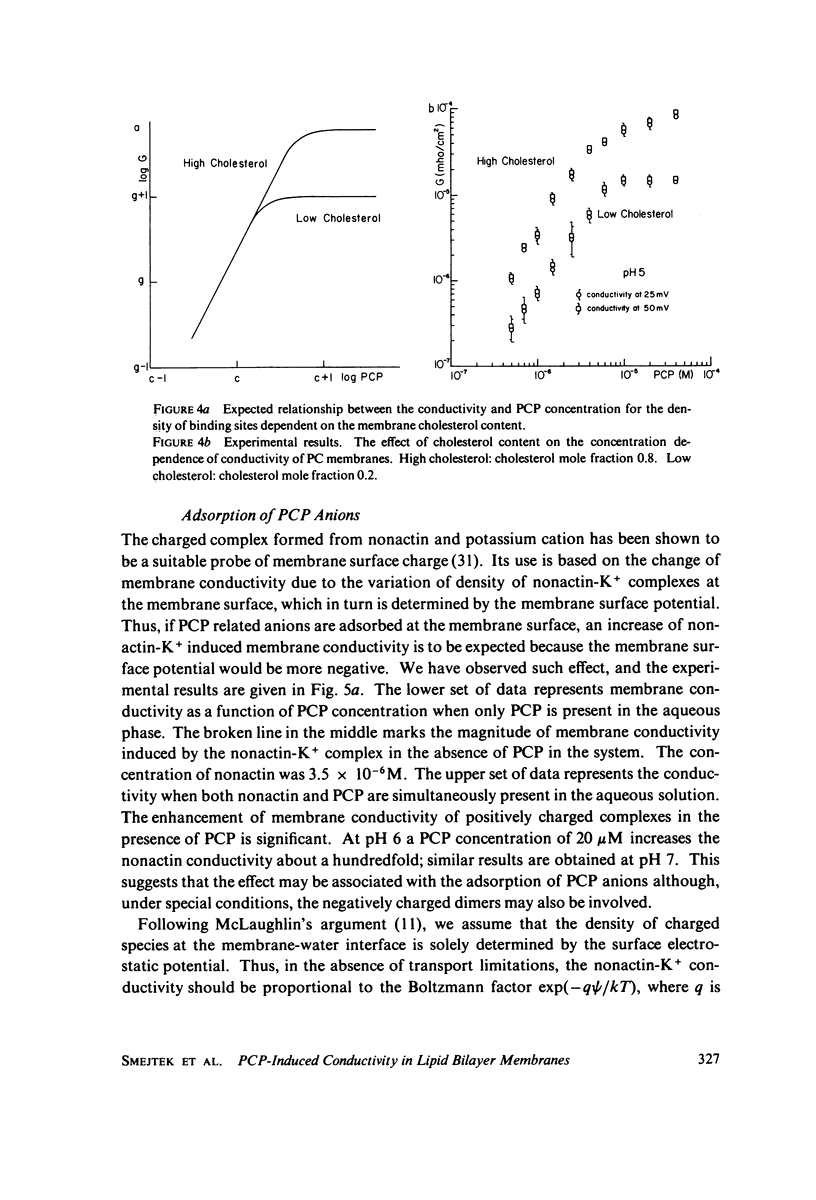
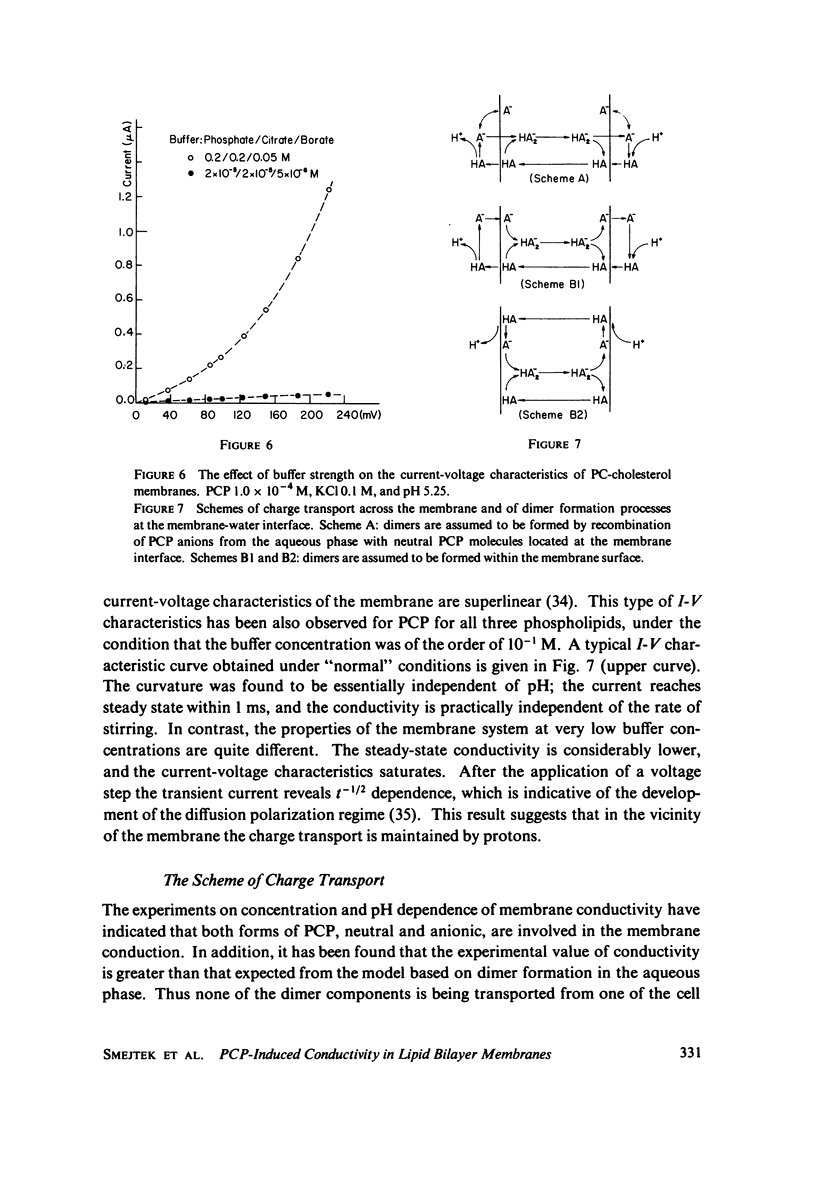
Electrical conductivity induced in thin lipid bilayer membranes by pentachlorophenol has been studied. The membranes were formed from phosphatidyl choline, phosphatidyl ethanolamine, or phosphatidyl glycerol and various amounts of cholesterol. The position and the magnitude of the maximum of the conductivity vs. pH curve depend on the type of lipids and cholesterol content. At low pentachlorophenol concentrations and low pH the concentration dependence of conductivity is quadratic and becomes linear at higher pH. Above 10(-5) M of pentachlorophenol the concentration dependence of the membrane conductivity tends to saturate. Presence of pentachlorophenol enhances membrane transport of nonactin-K+ complex. Increase of cholesterol content increases pentachlorophenol induced conductivity in all membranes and shifts the conductivity toward lower pH. For phosphatidyl choline the largest rate of change of membrane conductivity with cholesterol occurs at 1:1 phospholipid to cholesterol molar ratio. Pentachlorophenol is found to be a class II uncoupler and the experimental results are consistent with the hypothesis that the membrane permeable species are dimers formed by combination of neutral and dissociated pentachlorophenol molecules. Several schemes of membrane conduction, including dimer formation in the aqueous phase as well as at the membrane-water interface have been considered. Arguments are given in favor of the formation of dimers within the membrane surface.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLACKMAN G. E., PARKE M. H., GARTON G. The physiological activity of substituted phenols. II. Relationships between physical properties and physiological activity. Arch Biochem Biophys. 1955 Jan;54(1):55–71. doi: 10.1016/0003-9861(55)90008-4. [DOI] [PubMed] [Google Scholar]
- Bakker E. P., van den Heuvel E. J., Wiechmann A. H., van Dam K. A comparison between the effectiveness of uncouplers of oxidative phosphorylation in mitochondria and in different artificial membrane systems. Biochim Biophys Acta. 1973 Jan 18;292(1):78–87. doi: 10.1016/0005-2728(73)90252-1. [DOI] [PubMed] [Google Scholar]
- Borisova M. P., Ermishkin L. N., Liberman E. A., Silberstein A. Y., Trofimov E. M. Mechanism of conductivity of bimolecular lipid membranes in the presence of tetrachlorotrifluoromethylbenzimidazole. J Membr Biol. 1974;18(3-4):243–261. doi: 10.1007/BF01870115. [DOI] [PubMed] [Google Scholar]
- Bunce A. S., Hider R. C. The composition of black lipid membranes formed from egg-yolk lecithin, cholesterol and n-decane. Biochim Biophys Acta. 1974 Sep 23;363(3):423–427. doi: 10.1016/0005-2736(74)90081-9. [DOI] [PubMed] [Google Scholar]
- Finkelstein A. Weak-acid uncouplers of oxidative phosphorylation. Mechanism of action on thin lipid membranes. Biochim Biophys Acta. 1970 Apr 7;205(1):1–6. doi: 10.1016/0005-2728(70)90055-1. [DOI] [PubMed] [Google Scholar]
- Foster M., McLaughlin S. Complexes between uncouplers of oxidative phosphorylation. J Membr Biol. 1974;17(2):155–180. doi: 10.1007/BF01870177. [DOI] [PubMed] [Google Scholar]
- Haydon D. A., Hladky S. B. Ion transport across thin lipid membranes: a critical discussion of mechanisms in selected systems. Q Rev Biophys. 1972 May;5(2):187–282. doi: 10.1017/s0033583500000883. [DOI] [PubMed] [Google Scholar]
- Hopfer U., Lehninger A. L., Thompson T. E. Protonic conductance across phospholipid bilayer membranes induced by uncoupling agents for oxidative phosphorylation. Proc Natl Acad Sci U S A. 1968 Feb;59(2):484–490. doi: 10.1073/pnas.59.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton A. W., Schuff A. R., McClure D. W. Ionic transport through model membranes. II. Function of cholesterol and steroid hormones. Biochim Biophys Acta. 1973 Aug 22;318(2):225–234. doi: 10.1016/0005-2736(73)90116-8. [DOI] [PubMed] [Google Scholar]
- Iaguzhinskii L. S., Ratnikova L. A., Kolesova G. M. Kolichestvennaia zavisimost' mezhdu velichinoi konstanty dissotsiatsii razobshchitelei i ikh éffektivnost'iu na mitokhondrial'nykh membranakh. Biofizika. 1973 May-Jun;18(3):460–465. [PubMed] [Google Scholar]
- Kolesova G. M., Boguslavskii L. I., Iaguzhinskii L. S. Vzaimodeistvie razobshchitelia okislitel'nogo fosforilirovaniia pentakhlor fenola s letsitinom. Dokl Akad Nauk SSSR. 1973;210(6):1453–1456. [PubMed] [Google Scholar]
- Le Blanc O. H., Jr Tetraphenylborate conductance through lipid bilayer membranes. Biochim Biophys Acta. 1969;193(2):350–360. doi: 10.1016/0005-2736(69)90195-3. [DOI] [PubMed] [Google Scholar]
- Liberman E. A., Topaly V. P. Selective transport of ions through bimolecular phospholipid membranes. Biochim Biophys Acta. 1968 Sep 17;163(2):125–136. doi: 10.1016/0005-2736(68)90089-8. [DOI] [PubMed] [Google Scholar]
- Markin V. S., Krishtalik L. I., Liberman E. A., Topaly V. P. O mekhanizme provodimosti iskusstvennykh fosfolipoidnykh membran v prisutstvii perenoschikov ionov. Biofizika. 1969 Mar-Apr;14(2):256–264. [PubMed] [Google Scholar]
- McLaughlin S. G., Szabo G., Eisenman G., Ciani S. M. Surface charge and the conductance of phospholipid membranes. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1268–1275. doi: 10.1073/pnas.67.3.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S. G., Szabo G., Eisenman G. Divalent ions and the surface potential of charged phospholipid membranes. J Gen Physiol. 1971 Dec;58(6):667–687. doi: 10.1085/jgp.58.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S. The mechanism of action of DNP on phospholipid bilayer membranes. J Membr Biol. 1972;9(4):361–372. [PubMed] [Google Scholar]
- Neumcke B. Diffusion polarization at lipid bilayer membranes. Biophysik. 1971;7(2):95–105. doi: 10.1007/BF01190141. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D. Cholesterol and cell membrane function: a hypothesis concerning etiology of atherosclerosis. J Theor Biol. 1974 Feb;43(2):329–337. doi: 10.1016/s0022-5193(74)80064-0. [DOI] [PubMed] [Google Scholar]
- SINGLETON W. S., GRAY M. S., BROWN M. L., WHITE J. L. CHROMATOGRAPHICALLY HOMOGENEOUS LECITHIN FROM EGG PHOSPHOLIPIDS. J Am Oil Chem Soc. 1965 Jan;42:53–56. doi: 10.1007/BF02558256. [DOI] [PubMed] [Google Scholar]
- Szabo G., Eisenman G., Laprade R., Ciani S. M., Krasne S. Experimentally observed effects of carriers on the electrical properties of bilayer membranes--equilibrium domain. With a contribution on the molecular basis of ion selectivity. Membranes. 1973;2:179–328. [PubMed] [Google Scholar]
- Ting H. P., Wilson D. F., Chance B. Effects of uncouplers of oxidative phosphorylation on the specific conductance of bimolecular lipid membranes. Arch Biochem Biophys. 1970 Nov;141(1):141–146. doi: 10.1016/0003-9861(70)90116-5. [DOI] [PubMed] [Google Scholar]


