Abstract
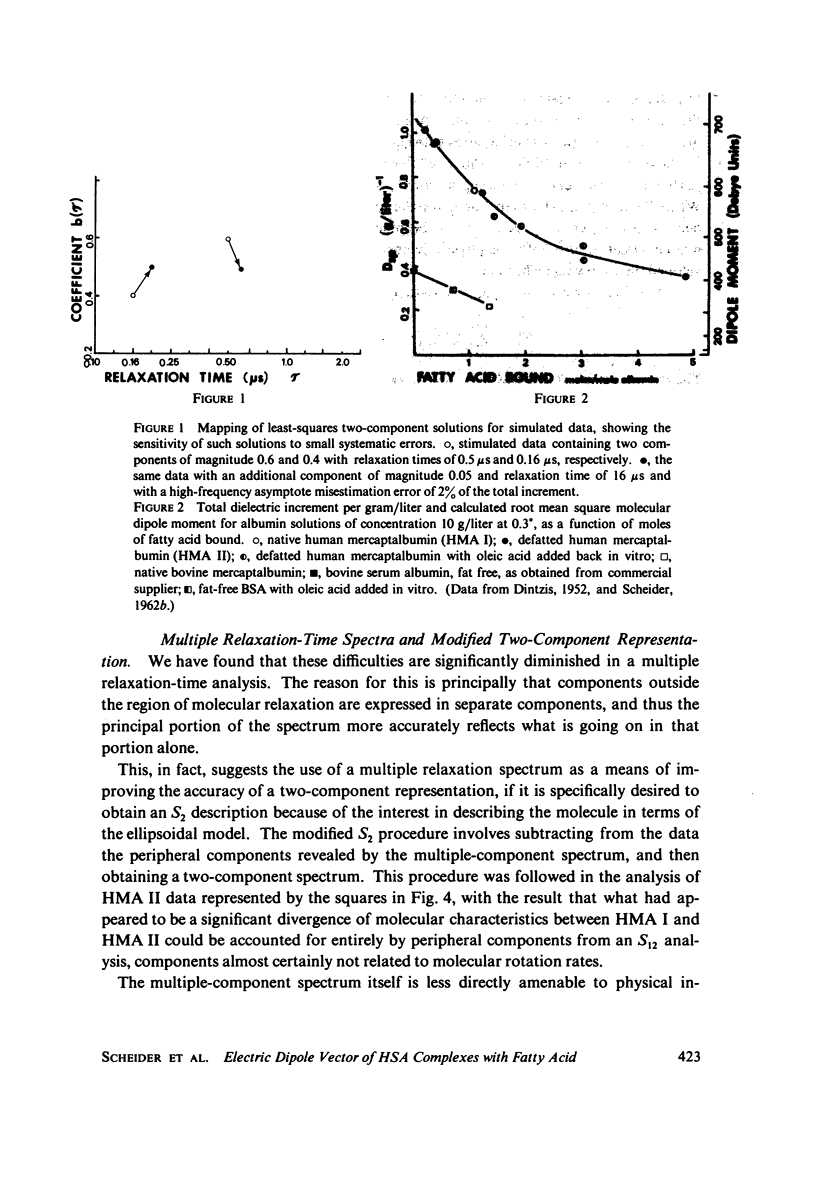
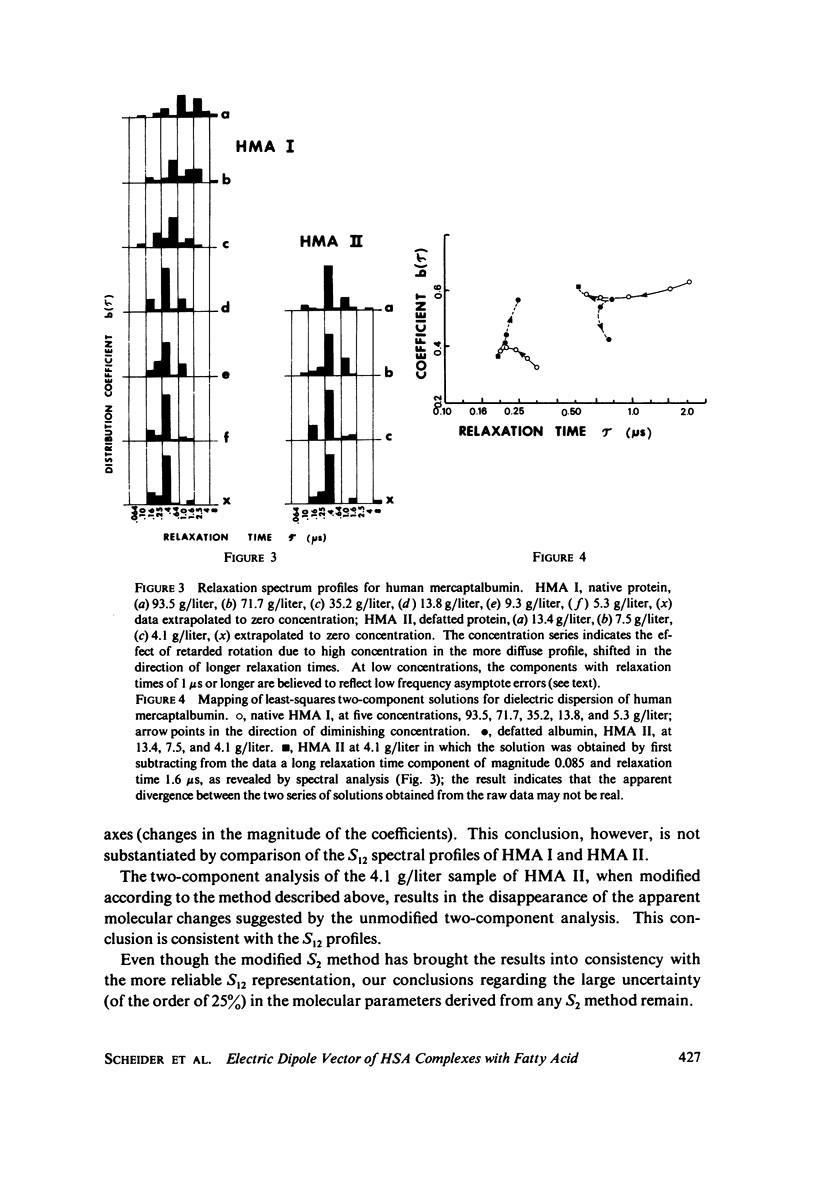
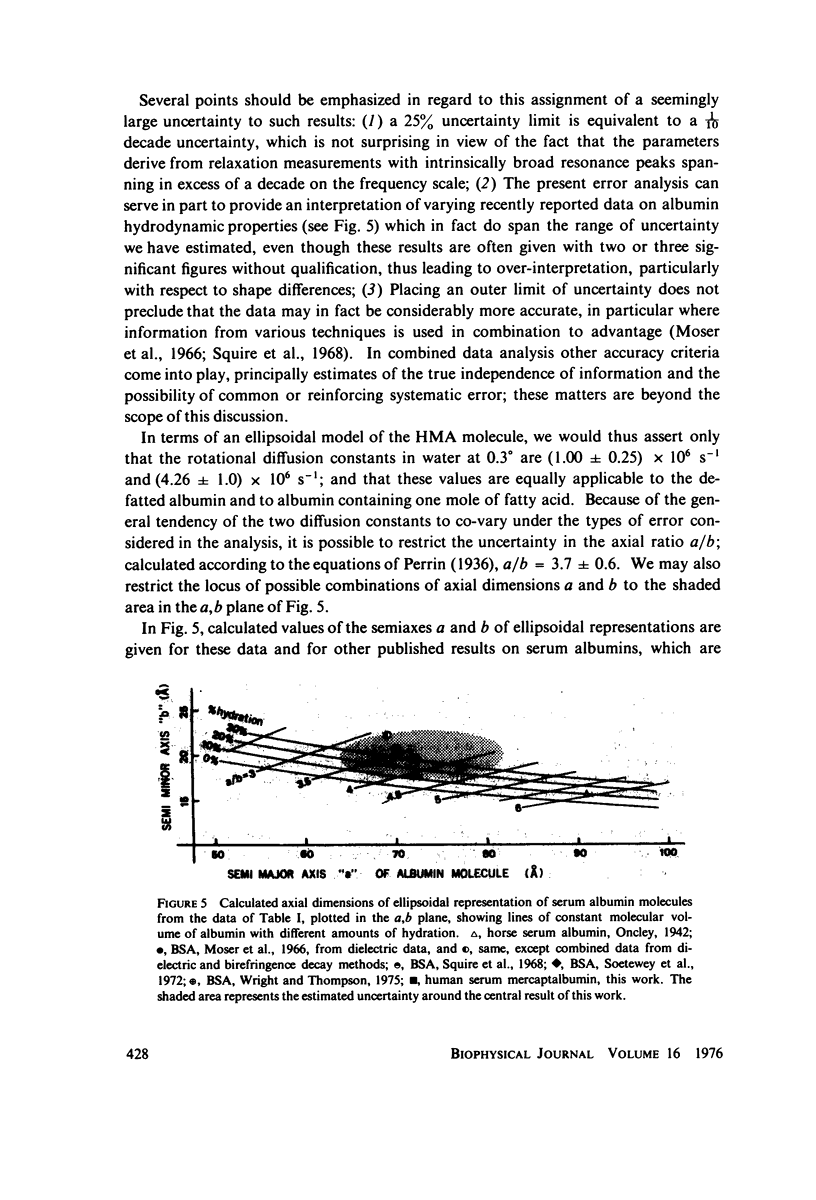
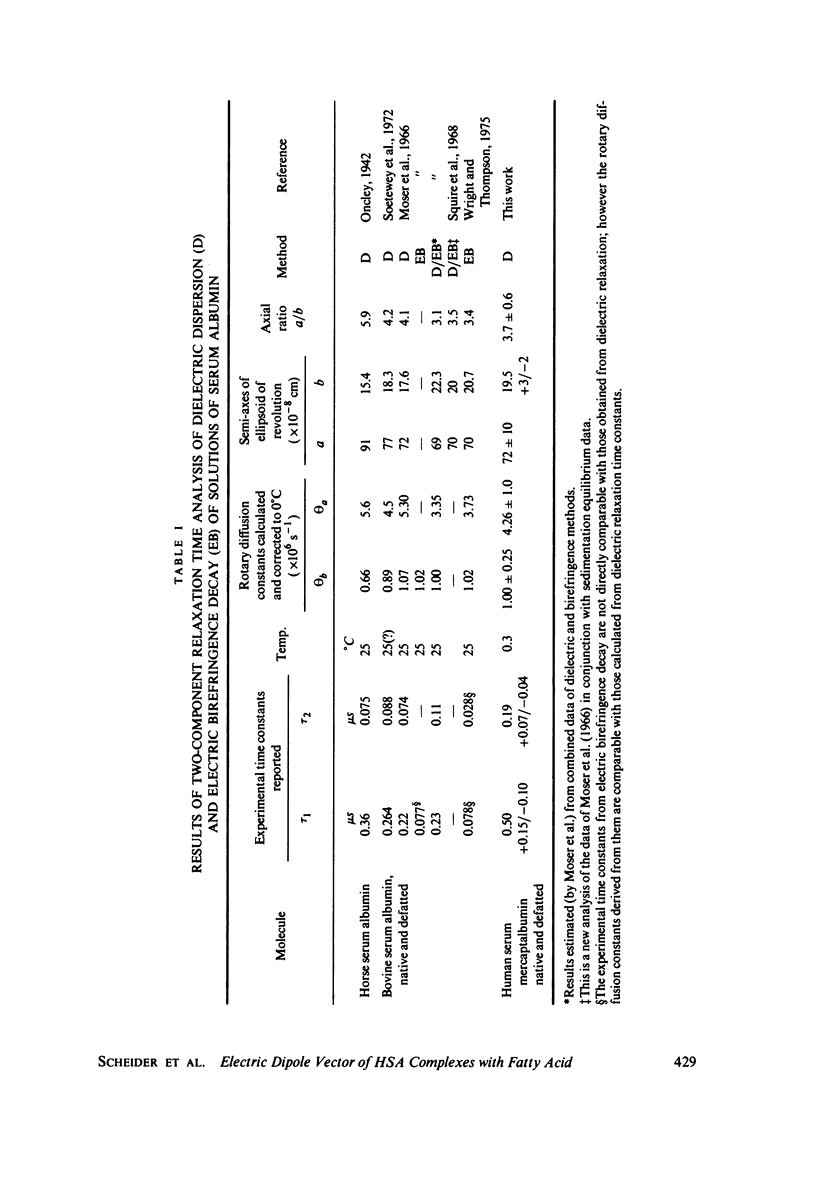
The magnitude of the electric dipole vector of human serum albumin, as measured by the dielectric increment of the isoionic solution, is found to be a sensitive, monotonic indicator of the number of moles (up to at least 5) of long chain fatty acid complexed. The sensitivity is about three times as great as it is in bovine albumin. New methods of analysis of the frequency dispersion of the dielectric constant were developed to ascertain if molecular shape changes also accompany the complexing with fatty acid. Direct two-component rotary diffusion constant analysis is found to be too strongly affected by cross modulation between small systematic errors and physically significant data components to be a reliable measure of structural modification. Multicomponent relaxation profiles are more useful as recognition patterns for structural comparisons, but the equations involved are ill-conditioned and solutions based on standard least-squares regression contain mathematical artifacts which mask the physically significant spectrum. By constraining the solution to non-negative coefficients, the magnitude of the artifacts is reduced to well below the magnitudes of the spectral components. Profiles calculated in this way show no evidence of significant dipole direction or molecular shape change as the albumin is complexed with 1 mol of fatty acid. In these experiments albumin was defatted by incubation with adipose tissue at physiological pH, which avoids passing the protein through the pH of the N-F transition usually required in defatting. Addition of fatty acid from soluion in small amounts of ethanol appears to form a complex indistinguishable from the "native" complex.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALLY P. R., CAHILL G. F., Jr, LEBOEUF B., RENOLD A. E. Studies on rat adipose tissue in vitro. V. Effects of glucose and insulin on the metabolism of palmitate-1-C14. J Biol Chem. 1960 Feb;235:333–336. [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- DOLE V. P. A relation between non-esterified fatty acids in plasma and the metabolism of glucose. J Clin Invest. 1956 Feb;35(2):150–154. doi: 10.1172/JCI103259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODMAN D. S. Preparation of human serum albumin free of long-chain fatty acids. Science. 1957 Jun 28;125(3261):1296–1297. doi: 10.1126/science.125.3261.1296. [DOI] [PubMed] [Google Scholar]
- GORDON R. S., Jr, CHERKES A. Production of unesterified fatty acids from isolated rat adipose tissue incubated in vitro. Proc Soc Exp Biol Med. 1958 Jan;97(1):150–151. doi: 10.3181/00379727-97-23672. [DOI] [PubMed] [Google Scholar]
- HUGHES W. L., DINTZIS H. M. CRYSTALLIZATION OF THE MERCURY DIMERS OF HUMAN AND BOVINE MERCAPTALBUMIN. J Biol Chem. 1964 Mar;239:845–849. [PubMed] [Google Scholar]
- Hendrickx H., Verbruggen R., Rosseneu-Motreff M. Y., Blaton V., Peeters H. The dipolar origin of protein relaxation. Biochem J. 1968 Dec;110(3):419–424. doi: 10.1042/bj1100419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgens J. A., Green C. The entry of palmitic acid into rat-liver cells. Biochem J. 1967 Aug;104(2):26P–26P. [PMC free article] [PubMed] [Google Scholar]
- Odell G. B. Influence of binding on the toxicity of bilirubin. Ann N Y Acad Sci. 1973 Nov 26;226:225–237. doi: 10.1111/j.1749-6632.1973.tb20484.x. [DOI] [PubMed] [Google Scholar]
- SCHEIDER W. Letter to the editor: computer method for fitting kinetic data. Biophys J. 1962 Nov;2:501–502. doi: 10.1016/s0006-3495(62)86870-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheider W. Dielectric Relaxation of Molecules with Fluctuating Dipole Moment. Biophys J. 1965 Sep;5(5):617–628. doi: 10.1016/s0006-3495(65)86740-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheider W., Fuller J. K. An effective method for defatting albumin using resin columns. Biochim Biophys Acta. 1970 Nov 17;221(2):376–378. doi: 10.1016/0005-2795(70)90280-1. [DOI] [PubMed] [Google Scholar]
- Soetewey F., Rosseneu-Motreff M., Lamote R., Peeters H. Size and shape determination of native and defatted bovine serum albumin monomers. II. Influence of the fatty acid content on the conformation of bovine serum albumin monomers. J Biochem. 1972 Apr;71(4):705–710. [PubMed] [Google Scholar]
- Sogami M., Petersen H. A., Foster J. F. The microheterogeneity of plasma albumins. V. Permutations in disulfide pairings as a probable source of microheterogeneity in bovine albumin. Biochemistry. 1969 Jan;8(1):49–58. doi: 10.1021/bi00829a008. [DOI] [PubMed] [Google Scholar]
- Spector A. A., John K. M. Effects of free fatty acid on the fluorescence of bovine serum albumin. Arch Biochem Biophys. 1968 Sep 20;127(1):65–71. doi: 10.1016/0003-9861(68)90202-6. [DOI] [PubMed] [Google Scholar]
- Spector A. A., John K., Fletcher J. E. Binding of long-chain fatty acids to bovine serum albumin. J Lipid Res. 1969 Jan;10(1):56–67. [PubMed] [Google Scholar]
- Spector A. A., Santos E. C., Ashbrook J. D., Fletcher J. E. Influence of free fatty acid concentration on drug binding to plasma albumin. Ann N Y Acad Sci. 1973 Nov 26;226:247–258. doi: 10.1111/j.1749-6632.1973.tb20486.x. [DOI] [PubMed] [Google Scholar]
- Wright A. K., Thompson M. R. Hydrodynamic structure of bovine serum albumin determined by transient electric birefringence. Biophys J. 1975 Feb;15(2 Pt 1):137–141. doi: 10.1016/s0006-3495(75)85797-3. [DOI] [PMC free article] [PubMed] [Google Scholar]



