Abstract
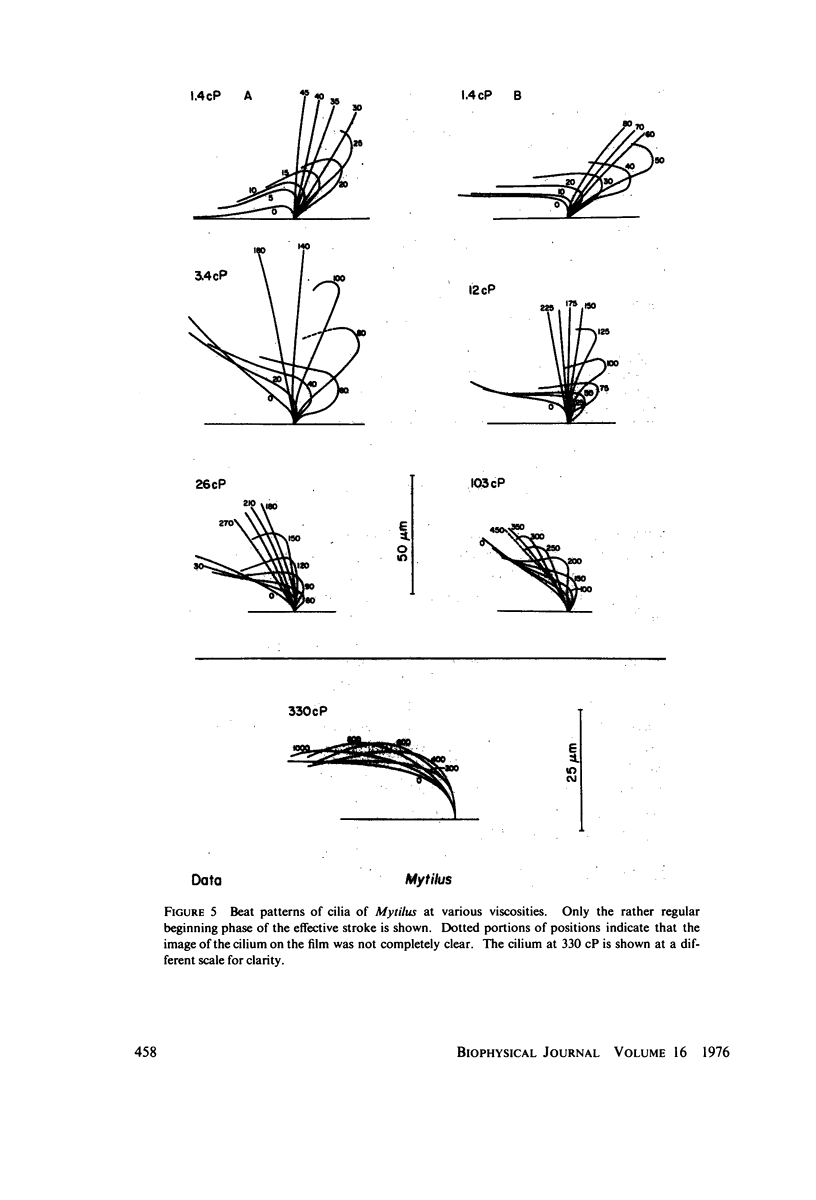
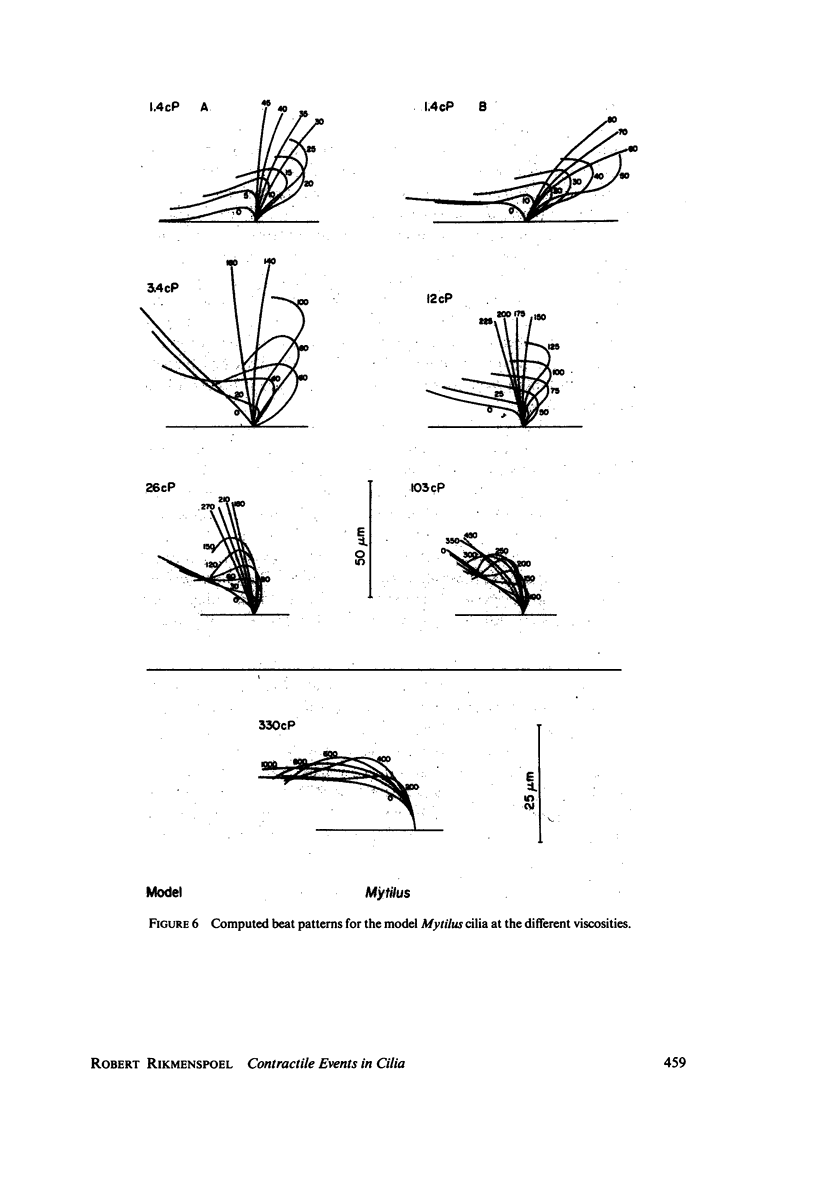
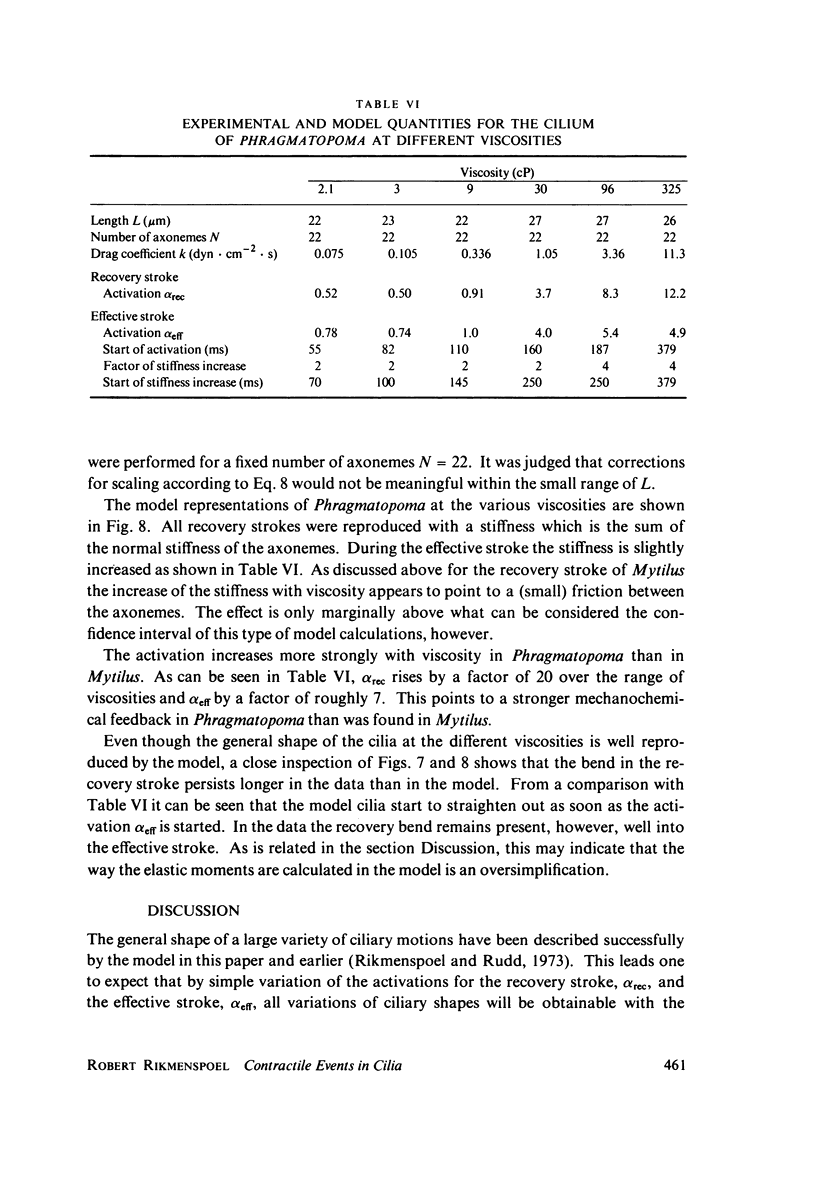
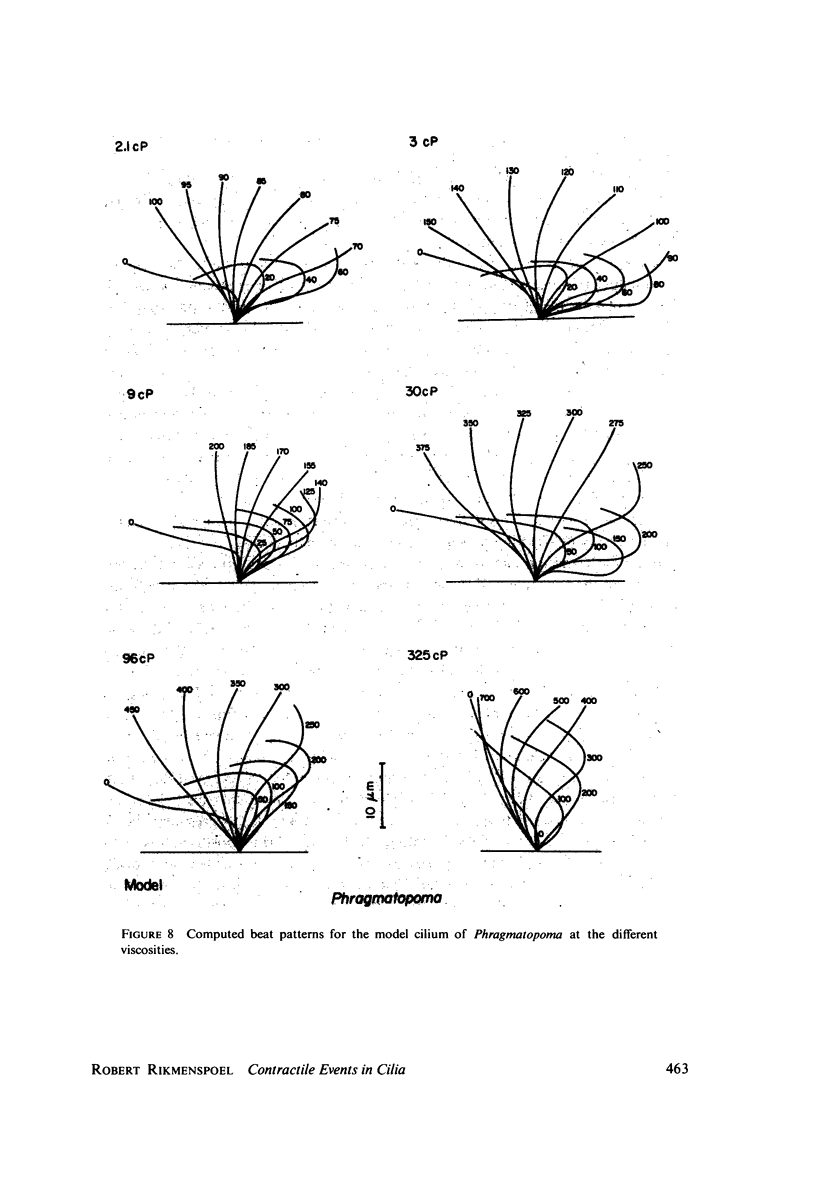
The motion of the abnormal cilia of Opalina and Mytilus can be described by the recently developed model for ciliary motion, provided the activation of the contractility during the effective stroke is reduced by three- to fivefold compared with that in the recovery stroke. The stiffness of the Mytilus cilium during the effective stroke is found several hundred times larger than that predicted by the model, however. The stiffness of the cilia of Paramecium, Opalina, Phragmatopoma, and of Mytilus in the recovery phase, is predicted approximately correctly by the model. The activation of contractility in Mytilus and Phragmatopoma cilia increases with the viscosity of the medium, as the velocity of the ciliary motion slows down. This leads to the equivalent of a force-velocity relation. The velocity of propagation of the bend in the cilia during the recovery stroke is shown to be dependent only on the elastic properties of the ciliary shaft, and to be independent of the contractile activiey.
Full text
PDF












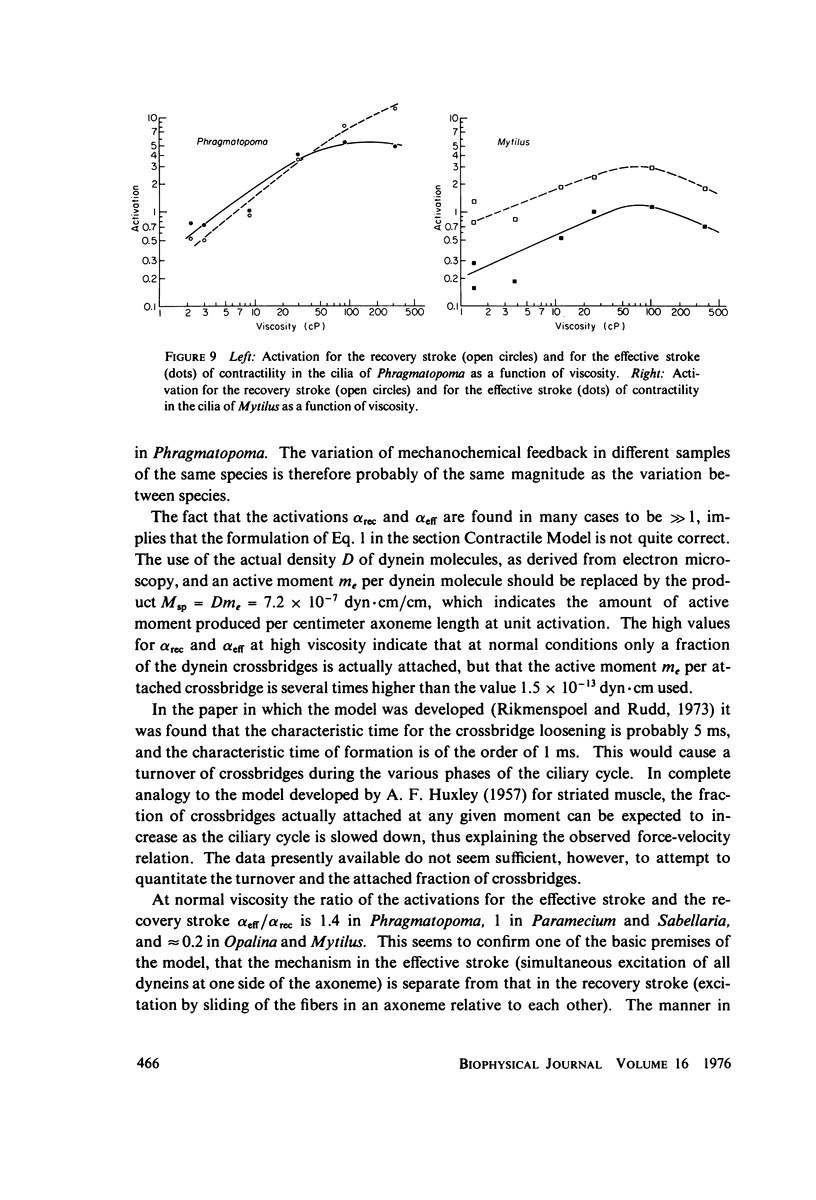
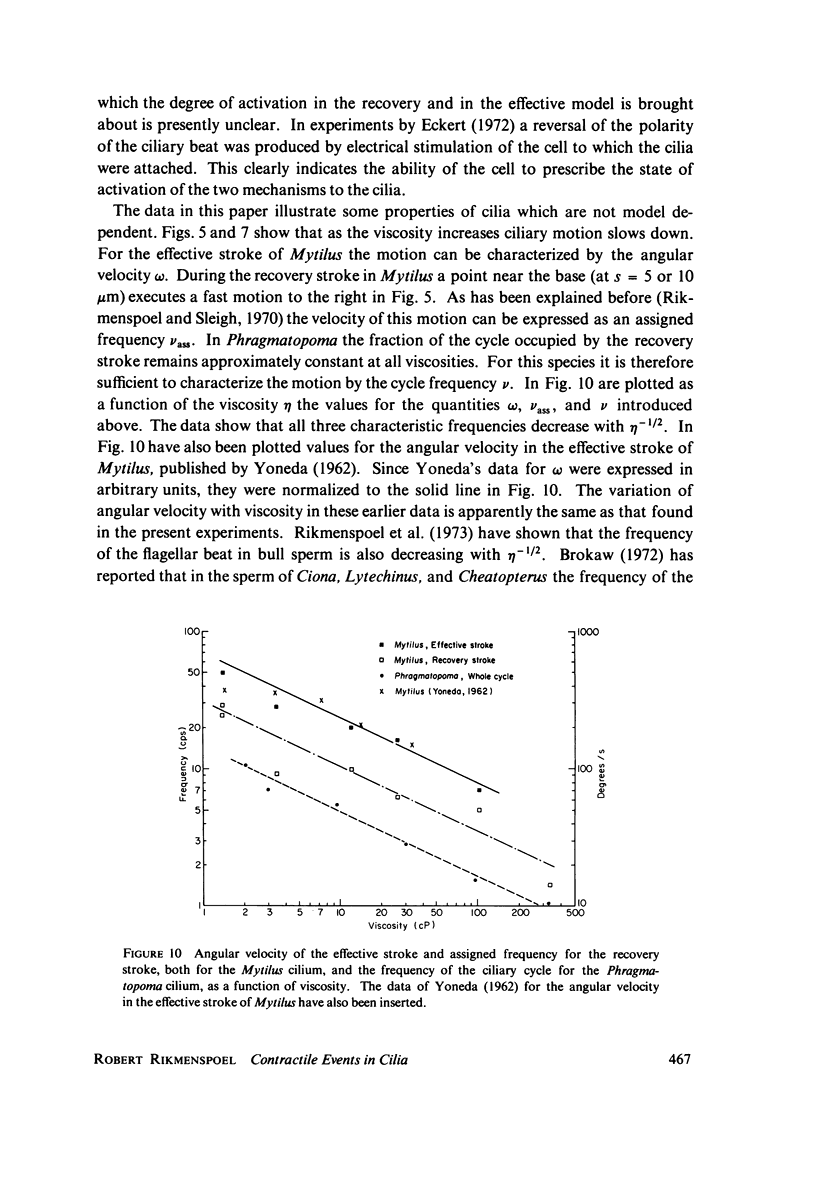
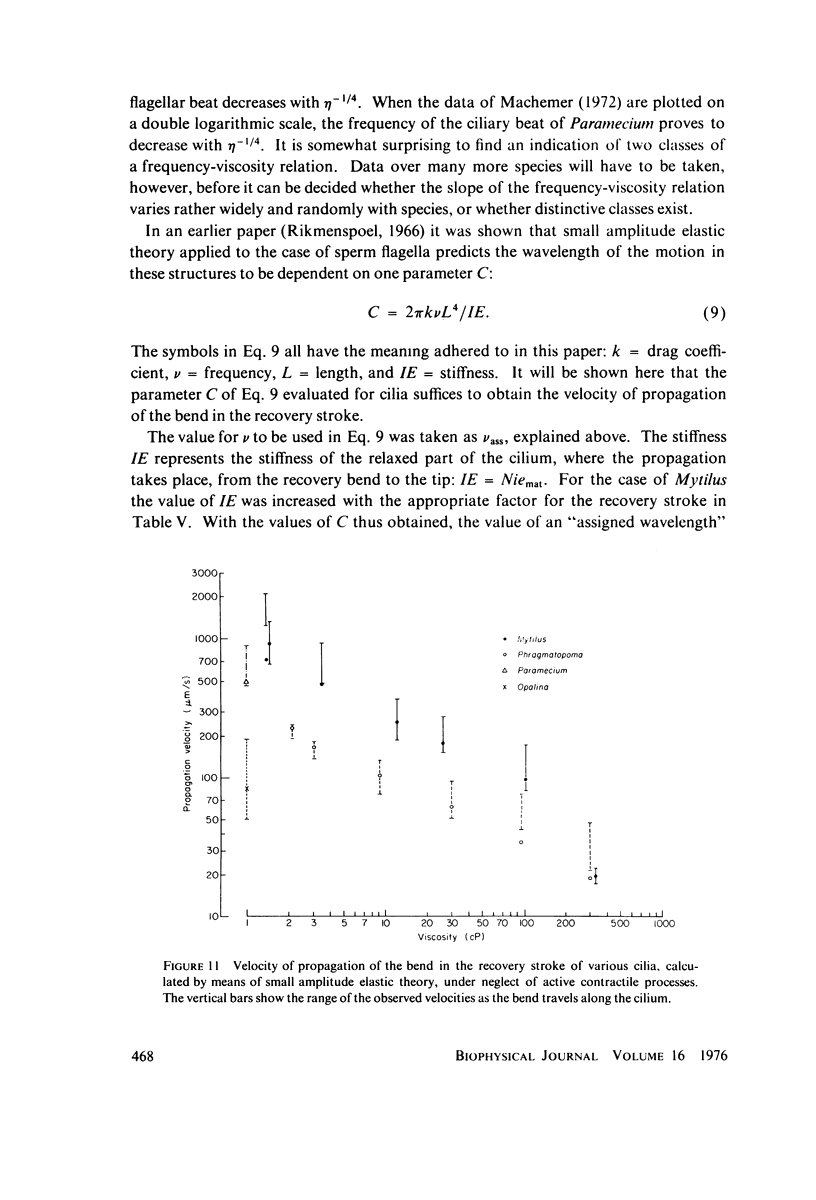


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baba S. A. Flexural rigidity and elastic constant of cilia. J Exp Biol. 1972 Apr;56(2):459–467. doi: 10.1242/jeb.56.2.459. [DOI] [PubMed] [Google Scholar]
- Blake J. R., Sleigh M. A. Mechanics of ciliary locomotion. Biol Rev Camb Philos Soc. 1974 Feb;49(1):85–125. doi: 10.1111/j.1469-185x.1974.tb01299.x. [DOI] [PubMed] [Google Scholar]
- Eckert R. Bioelectric control of ciliary activity. Science. 1972 May 5;176(4034):473–481. doi: 10.1126/science.176.4034.473. [DOI] [PubMed] [Google Scholar]
- GIBBONS I. R. The relationship between the fine structure and direction of beat in gill cilia of a lamellibranch mollusc. J Biophys Biochem Cytol. 1961 Oct;11:179–205. doi: 10.1083/jcb.11.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horridge G. A., Tamm S. L. Critical point drying for scanning electron microscopic sthdy of ciliary motion. Science. 1969 Feb 21;163(3869):817–818. doi: 10.1126/science.163.3869.817. [DOI] [PubMed] [Google Scholar]
- Machemer H. Ciliary activity and the origin of metachrony in Paramecium: effects of increased viscosity. J Exp Biol. 1972 Aug;57(1):239–259. doi: 10.1242/jeb.57.1.239. [DOI] [PubMed] [Google Scholar]
- RIKMENSPOEL R., van HERPEN, EIJKHOUT P. Cinematographic observations of the movements of bull sperm cells. Phys Med Biol. 1960 Oct;5:167–181. doi: 10.1088/0031-9155/5/2/306. [DOI] [PubMed] [Google Scholar]
- Rikmenspoel R. Contractile mechanisms in flagella. Biophys J. 1971 May;11(5):446–463. doi: 10.1016/S0006-3495(71)86227-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikmenspoel R. Elastic properties of the sea urchin sperm flagellum. Biophys J. 2008 Dec 31;6(4):471–479. doi: 10.1016/S0006-3495(66)86670-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikmenspoel R., Jacklet A. C., Orris S. E., Lindemann C. B. Control of bull sperm motility. Effects of viscosity, KCN and thiourea. J Mechanochem Cell Motil. 1973 May;2(1):7–24. [PubMed] [Google Scholar]
- Rikmenspoel R., Rudd W. G. The contractile mechanism in cilia. Biophys J. 1973 Sep;13(9):955–993. doi: 10.1016/S0006-3495(73)86037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikmenspoel R., Sleigh M. A. Bending moments and elastic constants in cilia. J Theor Biol. 1970 Jul;28(1):81–100. doi: 10.1016/0022-5193(70)90065-2. [DOI] [PubMed] [Google Scholar]



