Abstract
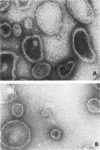
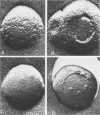
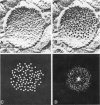
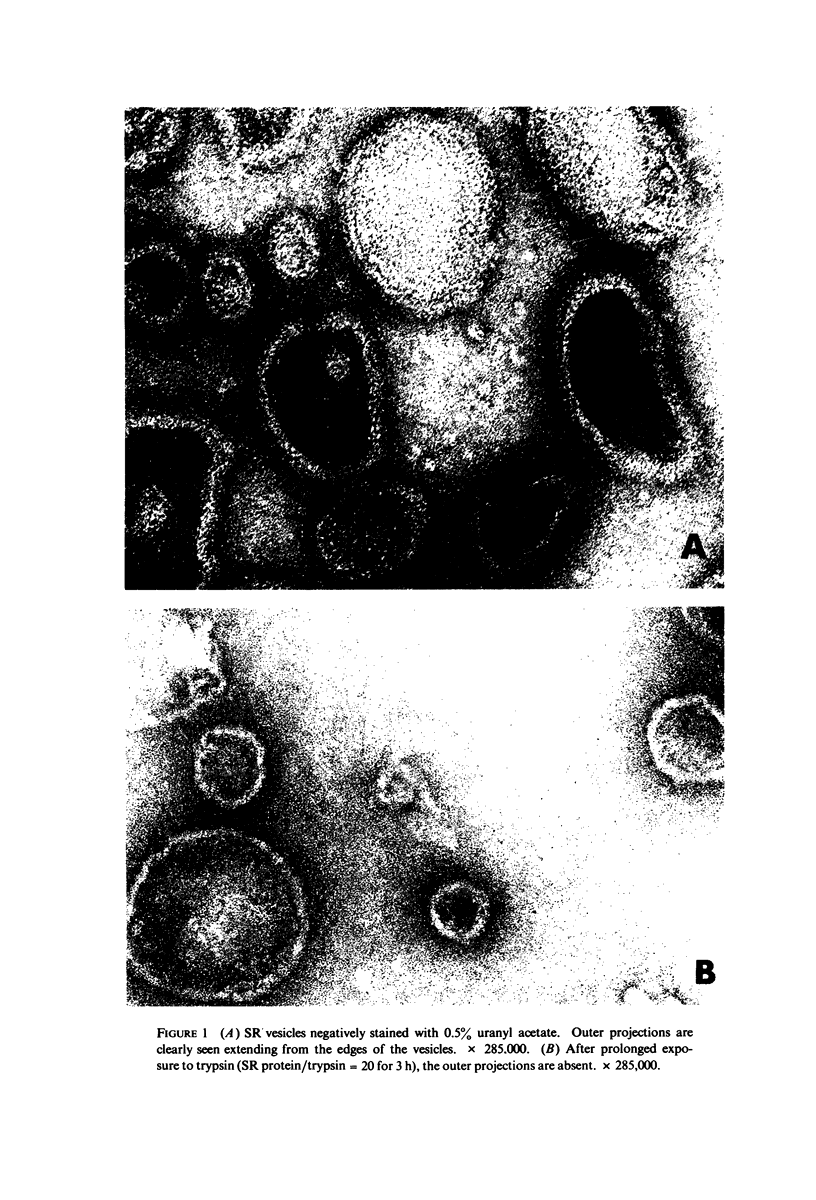
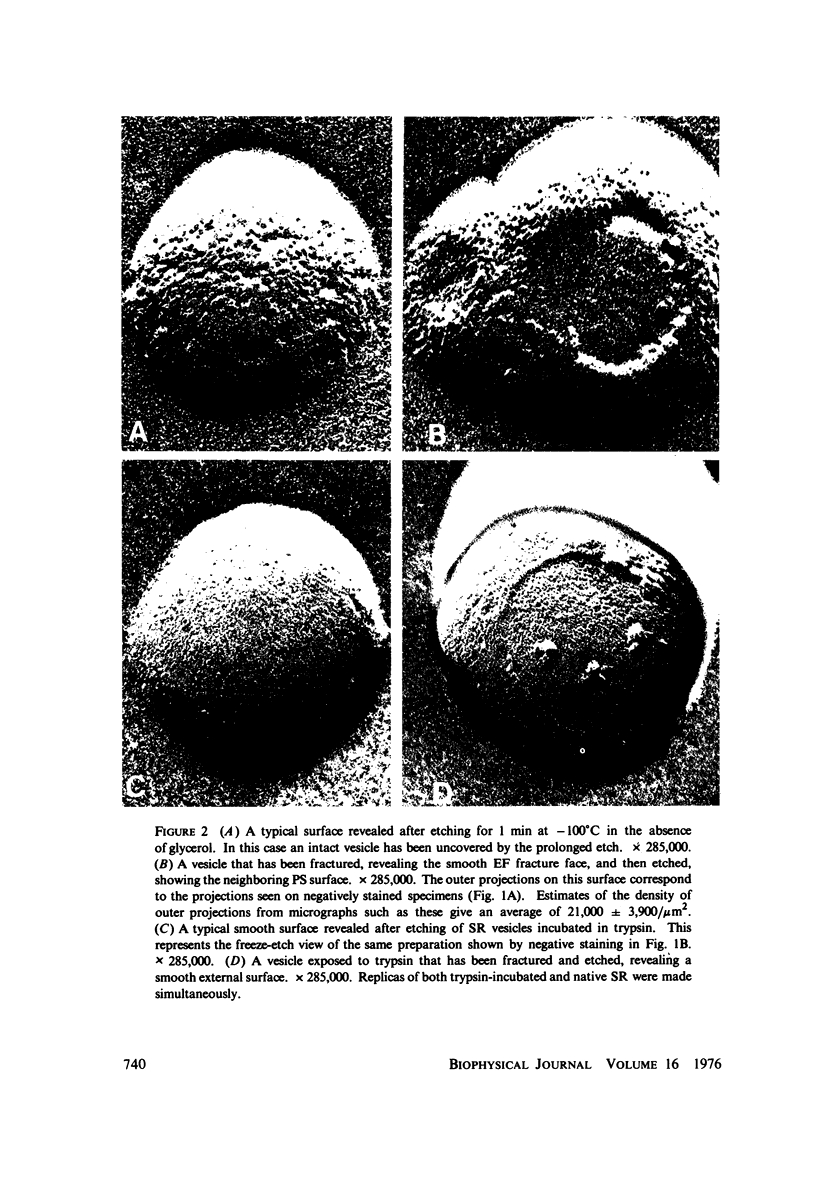
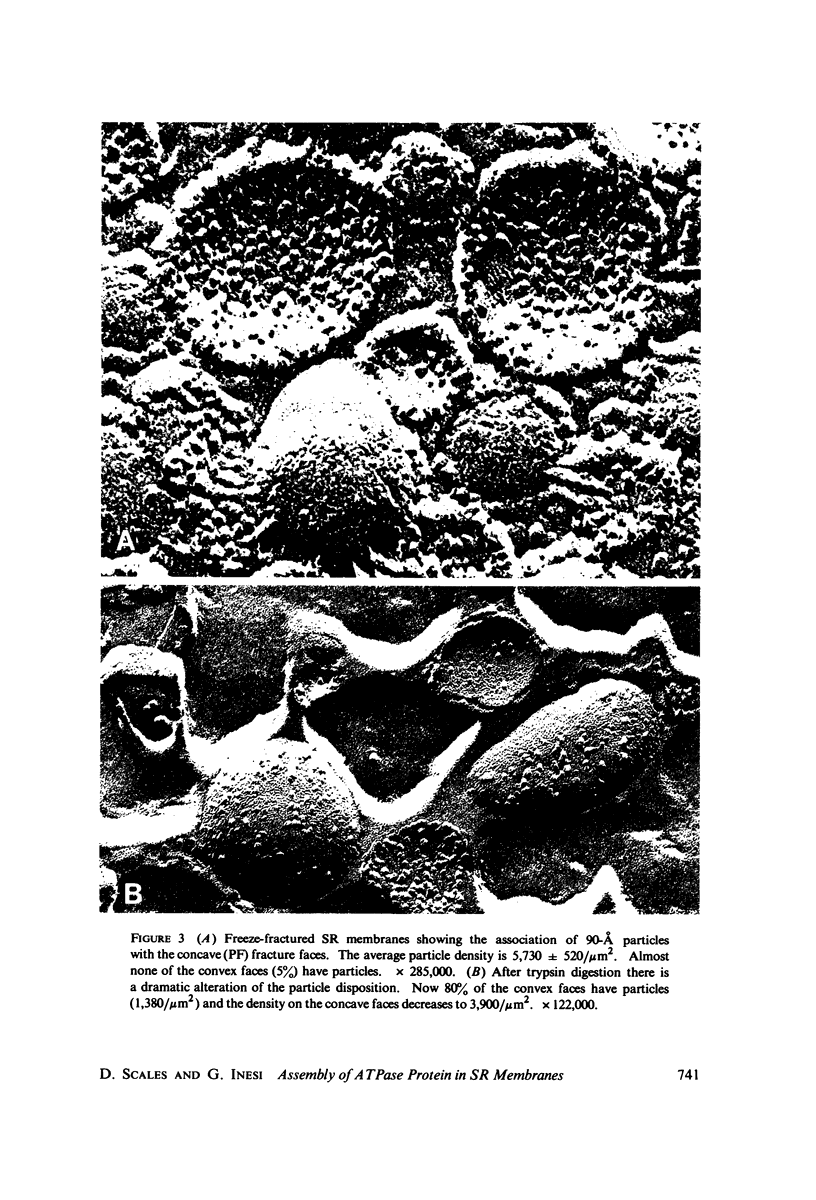
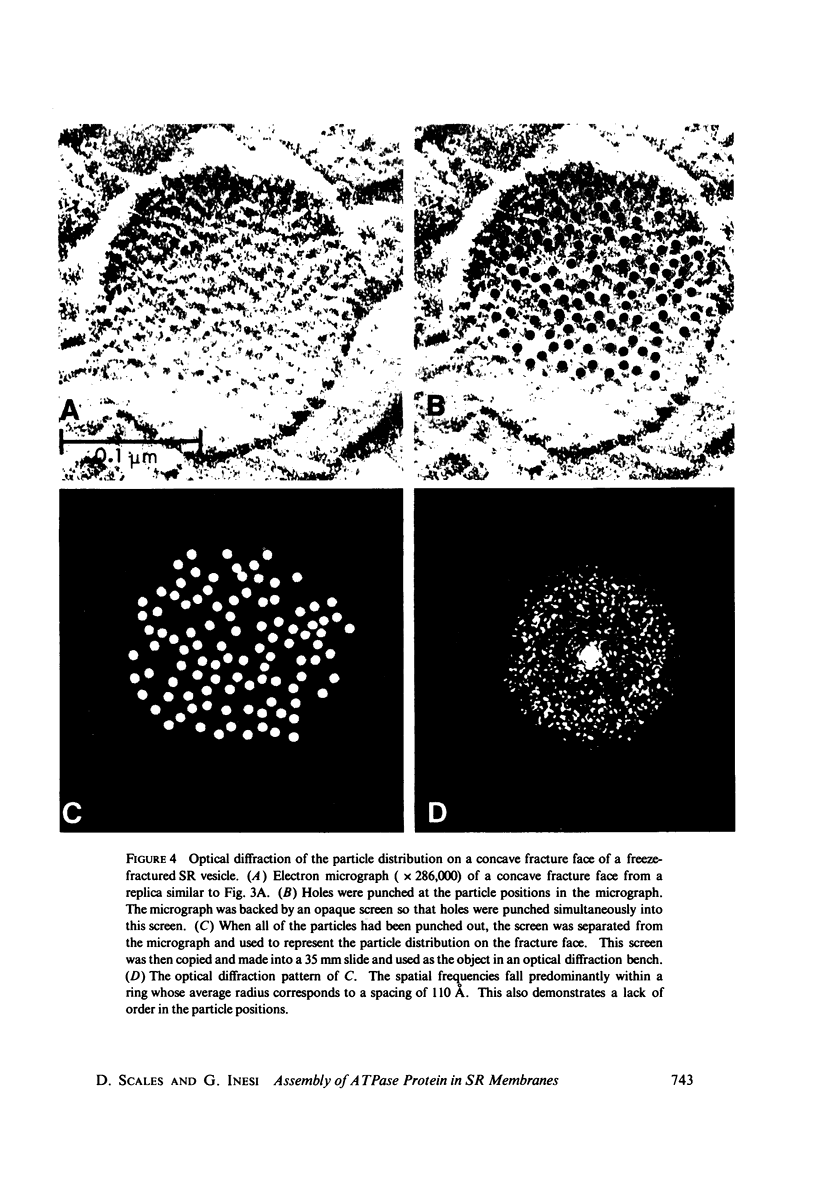
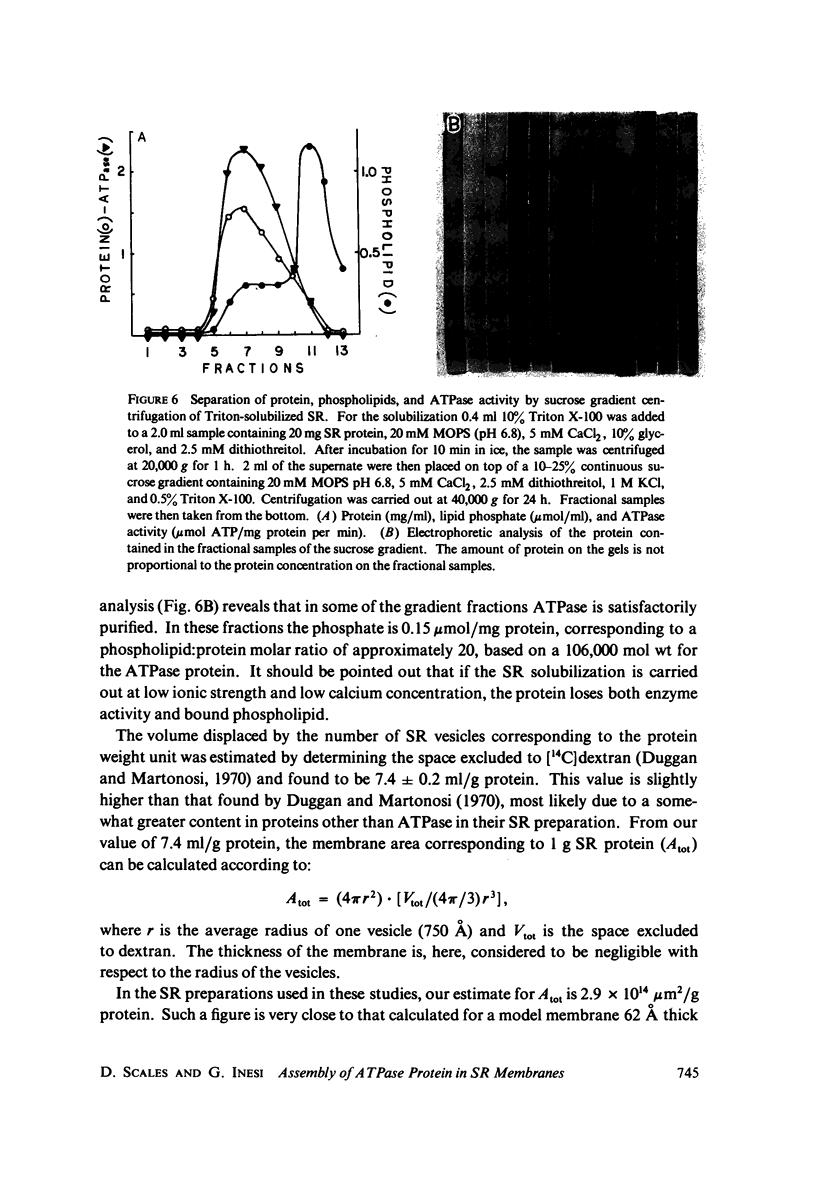
Three specimen preparation techniques for electron microscopy were used to investigate the incorporation of the ATPase polypeptide chains in the membranes of fragmented sarcoplasmic reticulum (SR) obtained from rabbit skeletal muscle. Observations were made of both normal vesicles and vesicles exposed to trypsin, which is known to cleave the ATPase protein and to alter the ultrastructure of the vesicles in predictable ways. Freeze-fracture replicas reveal the typical 90-A particles on the concave (PF) faces with a density of 5,730 +/- 520/mum2. On the other hand both negatively stained and deeply etched preparations display outer projections, which are absent on trypsin-incubated vesicles. The etched specimens afford for the first time top views of the vesicles in the absence of any stain. These views reveal outer projections on the PS surface with a density of 21,000 +/- 3,900/mum2, a value nearly approximating the density of the ATPase polypeptide chains (106,000 mol wt) calculated on the basis of protein and membrane area determinations. On the other hand, this value is three to four times higher than that found for the density of the 90-A particles on the concave fracture faces. Since both outer projections and 90-A particles are identified with the ATPase protein, it is suggested that the ATPase polypeptide chains are amphiphilic molecules, with polar ends protruding individually as outer projections on the surface of the vesicles, and hydrophobic ends appearing as 90-A particles on the concave fracture faces. The discrepancy between the densities of the outer projections and the 90-A particles may be attributed either to variable penetration of the polypeptide chains into the membrane bilayer, or to formation of oligomers containing three or four hydrophobic ends and appearing as single 90-A particles. Each ATPase chain forms a complex with 20-30 phospholipid molecules. The remaining phospholipids (approximately 70% of the total SR phospholipids) account for less than half the membrane volume. It is proposed that the outer leaflet of the SR membrane is prevalently composed of the ATPase lipoprotein complex, and the inner leaflet is mostly a phospholipid monolayer.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baskin R. J. Ultrastructure and calcium transport in crustacean muscle microsomes. J Cell Biol. 1971 Jan;48(1):49–60. doi: 10.1083/jcb.48.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin R. J. Ultrastructure and calcium transport in microsomes from developing muscle. J Ultrastruct Res. 1974 Dec;49(3):348–371. doi: 10.1016/s0022-5320(74)90050-1. [DOI] [PubMed] [Google Scholar]
- Davis D. G., Inesi G. Proton nuclear magnetic resonance studies of sarcoplasmic reticulum membranes. Correlation of the temperature-dependent Ca 2+ efflux with a reversible structural transition. Biochim Biophys Acta. 1971 Jul 6;241(1):1–8. doi: 10.1016/0005-2736(71)90297-5. [DOI] [PubMed] [Google Scholar]
- Deamer D. W., Baskin R. J. Ultrastructure of sarcoplasmic reticulum preparations. J Cell Biol. 1969 Jul;42(1):296–307. doi: 10.1083/jcb.42.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan P. F., Martonosi A. Sarcoplasmic reticulum. IX. The permeability of sarcoplasmic reticulum membranes. J Gen Physiol. 1970 Aug;56(2):147–167. doi: 10.1085/jgp.56.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebashi S., Lipmann F. ADENOSINE TRIPHOSPHATE-LINKED CONCENTRATION OF CALCIUM IONS IN A PARTICULATE FRACTION OF RABBIT MUSCLE. J Cell Biol. 1962 Sep 1;14(3):389–400. doi: 10.1083/jcb.14.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eletr S., Inesi G. Phospholipid orientation in sarcoplasmic membranes: spin-label ESR and proton MNR studies. Biochim Biophys Acta. 1972 Sep 1;282(1):174–179. doi: 10.1016/0005-2736(72)90321-5. [DOI] [PubMed] [Google Scholar]
- FINCH J. T., KLUG A., STRETTON A. O. THE STRUCTURE OF THE "POLYHEADS" OF T4 BACTERIOPHAGE. J Mol Biol. 1964 Dec;10:570–575. doi: 10.1016/s0022-2836(64)80082-6. [DOI] [PubMed] [Google Scholar]
- Franzini-Armstron C. Freeze fracture of skeletal muscle from the Tarantula spider. Structural differentiations of sarcoplasmic reticulum and transverse tubular system membranes. J Cell Biol. 1974 May;61(2):501–513. doi: 10.1083/jcb.61.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASSELBACH W., MAKINOSE M. [The calcium pump of the "relaxing granules" of muscle and its dependence on ATP-splitting]. Biochem Z. 1961;333:518–528. [PubMed] [Google Scholar]
- Hardwicke P. M., Green N. M. The effect of delipidation on the adenosine triphosphatase of sarcoplasmic reticulum. Electron microscopy and physical properties. Eur J Biochem. 1974 Feb 15;42(1):183–193. doi: 10.1111/j.1432-1033.1974.tb03328.x. [DOI] [PubMed] [Google Scholar]
- Hasselbach W., Elfvin L. G. Structural and chemical asymmetry of the calcium-transporting membranes of the sarcotubular system as revealed by electron microscopy. J Ultrastruct Res. 1967 Mar;17(5):598–622. doi: 10.1016/s0022-5320(67)80143-6. [DOI] [PubMed] [Google Scholar]
- Inesi G. Active transport of calcium ion in sarcoplasmic membranes. Annu Rev Biophys Bioeng. 1972;1:191–210. doi: 10.1146/annurev.bb.01.060172.001203. [DOI] [PubMed] [Google Scholar]
- Inesi G., Asai H. Trypsin digestion of fragmented sarcoplasmic reticulum. Arch Biochem Biophys. 1968 Aug;126(2):469–477. doi: 10.1016/0003-9861(68)90431-1. [DOI] [PubMed] [Google Scholar]
- Inesi G., Scales D. Tryptic cleavage of sarcoplasmic reticulum protein. Biochemistry. 1974 Jul 30;13(16):3298–3306. doi: 10.1021/bi00713a019. [DOI] [PubMed] [Google Scholar]
- Jilka R. L., Martonosi A. N., Tillack T. W. Effect of the purified (Mg2+ + Ca2+)-activated ATPase of sarcoplasmic reticulum upon the passive Ca2+ permeability and ultrastructure of phospholipid vesicles. J Biol Chem. 1975 Sep 25;250(18):7511–7524. [PubMed] [Google Scholar]
- KLUG A., BERGER J. E. AN OPTICAL METHOD FOR THE ANALYSIS OF PERIODICITIES IN ELECTRON MICROGRAPHS, AND SOME OBSERVATIONS ON THE MECHANISM OF NEGATIVE STAINING. J Mol Biol. 1964 Dec;10:565–569. doi: 10.1016/s0022-2836(64)80081-4. [DOI] [PubMed] [Google Scholar]
- Liu S. C., Worthington C. R. Electron density levels of sarcoplasmic reticulum membranes. Arch Biochem Biophys. 1974 Jul;163(1):332–342. doi: 10.1016/0003-9861(74)90484-6. [DOI] [PubMed] [Google Scholar]
- MARTONOSI A. ROLE OF PHOSPHOLIPIDS IN ATPASE ACTIVITY AND CA TRANSPORT OF FRAGMENTED SARCOPLASMIC RETICULUM. Fed Proc. 1964 Sep-Oct;23:913–921. [PubMed] [Google Scholar]
- MacLennan D. H. Purification and properties of an adenosine triphosphatase from sarcoplasmic reticulum. J Biol Chem. 1970 Sep 10;245(17):4508–4518. [PubMed] [Google Scholar]
- MacLennan D. H., Wong P. T. Isolation of a calcium-sequestering protein from sarcoplasmic reticulum. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1231–1235. doi: 10.1073/pnas.68.6.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martonosi A., Halpin R. A. Sarcoplasmic reticulum. X. The protein composition of sarcoplasmic reticulum membranes. Arch Biochem Biophys. 1971 May;144(1):66–77. doi: 10.1016/0003-9861(71)90455-3. [DOI] [PubMed] [Google Scholar]
- Martonosi A. Membrane transport during development in animals. Biochim Biophys Acta. 1975 Oct 31;415(3):311–333. doi: 10.1016/0304-4157(75)90012-x. [DOI] [PubMed] [Google Scholar]
- McConnell H. M., Wright K. L., McFarland B. G. The fraction of the lipid in a biological membrane that is in a fluid state: a spin label assay. Biochem Biophys Res Commun. 1972 Apr 14;47(1):273–281. doi: 10.1016/s0006-291x(72)80039-1. [DOI] [PubMed] [Google Scholar]
- McFarland B. H., Inesi G. Solubilization of sarcoplasmic reticulum with Triton X-100. Arch Biochem Biophys. 1971 Aug;145(2):456–464. doi: 10.1016/s0003-9861(71)80005-x. [DOI] [PubMed] [Google Scholar]
- McFarland B. H., Inesi G. Studies of solubilized sarcoplasmic reticulum. Biochem Biophys Res Commun. 1970 Oct 9;41(1):239–243. doi: 10.1016/0006-291x(70)90494-8. [DOI] [PubMed] [Google Scholar]
- Meissner G., Fleischer S. Ca 2+ uptake in reconstituted sarcoplasmic reticulum vesicles. Biochem Biophys Res Commun. 1973 Jun 8;52(3):913–920. doi: 10.1016/0006-291x(73)91024-3. [DOI] [PubMed] [Google Scholar]
- Meissner G., Fleischer S. Characterization of sarcoplasmic reticulum from skeletal muscle. Biochim Biophys Acta. 1971 Aug 13;241(2):356–378. doi: 10.1016/0005-2736(71)90036-8. [DOI] [PubMed] [Google Scholar]
- Meissner G. Isolation and characterization of two types of sarcoplasmic reticulum vesicles. Biochim Biophys Acta. 1975 Apr 21;389(1):51–68. doi: 10.1016/0005-2736(75)90385-5. [DOI] [PubMed] [Google Scholar]
- Ostwald T. J., MacLennan D. H. Isolation of a high affinity calcium-binding protein from sarcoplasmic reticulum. J Biol Chem. 1974 Feb 10;249(3):974–979. [PubMed] [Google Scholar]
- Packer L., Mehard C. W., Meissner G., Zahler W. L., Fleischer S. The structural role of lipids in mitochondrial and sarcoplasmic reticulum membranes. Freeze-fracture electron microscopy studies. Biochim Biophys Acta. 1974 Sep 6;363(2):159–181. doi: 10.1016/0005-2736(74)90056-x. [DOI] [PubMed] [Google Scholar]
- Racker E. Reconstitution of a calcium pump with phospholipids and a purified Ca ++ - adenosine triphosphatase from sacroplasmic reticulum. J Biol Chem. 1972 Dec 25;247(24):8198–8200. [PubMed] [Google Scholar]
- Sarzala M. G., Pilarska M., Zubrzycka E., Michalak M. Changes in the structure, composition and function of sarcoplasmic-reticulum membrane during development. Eur J Biochem. 1975 Sep 1;57(1):25–34. doi: 10.1111/j.1432-1033.1975.tb02273.x. [DOI] [PubMed] [Google Scholar]
- Stewart P. S., MacLennan D. H. Surface particles of sarcoplasmic reticulum membranes. Structural features of the adenosine triphosphatase. J Biol Chem. 1974 Feb 10;249(3):985–993. [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Green N. M. Studies on the location and orientation of proteins in the sarcoplasmic reticulum. Eur J Biochem. 1973 Dec 17;40(2):403–413. doi: 10.1111/j.1432-1033.1973.tb03209.x. [DOI] [PubMed] [Google Scholar]
- Tillack T. W., Boland R., Martonosi A. The ultrastructure of developing sarcoplasmic reticulum. J Biol Chem. 1974 Jan 25;249(2):624–633. [PubMed] [Google Scholar]
- Warren G. B., Toon P. A., Birdsall N. J., Lee A. G., Metcalfe J. C. Complete control of the lipid environment of membrane-bound proteins: application to a calcium transport system. FEBS Lett. 1974 Apr 15;41(1):122–124. doi: 10.1016/0014-5793(74)80969-5. [DOI] [PubMed] [Google Scholar]