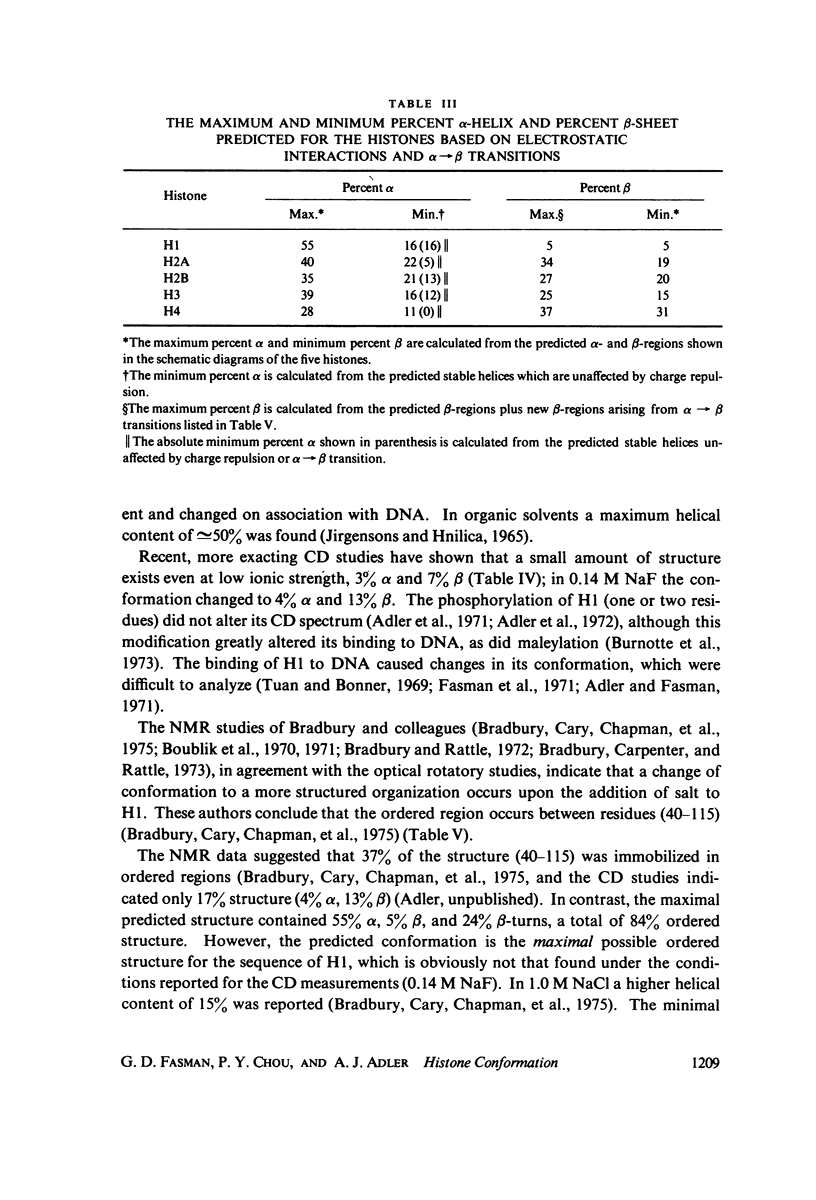
Abstract
The secondary structures of the histones, H1, H2A, H2B, H3, and H4 have been predicted utilizing the predictive scheme of Chou and Fasman (Biochemistry 13:211, 222[1974]) and a new set of conformational parameters based on the X-ray data of 29 protein structures. The alpha-helical, beta-sheet, reverse beta-turns, and random coil regions of these proteins are carefully delineated. Structures are specified which are most probably under various environmental conditions, i.e., for changes in ionic strength, association between histones and in association with DNA. Potential conformational changes within these histones are also predicted.
Full text
PDF
































Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler A. J., Fasman G. D. Circular dichroism studies of lysine-rich histone f-1-deoxyribonucleic acid complexes. Effect of salts and dioxane upon conformation. J Phys Chem. 1971 May 13;75(10):1516–1526. doi: 10.1021/j100680a024. [DOI] [PubMed] [Google Scholar]
- Adler A. J., Fasman G. D., Wangh L. J., Allfrey V. G. Altered conformational effects of naturally acetylated histone f2al (IV) in f2al-deoxyribonucleic acid complexes. Circular dichroism studies. J Biol Chem. 1974 May 10;249(9):2911–2914. [PubMed] [Google Scholar]
- Adler A. J., Fulmer A. W., Fasman G. D. Interaction of histone f2al fragments with deoxyribonucleic acid. Circular dichroism and thermal denaturation studies. Biochemistry. 1975 Apr 8;14(7):1445–1454. doi: 10.1021/bi00678a015. [DOI] [PubMed] [Google Scholar]
- Adler A. J., Langan T. A., Fasman G. D. Complexes of deoxyribonucleic acid with lysine-rich (f1) histone phosphorylated at two separate sites: circular dichroism studies. Arch Biochem Biophys. 1972 Dec;153(2):769–777. doi: 10.1016/0003-9861(72)90397-9. [DOI] [PubMed] [Google Scholar]
- Adler A. J., Moran E. C., Fasman G. D. Complexes of DNA with histones f2a2 and f3. Circular dichroism studies. Biochemistry. 1975 Sep 23;14(19):4179–4185. doi: 10.1021/bi00690a005. [DOI] [PubMed] [Google Scholar]
- Adler A. J., Ross D. G., Chen K., Stafford P. A., Woiszwillo M. J., Fasman G. D. Interaction of deoxyribonucleic acid with histone f2b and its half-molecules. Circular dichroism studies. Biochemistry. 1974 Jan 29;13(3):616–623. doi: 10.1021/bi00700a033. [DOI] [PubMed] [Google Scholar]
- Adler A. J., Schaffhausen B., Langan T. A., Fasman G. D. Altered conformational effects of phosphorylated lysine-rich histone (f-1) in f-1--deoxyribonucleic acid complexes. Circular dichroism and immunological studies. Biochemistry. 1971 Mar 2;10(5):909–913. doi: 10.1021/bi00781a028. [DOI] [PubMed] [Google Scholar]
- Anderson R. A., Nakashima Y., Coleman J. E. Chemical modifications of functional residues of fd gene 5 DNA-binding protein. Biochemistry. 1975 Mar 11;14(5):907–917. doi: 10.1021/bi00676a006. [DOI] [PubMed] [Google Scholar]
- Baldwin J. P., Boseley P. G., Bradbury E. M., Ibel K. The subunit structure of the eukaryotic chromosome. Nature. 1975 Jan 24;253(5489):245–249. doi: 10.1038/253245a0. [DOI] [PubMed] [Google Scholar]
- Boublik M., Bradbury E. M., Crane-Robinson C., Rattle H. W. Proton magnetic resonance ttudies of the interactions of histones F1 and F2B with DNA. Nat New Biol. 1971 Feb 3;229(5):149–150. doi: 10.1038/newbio229149a0. [DOI] [PubMed] [Google Scholar]
- Boublík M., Bradbury E. M., Crane-Robinson C. An investigation of the conformational changes in histones F1 and F2a1 by proton magnetic resonance spectroscopy. Eur J Biochem. 1970 Jul;14(3):486–497. doi: 10.1111/j.1432-1033.1970.tb00315.x. [DOI] [PubMed] [Google Scholar]
- Boublík M., Bradbury E. M., Crane-Robinson C., Johns E. W. An investigation of the conformational changes of histone F2b by high resolution nuclear magnetic resonance. Eur J Biochem. 1970 Nov;17(1):151–159. doi: 10.1111/j.1432-1033.1970.tb01147.x. [DOI] [PubMed] [Google Scholar]
- Bradbury E. M., Carpenter B. G., Rattle H. W. Magnetic resonance studies of deoxyribonucleoprotein. Nature. 1973 Jan 12;241(5385):123–126. doi: 10.1038/241123a0. [DOI] [PubMed] [Google Scholar]
- Bradbury E. M., Cary P. D., Chapman G. E., Crane-Robinson C., Danby S. E., Rattle H. W., Boublik M., Palau J., Aviles F. J. Studies on the role and mode of operation of the very-lysine-rich histone H1 (F1) in eukaryote chromatin. The conformation of histone H1. Eur J Biochem. 1975 Apr 1;52(3):605–613. doi: 10.1111/j.1432-1033.1975.tb04032.x. [DOI] [PubMed] [Google Scholar]
- Bradbury E. M., Cary P. D., Crane-Robinson C., Rattle H. W., Boublik M., Sautière P. Conformations and interactions of histone H2A (F2A2, ALK). Biochemistry. 1975 May 6;14(9):1876–1885. doi: 10.1021/bi00680a012. [DOI] [PubMed] [Google Scholar]
- Bradbury E. M., Cary P. D., Crane-Robinson C., Rattle H. W. Conformations and interactions of histones and their role in chromosome structure. Ann N Y Acad Sci. 1973 Dec 31;222:266–289. doi: 10.1111/j.1749-6632.1973.tb15268.x. [DOI] [PubMed] [Google Scholar]
- Bradbury E. M., Cary P. D., Crane-Robinson C., Riches P. L., Johns E. W. Nuclear-magnetic resonance and optical-spectroscopic studies of conformation and interactions in the cleaved halves of histone F2B. Eur J Biochem. 1972 Apr 24;26(4):482–489. doi: 10.1111/j.1432-1033.1972.tb01790.x. [DOI] [PubMed] [Google Scholar]
- Bradbury E. M., Chapman G. E., Danby S. E., Hartman P. G., Riches P. L. Studies on the role and mode of operation of the very-lysine-rich histone H1 (F1) in eukaryote chromatin. The properties of the N-terminal and C-terminal halves of histone H1. Eur J Biochem. 1975 Sep 15;57(2):521–528. doi: 10.1111/j.1432-1033.1975.tb02327.x. [DOI] [PubMed] [Google Scholar]
- Bradbury E. M., Rattle H. W. Simple computer-aided approach for the analyses of the nuclear-magnetic-resonance spectra of histones. Fractions F1, Fsa1, F2B, cleaved halves of F2B and F2B-DNA. Eur J Biochem. 1972 May 23;27(2):270–281. doi: 10.1111/j.1432-1033.1972.tb01836.x. [DOI] [PubMed] [Google Scholar]
- Burnotte J., Stollar B. D., Fasman G. D. Immunological and circular dichroism studies of maleylated f-1 (A) histone and complexes with DNA. Arch Biochem Biophys. 1973 Apr;155(2):428–435. doi: 10.1016/0003-9861(73)90133-1. [DOI] [PubMed] [Google Scholar]
- Bustin M., Rall S. C., Stellwagen R. H., Cole R. D. Histone structure: asymmetric distribution of lysine residues in lysine-rich histone. Science. 1969 Jan 24;163(3865):391–393. doi: 10.1126/science.163.3865.391-a. [DOI] [PubMed] [Google Scholar]
- Chen Y. H., Yang J. T., Martinez H. M. Determination of the secondary structures of proteins by circular dichroism and optical rotatory dispersion. Biochemistry. 1972 Oct 24;11(22):4120–4131. doi: 10.1021/bi00772a015. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Adler A. J., Fasman G. D. Conformational prediction and circular dichroism studies on the lac repressor. J Mol Biol. 1975 Jul 25;96(1):29–45. doi: 10.1016/0022-2836(75)90180-1. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Conformational parameters for amino acids in helical, beta-sheet, and random coil regions calculated from proteins. Biochemistry. 1974 Jan 15;13(2):211–222. doi: 10.1021/bi00699a001. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. The conformation of glucagon: predictions and consequences. Biochemistry. 1975 Jun 3;14(11):2536–2541. doi: 10.1021/bi00682a037. [DOI] [PubMed] [Google Scholar]
- Clark V. M., Lilley D. M., Howarth O. W., Richards B. M., Pardon J. F. The structure and properties of histone F2a comprising the heterologous group F2a1 and F2a2 studied by 13C nuclear magnetic resonance. Nucleic Acids Res. 1974 Jul;1(7):865–880. doi: 10.1093/nar/1.7.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Anna J. A., Jr, Isenberg I. A complex of histones IIb2 and IV. Biochemistry. 1973 Mar 13;12(6):1035–1043. doi: 10.1021/bi00730a003. [DOI] [PubMed] [Google Scholar]
- D'Anna J. A., Jr, Isenberg I. A histone cross-complexing pattern. Biochemistry. 1974 Nov 19;13(24):4992–4997. doi: 10.1021/bi00721a019. [DOI] [PubMed] [Google Scholar]
- D'Anna J. A., Jr, Isenberg I. Conformational changes of histone ARE(F3, III). Biochemistry. 1974 Nov 19;13(24):4987–4992. doi: 10.1021/bi00721a018. [DOI] [PubMed] [Google Scholar]
- D'Anna J. A., Jr, Isenberg I. Conformational changes of histone LAK (f2a2). Biochemistry. 1974 May 7;13(10):2093–2098. doi: 10.1021/bi00707a015. [DOI] [PubMed] [Google Scholar]
- D'Anna J. A., Jr, Isenberg I. Fluorescence anisotropy and circular dichroism study of conformational changes in histone IIb2. Biochemistry. 1972 Oct 24;11(22):4017–4025. doi: 10.1021/bi00772a002. [DOI] [PubMed] [Google Scholar]
- DeLange R. J., Fambrough D. M., Smith E. L., Bonner J. Calf and pea histone IV. II. The complete amino acid sequence of calf thymus histone IV; presence of epsilon-N-acetyllysine. J Biol Chem. 1969 Jan 25;244(2):319–334. [PubMed] [Google Scholar]
- DeLange R. J., Hooper J. A., Smith E. L. Complete amino-acid sequence of calf-thymus histone 3. Proc Natl Acad Sci U S A. 1972 Apr;69(4):882–884. doi: 10.1073/pnas.69.4.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diggle J. H., Peacocke A. R. The molecular weights and association of the histones of chicken erythrocytes. FEBS Lett. 1971 Oct 15;18(1):138–140. doi: 10.1016/0014-5793(71)80429-5. [DOI] [PubMed] [Google Scholar]
- Dunn B. M., Chaiken I. M. Relationship between alpha-helical propensity and formation of the ribonuclease-S complex. J Mol Biol. 1975 Jul 15;95(4):497–511. doi: 10.1016/0022-2836(75)90313-7. [DOI] [PubMed] [Google Scholar]
- Ewars P. A., Sooter K. V. Ultracentrifuge tudies of histone fractions from calf thymus deoxyribonucleoprotein. Biochem J. 1969 Sep;114(2):227–235. doi: 10.1042/bj1140227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasman G. D., Schaffhausen B., Goldsmith L., Adler A. Conformational changes associated with f-1 histone-deoxyribonucleic acid complexes. Circular dichroism studies. Biochemistry. 1970 Jul 7;9(14):2814–2822. doi: 10.1021/bi00816a010. [DOI] [PubMed] [Google Scholar]
- Fasman G. D., Valenzuela M. S., Adler A. J. Complexes of deoxyribonucleic acid with fragments of lysine-rich histone (f-1). Circular dichroism studies. Biochemistry. 1971 Sep 28;10(20):3795–3801. doi: 10.1021/bi00796a024. [DOI] [PubMed] [Google Scholar]
- Garel A., Kovacs A. M., Champagne M., Daune M. Comparison between histones FV and F2a2 of chicken erythrocyte. I. Structure, stability and conformation of the free proteins. Biochim Biophys Acta. 1975 Jun 2;395(1):5–15. doi: 10.1016/0005-2787(75)90228-2. [DOI] [PubMed] [Google Scholar]
- Garel A., Kovacs A. M., Champagne M., Daune M. Comparison between histones FV and F2a2 of chicken erythrocyte. II. Interaction with homologous DNA. Biochim Biophys Acta. 1975 Jun 2;395(1):16–27. doi: 10.1016/0005-2787(75)90229-4. [DOI] [PubMed] [Google Scholar]
- Greenfield N., Fasman G. D. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry. 1969 Oct;8(10):4108–4116. doi: 10.1021/bi00838a031. [DOI] [PubMed] [Google Scholar]
- Hardman K. D., Ainsworth C. F. Structure of concanavalin A at 2.4-A resolution. Biochemistry. 1972 Dec 19;11(26):4910–4919. doi: 10.1021/bi00776a006. [DOI] [PubMed] [Google Scholar]
- Hyde J. E., Walker I. O. Covalent cross-linking of histones in chromatin. FEBS Lett. 1975 Feb 1;50(2):150–154. doi: 10.1016/0014-5793(75)80477-7. [DOI] [PubMed] [Google Scholar]
- Iwai K., Ishikawa K., Hayashi H. Amino-acid sequence of slightly lysine-rich histone. Nature. 1970 Jun 13;226(5250):1056–1058. doi: 10.1038/2261056b0. [DOI] [PubMed] [Google Scholar]
- Jirgensons B., Hnilica L. S. The conformational changes of calf-thymus histone fractions as determined by the optical rotary dispersion. Biochim Biophys Acta. 1965 Sep 27;109(1):241–249. doi: 10.1016/0926-6585(65)90108-1. [DOI] [PubMed] [Google Scholar]
- Jones G. M., Rall S. C., Cole R. D. Extension of the amino acid sequence of a lysine-rich histone. J Biol Chem. 1974 Apr 25;249(8):2548–2553. [PubMed] [Google Scholar]
- Kelley R. I. Isolation of a histone IIb1-IIb2 complex. Biochem Biophys Res Commun. 1973 Oct 15;54(4):1588–1594. doi: 10.1016/0006-291x(73)91168-6. [DOI] [PubMed] [Google Scholar]
- Kornberg R. D. Chromatin structure: a repeating unit of histones and DNA. Science. 1974 May 24;184(4139):868–871. doi: 10.1126/science.184.4139.868. [DOI] [PubMed] [Google Scholar]
- Kornberg R. D., Thomas J. O. Chromatin structure; oligomers of the histones. Science. 1974 May 24;184(4139):865–868. doi: 10.1126/science.184.4139.865. [DOI] [PubMed] [Google Scholar]
- Lewis P. N., Bradbury M., Crane-Robinson C. Ionic strength induced structure in histone H4 and its fragments. Biochemistry. 1975 Jul 29;14(15):3391–3400. doi: 10.1021/bi00686a016. [DOI] [PubMed] [Google Scholar]
- Li H. J., Isenberg I., Johnson W. C., Jr Absorption and circular dichroism studies on nucleohistone IV. Biochemistry. 1971 Jun 22;10(13):2587–2593. doi: 10.1021/bi00789a027. [DOI] [PubMed] [Google Scholar]
- Li H. J., Wickett R., Craig A. M., Isenberg I. Conformational changes in histone IV. Biopolymers. 1972 Feb;11(2):375–397. doi: 10.1002/bip.1972.360110206. [DOI] [PubMed] [Google Scholar]
- Lim V. I. Structural principles of the globular organization of protein chains. A stereochemical theory of globular protein secondary structure. J Mol Biol. 1974 Oct 5;88(4):857–872. doi: 10.1016/0022-2836(74)90404-5. [DOI] [PubMed] [Google Scholar]
- Matthews B. W. Comparison of the predicted and observed secondary structure of T4 phage lysozyme. Biochim Biophys Acta. 1975 Oct 20;405(2):442–451. doi: 10.1016/0005-2795(75)90109-9. [DOI] [PubMed] [Google Scholar]
- Maxfield F. R., Scheraga H. A. The effect of neighboring charges on the helix forming ability of charged amino acids in proteins. Macromolecules. 1975 Jul-Aug;8(4):491–493. doi: 10.1021/ma60046a022. [DOI] [PubMed] [Google Scholar]
- Ogawa Y., Quagliarotti G., Jordan J., Taylor C. W., Starbuck W. C., Busch H. Structural analysis of the glycine-rich, arginine-rich histone. 3. Sequence of the amino-terminal half of the molecule containing the modified lysine residues and the total sequence. J Biol Chem. 1969 Aug 25;244(16):4387–4392. [PubMed] [Google Scholar]
- Oh Y. H. Spectroscopic studies of five purified histones from calf thymus. J Biol Chem. 1970 Dec 10;245(23):6404–6416. [PubMed] [Google Scholar]
- Palau J., Padrós E. Behaviour of tyrosyl residues of calf-thymus histone F3. Difference-spectroscopy studies. Eur J Biochem. 1975 Apr 1;52(3):555–560. doi: 10.1111/j.1432-1033.1975.tb04026.x. [DOI] [PubMed] [Google Scholar]
- Palau J., Puigdoménech P. The structural code for proteins: zonal distribution of amino acid residues and stabilization of helices by hydrophobic triplets. J Mol Biol. 1974 Sep 15;88(2):457–469. doi: 10.1016/0022-2836(74)90495-1. [DOI] [PubMed] [Google Scholar]
- Pekary A. E., Chan S. I., Hsu C. J., Wagner T. E. Nuclear magnetic resonance studies on the solution conformation of histone IV fragments obtained by cyanogen bromide cleavage. Biochemistry. 1975 Mar 25;14(6):1184–1189. doi: 10.1021/bi00677a013. [DOI] [PubMed] [Google Scholar]
- Pekary A. E., Li H. J., Chan S. I., Hsu C. J., Wagner T. E. Nuclear magnetic resonance studies of histone IV solution conformation. Biochemistry. 1975 Mar 25;14(6):1177–1184. doi: 10.1021/bi00677a012. [DOI] [PubMed] [Google Scholar]
- Rall S. C., Cole R. D. Amino acid sequence and sequence variability of the amino-terminal regions of lysine-rich histones. J Biol Chem. 1971 Dec 10;246(23):7175–7190. [PubMed] [Google Scholar]
- Roark D. E., Geoghegan T. E., Keller G. H. A two-subunit histone complex from calf thymus. Biochem Biophys Res Commun. 1974 Jul 24;59(2):542–547. doi: 10.1016/s0006-291x(74)80014-8. [DOI] [PubMed] [Google Scholar]
- Rubin R. L., Moudrianakis E. N. The F3-F2a1 complex as a unit in the self-assembly of nucleoproteins. Biochemistry. 1975 Apr 22;14(8):1718–1726. doi: 10.1021/bi00679a026. [DOI] [PubMed] [Google Scholar]
- Sautière P., Tyrou D., Laine B., Mizon J., Ruffin P., Biserte G. Covalent structure of calf-thymus ALK-histone. Eur J Biochem. 1974 Feb 1;41(3):563–576. doi: 10.1111/j.1432-1033.1974.tb03298.x. [DOI] [PubMed] [Google Scholar]
- Schulz G. E., Barry C. D., Friedman J., Chou P. Y., Fasman G. D., Finkelstein A. V., Lim V. I., Pititsyn O. B., Kabat E. A., Wu T. T. Comparison of predicted and experimentally determined secondary structure of adenyl kinase. Nature. 1974 Jul 12;250(462):140–142. doi: 10.1038/250140a0. [DOI] [PubMed] [Google Scholar]
- Senior M. B., Olins D. E. Effect of formaldehyde on the circular dichroism of chicken erythrocyte chromatin. Biochemistry. 1975 Jul 29;14(15):3332–3337. doi: 10.1021/bi00686a007. [DOI] [PubMed] [Google Scholar]
- Shih T. Y., Fasman G. D. Circular dichroism studies of deoxyribonucleic acid complexes with arginine-rich histone IV (f2al). Biochemistry. 1971 Apr 27;10(9):1675–1683. doi: 10.1021/bi00785a027. [DOI] [PubMed] [Google Scholar]
- Shih T. Y., Fasman G. D. Circular dichroism studies of histone-deoxyribonucleic acid complexes. A comparison of complexes with histone I (f-1), histone IV (f2al), and their mixtures. Biochemistry. 1972 Feb 1;11(3):398–404. doi: 10.1021/bi00753a016. [DOI] [PubMed] [Google Scholar]
- Skandrani E., Mizon J., Sautière P., Biserte G. Etude de la fraction F2b des histones de thymus de veau. Biochimie. 1972;54(10):1267–1272. doi: 10.1016/s0300-9084(72)80067-1. [DOI] [PubMed] [Google Scholar]
- Slayter H. S., Shih T. Y., Adler A. J., Fasman G. D. Electron microscopy and circular dichroism studies on chromatin. Biochemistry. 1972 Aug 1;11(16):3044–3054. doi: 10.1021/bi00766a016. [DOI] [PubMed] [Google Scholar]
- Smerdon M. J., Isenberg I. Conformational changes in histone GRK(f2a-1). Biochemistry. 1974 Sep 10;13(19):4046–4049. doi: 10.1021/bi00716a036. [DOI] [PubMed] [Google Scholar]
- Smerdon M. J., Isenberg I. The effect of temperature on histone GRK aggregation. Biochem Biophys Res Commun. 1973 Dec 10;55(3):1029–1034. doi: 10.1016/0006-291x(73)91245-x. [DOI] [PubMed] [Google Scholar]
- Snell C. R., Smyth D. G. Proinsulin: a proposed three-dimensional structure. J Biol Chem. 1975 Aug 25;250(16):6291–6295. [PubMed] [Google Scholar]
- Tuan D. Y., Bonner J. Optical absorbance and optical rotatory dispersion studies on calf thymus nucleohistone. J Mol Biol. 1969 Oct 14;45(1):59–76. doi: 10.1016/0022-2836(69)90209-5. [DOI] [PubMed] [Google Scholar]
- Van Lente F., Jackson J. F., Weintraub H. Identification of specific crosslinked histones after treatment of chromatin with formaldehyde. Cell. 1975 May;5(1):45–50. doi: 10.1016/0092-8674(75)90090-2. [DOI] [PubMed] [Google Scholar]
- Wagner T. E., Vandegrift V. Circular dichrosim studies of calf thymus Ca 2+ nucleohistone IV. Biochemistry. 1972 Apr 11;11(8):1431–1436. doi: 10.1021/bi00758a016. [DOI] [PubMed] [Google Scholar]
- Wickett R. R., Li H. J., Isenberg I. Salt effects on histone IV conformation. Biochemistry. 1972 Aug 1;11(16):2952–2957. doi: 10.1021/bi00766a005. [DOI] [PubMed] [Google Scholar]
- Wootton J. C. The coenzyme-binding domains of glutamate dehydrogenases. Nature. 1974 Dec 13;252(5484):542–546. doi: 10.1038/252542a0. [DOI] [PubMed] [Google Scholar]
- Yeoman L. C., Olson M. O., Sugano N., Jordan J. J., Taylor D. W., Starbuck W. C., Busch H. Amino acid sequence of the center of the arginine-lysine-rich histone from calf thymus. The total sequence. J Biol Chem. 1972 Oct 10;247(19):6018–6023. [PubMed] [Google Scholar]
- Ziccardi R., Schumaker V. Interaction of histone f2al with T7 deoxyribonucleic acid. Cooperativity of histone binding. Biochemistry. 1973 Aug 14;12(17):3231–3235. doi: 10.1021/bi00741a014. [DOI] [PubMed] [Google Scholar]


