Abstract
Fluorescence photobleaching recovery (FPR) denotes a method for measuring two-dimensional lateral mobility of fluorescent particles, for example, the motion of fluorescently labeled molecules in approximately 10 mum2 regions of a single cell surface. A small spot on the fluorescent surface is photobleached by a brief exposure to an intense focused laser beam, and the subsequent recovery of the fluorescence is monitored by the same, but attenuated, laser beam. Recovery occurs by replenishment of intact fluorophore in the bleached spot by lateral transport from the surrounding surface. We present the theoretical basis and some practical guidelines for simple, rigorous analysis of FPR experiments. Information obtainable from FPR experiments includes: (a) identification of transport process type, i.e. the admixture of random diffusion and uniform directed flow; (b) determination of the absolute mobility coefficient, i.e. the diffusion constant and/or flow velocity; and (c) the fraction of total fluorophore which is mobile. To illustrate the experimental method and to verify the theory for diffusion, we describe some model experiments on aqueous solutions of rhodamine 6G.
Full text
PDF


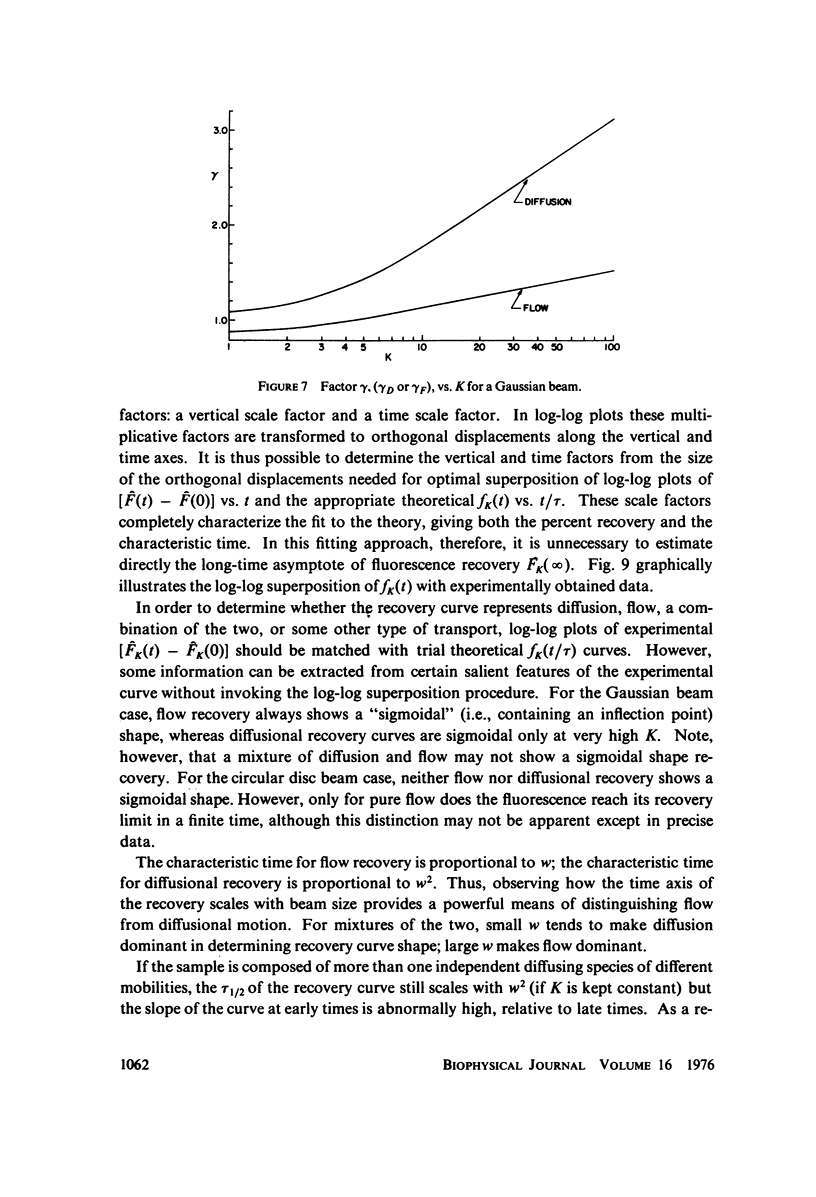


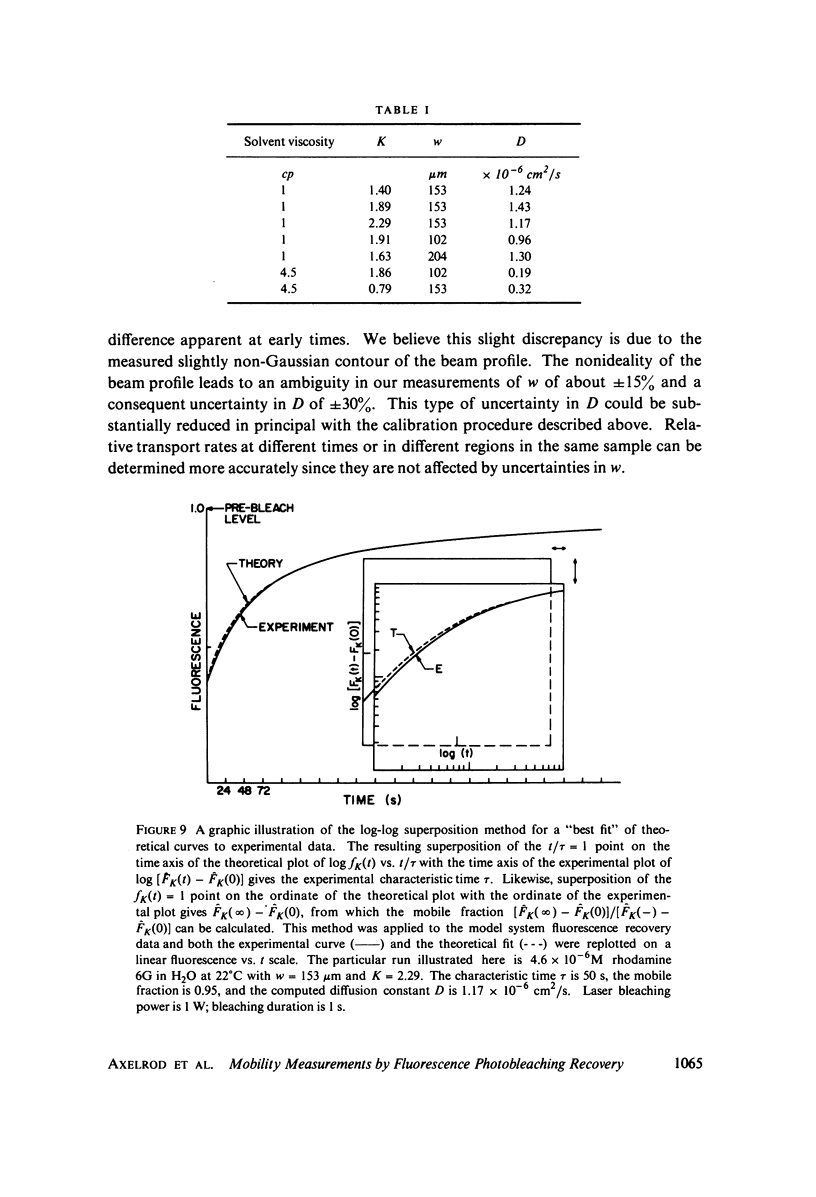




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Edidin M. Rotational and translational diffusion in membranes. Annu Rev Biophys Bioeng. 1974;3(0):179–201. doi: 10.1146/annurev.bb.03.060174.001143. [DOI] [PubMed] [Google Scholar]
- Edidin M., Zagyansky Y., Lardner T. J. Measurement of membrane protein lateral diffusion in single cells. Science. 1976 Feb 6;191(4226):466–468. doi: 10.1126/science.1246629. [DOI] [PubMed] [Google Scholar]
- Elson E. L., Webb W. W. Concentration correlation spectroscopy: a new biophysical probe based on occupation number fluctuations. Annu Rev Biophys Bioeng. 1975;4(00):311–334. doi: 10.1146/annurev.bb.04.060175.001523. [DOI] [PubMed] [Google Scholar]
- Jacobson K., Wu E., Poste G. Measurement of the translational mobility of concanavalin A in glycerol-saline solutions and on the cell surface by fluorescence recovery after photobleaching. Biochim Biophys Acta. 1976 Apr 16;433(1):215–222. doi: 10.1016/0005-2736(76)90189-9. [DOI] [PubMed] [Google Scholar]
- Magde D., Elson E. L., Webb W. W. Fluorescence correlation spectroscopy. II. An experimental realization. Biopolymers. 1974 Jan;13(1):29–61. doi: 10.1002/bip.1974.360130103. [DOI] [PubMed] [Google Scholar]
- Peters R., Peters J., Tews K. H., Bähr W. A microfluorimetric study of translational diffusion in erythrocyte membranes. Biochim Biophys Acta. 1974 Nov 15;367(3):282–294. doi: 10.1016/0005-2736(74)90085-6. [DOI] [PubMed] [Google Scholar]
- Poo M., Cone R. A. Lateral diffusion of rhodopsin in the photoreceptor membrane. Nature. 1974 Feb 15;247(5441):438–441. doi: 10.1038/247438a0. [DOI] [PubMed] [Google Scholar]
- Yahara I., Edelman G. M. Restriction of the mobility of lymphocyte immunoglobulin receptors by concanavalin A. Proc Natl Acad Sci U S A. 1972 Mar;69(3):608–612. doi: 10.1073/pnas.69.3.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagyansky Y., Edidin M. Lateral diffusion of concanavalin A receptors in the plasma membrane of mouse fibroblasts. Biochim Biophys Acta. 1976 Apr 16;433(1):209–214. doi: 10.1016/0005-2736(76)90188-7. [DOI] [PubMed] [Google Scholar]



