Abstract
Previous work has shown that the application of the Joule heating temperature jump technique of Eigen and de Maeyer to an istonic suspension of human erythrocytes induced an interiorization of [3H-A1glucose and a hemolysis of the red cells (Tsong, T.Y., and E. Kingsley, J. Biol. Chem. 250:786 [1975]). The result was interpreted as due to the thermal osmosis effect. Further considerations of the various effects of the Joule heating technique indicate that the hemolysis of the red cells may also be caused by the rapid dielectric perturbation of the cell membranes. By means of turbidity measurements of the suspensions we have detected at least four relaxation times. Two of the faster ones (tau1 approximately 20 mus and tau2 approximately 5 ms) are tentatively attributed to water relaxations in the membrane structures. The other two are attributed to membrane ruptures (tlag approximately 0.1s) and the hemolysis reaction (tau3 approximately 0.5 s). Studies with the erythrocytes from different hematological disorders indicate that whereas the two slower relaxations are sensitive to the overall physical property of the red cell membranes the two faster relaxations are not. These observations are consistent with the above assignment of the relaxation processes. The apparent activation energies are, above assignment of the relaxation processes. The apparent activation energies are, respectively, 8.4, 12.0, and 11.8 kcal/mol for the tau1, tau2, and tau3 reactions. Experiments with erythrocyte ghosts indicate a single relaxation for the water permeation, and biphasic kinetics for the membrane rupture and resealing reactions. The phenomena reported here may contribute to our understanding of water transport and molecular release in cellular systems.
Full text
PDF




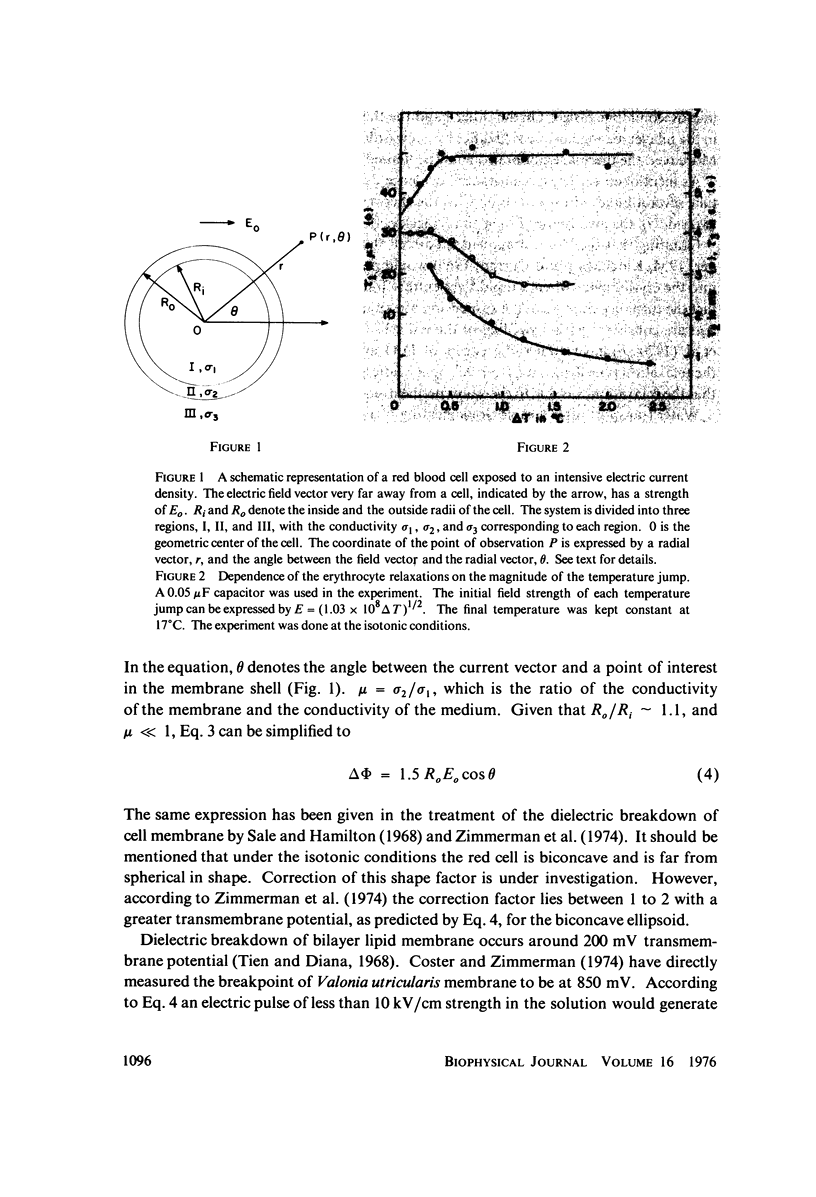

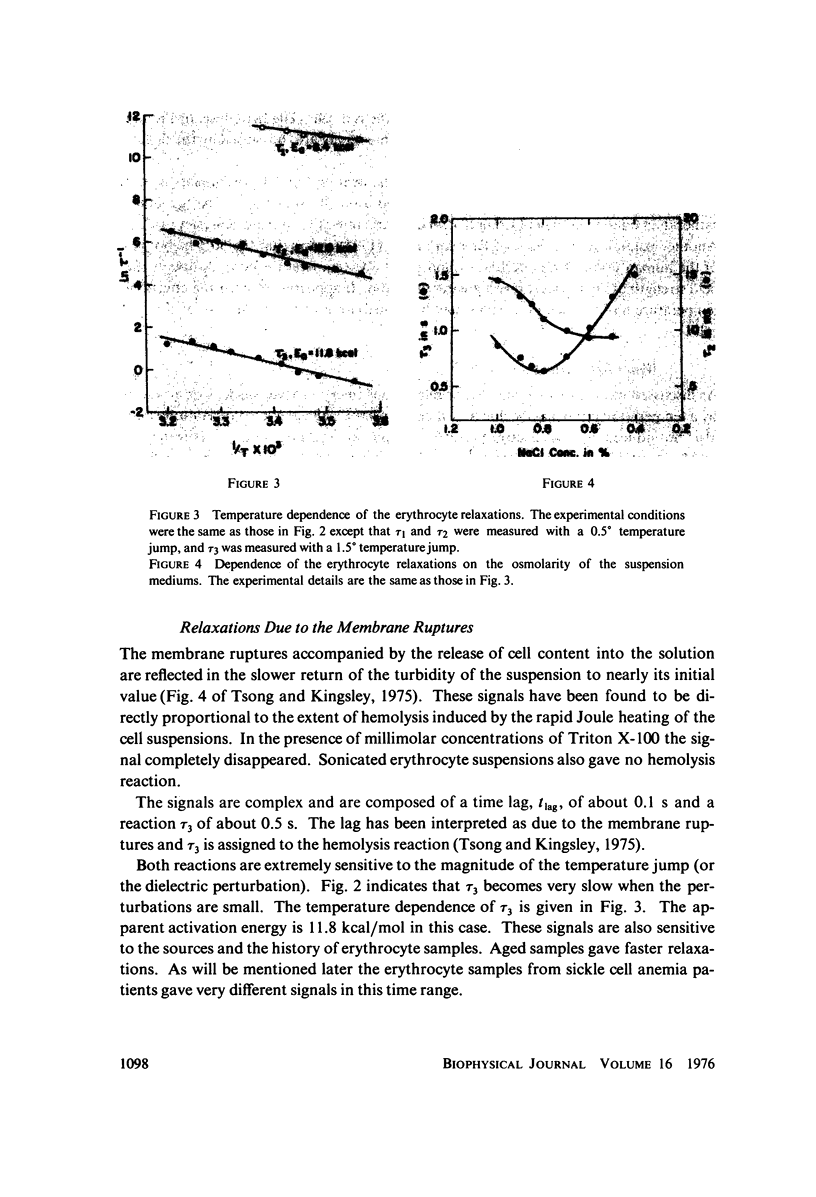
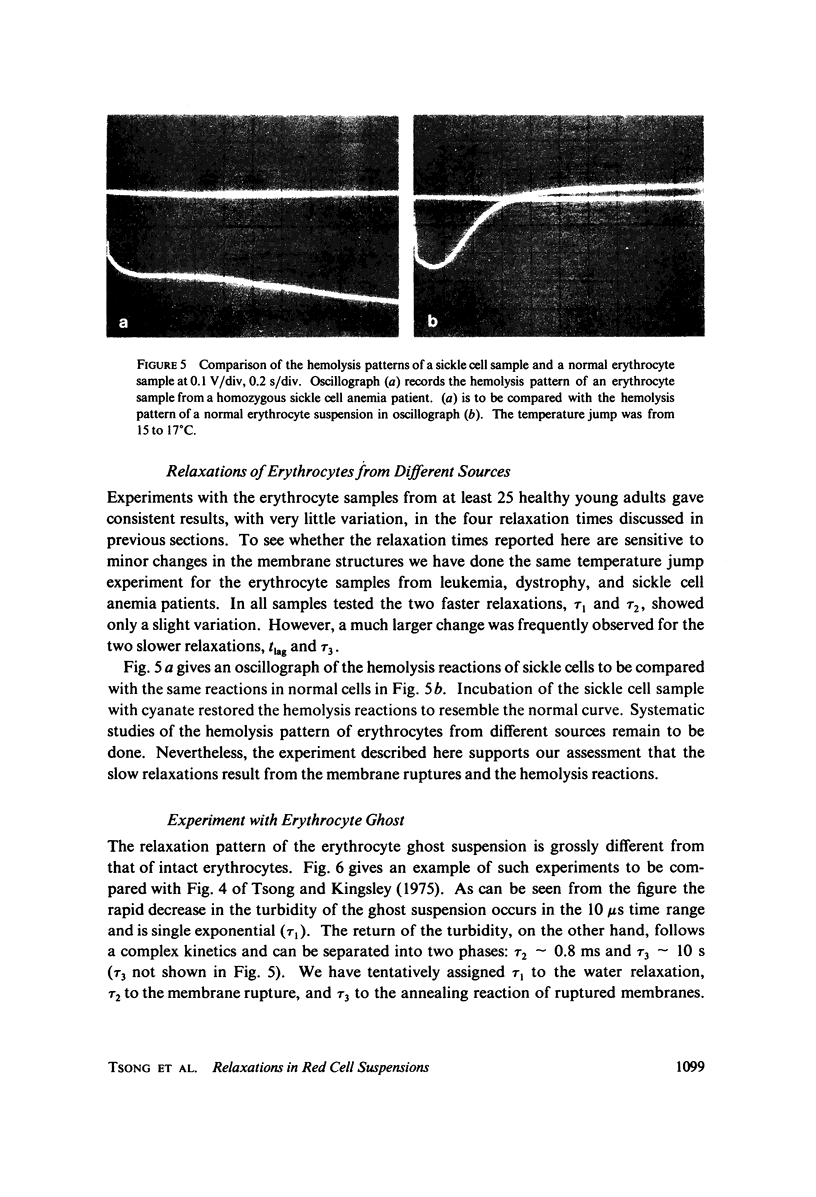
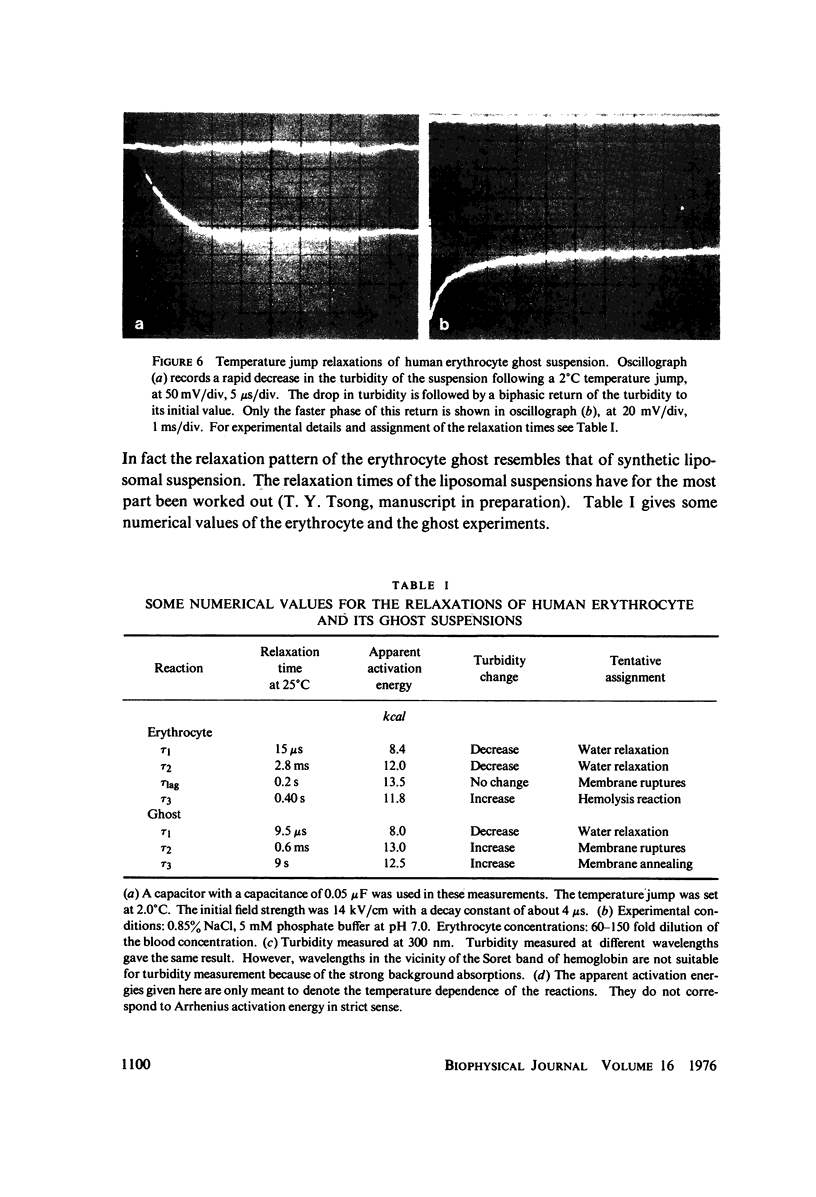




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldo J. H., Manuck B. A., Priestley E. B., Sykes B. D. An intracavity laser temperature jump apparatus and its application to the interaction of methylisonitrile with hemoglobin beta chains. J Am Chem Soc. 1975 Apr 2;97(7):1684–1688. doi: 10.1021/ja00840a010. [DOI] [PubMed] [Google Scholar]
- Burka E. R., Schreml W., Kick C. J. Membrane-bound ribonucleic acid in mammalian erythroid cells. Biochemistry. 1967 Sep;6(9):2840–2847. doi: 10.1021/bi00861a026. [DOI] [PubMed] [Google Scholar]
- Coster H. G., Simmermann U. The mechanism of electrical breakdown in the membranes of Valonai utricularis. J Membr Biol. 1975 Jun 3;22(1):73–90. doi: 10.1007/BF01868164. [DOI] [PubMed] [Google Scholar]
- Coster H. G., Zimmermann U. Dielectric breakdown in the membranes of Valonia utricularis. The role of energy dissipation. Biochim Biophys Acta. 1975 Mar 25;382(3):410–418. doi: 10.1016/0005-2736(75)90281-3. [DOI] [PubMed] [Google Scholar]
- Hammes G. G., Tallman D. E. Application of the temperature-jump technique to the study of phospholipid dispersions. J Am Chem Soc. 1970 Oct 7;92(20):6042–6046. doi: 10.1021/ja00723a038. [DOI] [PubMed] [Google Scholar]
- Owen J. D., Bennion B. C., Holmes L. P., Eyring E. M., Berg M. W., Lords J. L. Temperature jump relaxations in aqueous saline suspensions of human erythrocytes. Biochim Biophys Acta. 1970 Mar 17;203(1):77–82. doi: 10.1016/0005-2736(70)90037-4. [DOI] [PubMed] [Google Scholar]
- Owen J. D., Hemmes P., Eyring E. M. Light scattering temperature jump relaxations in mixed solvent suspensions of phosphatidylcholine vesicles. Biochim Biophys Acta. 1970 Dec 1;219(2):276–282. doi: 10.1016/0005-2736(70)90206-3. [DOI] [PubMed] [Google Scholar]
- Riemann F., Zimmermann U., Pilwat G. Release and uptake of haemoglobin and ions in red blood cells induced by dielectric breakdown. Biochim Biophys Acta. 1975 Jul 3;394(3):449–462. doi: 10.1016/0005-2736(75)90296-5. [DOI] [PubMed] [Google Scholar]
- Sale A. J., Hamilton W. A. Effects of high electric fields on micro-organisms. 3. Lysis of erythrocytes and protoplasts. Biochim Biophys Acta. 1968 Aug;163(1):37–43. doi: 10.1016/0005-2736(68)90030-8. [DOI] [PubMed] [Google Scholar]
- Seufert W. D. Model membranes: spherical shells bounded by one bimolecular layer of phospholipids. Biophysik. 1970;7(1):60–73. doi: 10.1007/BF01189465. [DOI] [PubMed] [Google Scholar]
- Solomon A. K. Properties of water in red cell and synthetic membranes. Biomembranes. 1972;3:299–330. doi: 10.1007/978-1-4684-0961-1_21. [DOI] [PubMed] [Google Scholar]
- Tien H. T., Diana A. L. Bimolecular lipid membranes: a review and a summary of some recent studies. Chem Phys Lipids. 1968 Feb;2(1):55–101. doi: 10.1016/0009-3084(68)90035-2. [DOI] [PubMed] [Google Scholar]
- Träuble H. Phasenumwandlungen in Lipiden. Mögliche Schaltprozesse in biologischen Membranen. Naturwissenschaften. 1971 Jun;58(6):277–284. doi: 10.1007/BF00624732. [DOI] [PubMed] [Google Scholar]
- Tsong T. Y. Effect of phase transition on the kinetics of dye transport in phospholipid bilater structures. Biochemistry. 1975 Dec 16;14(25):5409–5414. doi: 10.1021/bi00696a004. [DOI] [PubMed] [Google Scholar]
- Tsong T. Y. Kinetics of the crystalline-liquid crystalline phase transition of dimyristoyl L-alpha-lecithin bilayers. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2684–2688. doi: 10.1073/pnas.71.7.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsong T. Y., Kingsley E. Hemolysis of human erythrocyte induced by a rapid temperature jump. J Biol Chem. 1975 Jan 25;250(2):786–789. [PubMed] [Google Scholar]
- Zimmermann U., Pilwat G., Riemann F. Dielectric breakdown of cell membranes. Biophys J. 1974 Nov;14(11):881–899. doi: 10.1016/S0006-3495(74)85956-4. [DOI] [PMC free article] [PubMed] [Google Scholar]




