Abstract
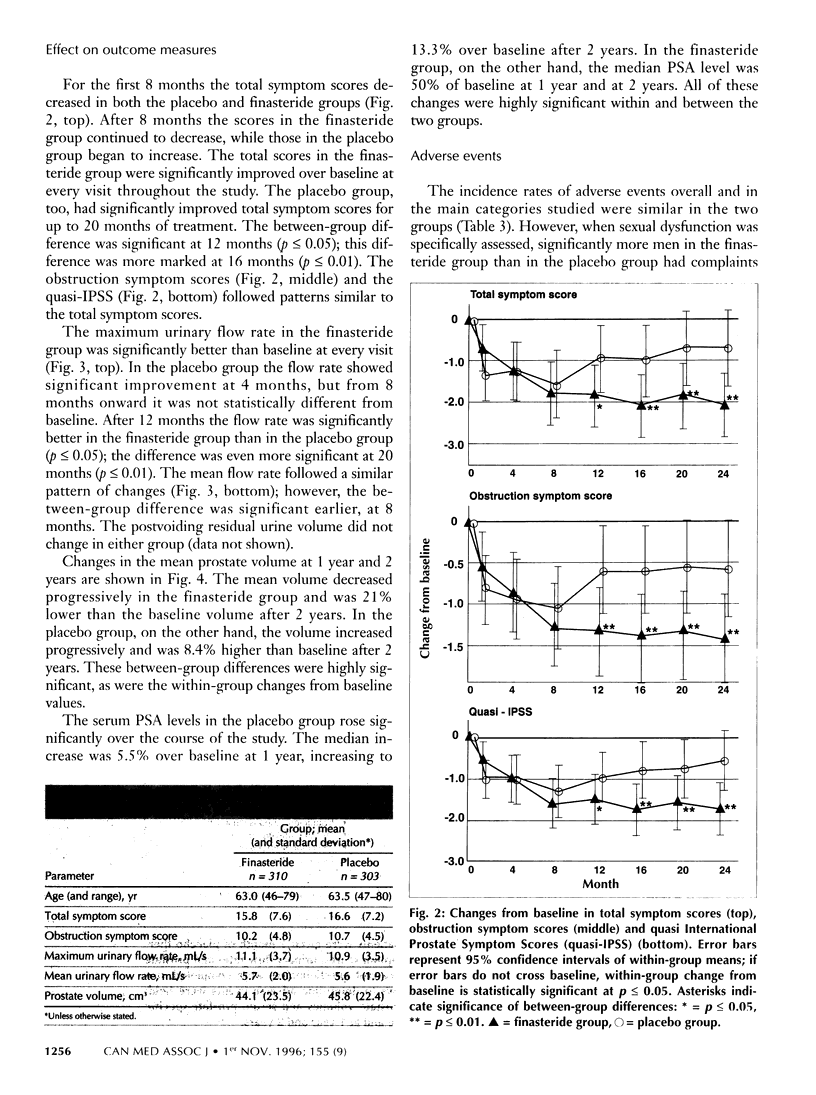
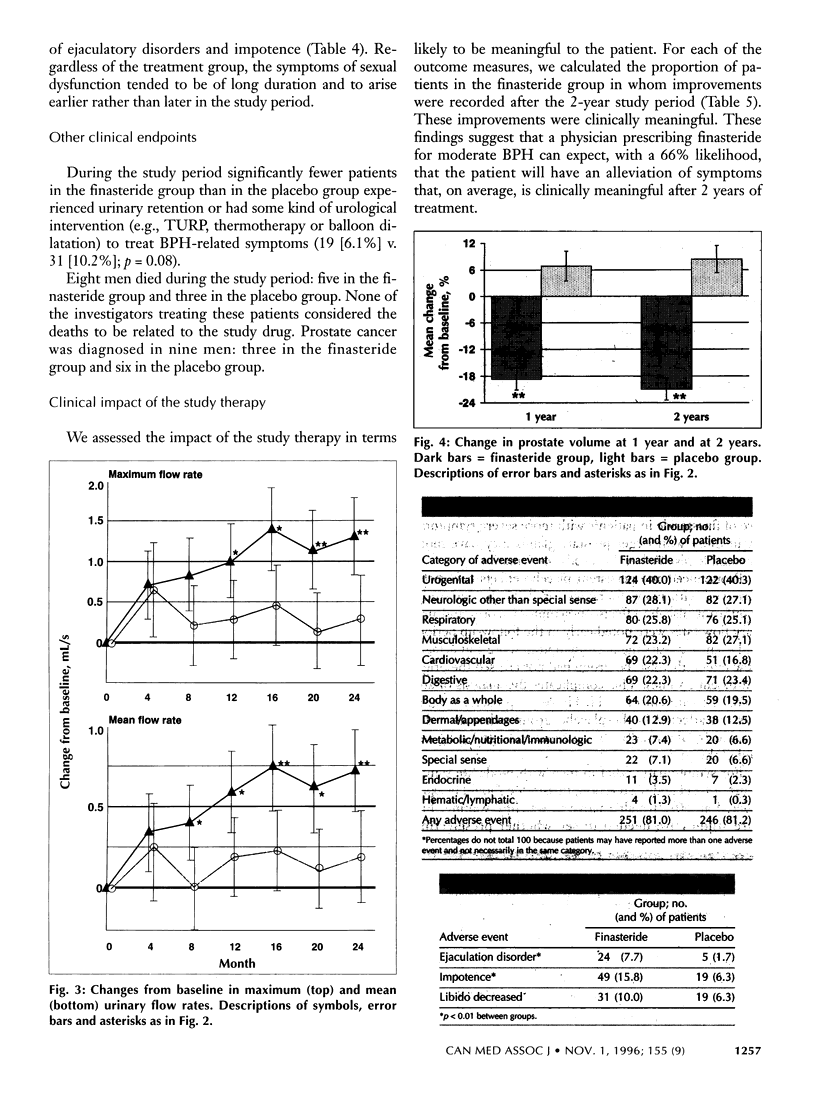
OBJECTIVE: To evaluate the efficacy and safety of 2 years' treatment of moderate benign prostatic hyperplasia (BPH) with finasteride. DESIGN: Double-blind, parallel-group, placebo-controlled, multicentre, prospective randomized study. SETTING: Outpatient care in 28 centres across Canada. PARTICIPANTS: Men aged 45 to 80, in good health, with moderate BPH and no evidence of prostate cancer. A total of 613 men were entered into the study; 472 completed the 2 years of treatment. INTERVENTION: After 1 month of receiving a placebo (run-in period), patients were given either finasteride (5 mg/d) or a placebo for 2 years. OUTCOME MEASURES: Efficacy: changes from baseline in BPH symptom scores, maximum urinary flow rates and prostate volume. Safety: onset, course and resolution of all adverse events during the treatment period. RESULTS: In the efficacy analyses the mean BPH symptom scores decreased 2.1 points (from 15.8 to 13.7) in the finasteride group, as compared with a decrease of 0.7 points (from 16.6 to 15.9) in the placebo group (P < or = 0.01). The maximum urinary flow rate increased by a mean of 1.4 mL/s (from 11.1 to 12.5 mL/s) in the finasteride group, as compared with an increase of 0.3 mL/s (from 10.9 to 11.2 mL/s) in the placebo group (p < or = 0.01). The mean prostate volume decreased by 21% (from a mean volume of 44.1 cm3 at baseline) in the treatment group; it increased by 8.4% (from a mean volume of 45.8 cm3 at baseline) in the placebo group (p < or = 0.01). In the safety analysis, the proportion of patients who experienced any adverse event was similar in the two groups (81.0% in the treatment group and 81.2% in the placebo group). However, the incidence of adverse events related to sexual dysfunction were significantly higher in the finasteride group than in the placebo group (ejaculation disorder 7.7% v. 1.7% and impotence 15.8% v. 6.3%; p < or = 0.01 for both parameters). CONCLUSION: Finasteride is a well-tolerated and effective alternative to watchful waiting in the treatment of moderate BPH.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry M. J., Cockett A. T., Holtgrewe H. L., McConnell J. D., Sihelnik S. A., Winfield H. N. Relationship of symptoms of prostatism to commonly used physiological and anatomical measures of the severity of benign prostatic hyperplasia. J Urol. 1993 Aug;150(2 Pt 1):351–358. doi: 10.1016/s0022-5347(17)35482-4. [DOI] [PubMed] [Google Scholar]
- Beisland H. O., Binkowitz B., Brekkan E., Ekman P., Kontturi M., Lehtonen T., Lundmo P., Pappas F., Round E., Shapiro D. Scandinavian clinical study of finasteride in the treatment of benign prostatic hyperplasia. Eur Urol. 1992;22(4):271–277. doi: 10.1159/000474771. [DOI] [PubMed] [Google Scholar]
- Bruchovsky N., Wilson J. D. The conversion of testosterone to 5-alpha-androstan-17-beta-ol-3-one by rat prostate in vivo and in vitro. J Biol Chem. 1968 Apr 25;243(8):2012–2021. [PubMed] [Google Scholar]
- Elhilali M. M., Ramsey E. W., Barkin J., Casey R. W., Boake R. C., Beland G., Fradet Y., Trachtenberg J., Orovan W. L., Schick E. A multicenter, randomized, double-blind, placebo-controlled study to evaluate the safety and efficacy of terazosin in the treatment of benign prostatic hyperplasia. Urology. 1996 Mar;47(3):335–342. doi: 10.1016/S0090-4295(99)80449-X. [DOI] [PubMed] [Google Scholar]
- Gormley G. J., Stoner E., Bruskewitz R. C., Imperato-McGinley J., Walsh P. C., McConnell J. D., Andriole G. L., Geller J., Bracken B. R., Tenover J. S. The effect of finasteride in men with benign prostatic hyperplasia. The Finasteride Study Group. N Engl J Med. 1992 Oct 22;327(17):1185–1191. doi: 10.1056/NEJM199210223271701. [DOI] [PubMed] [Google Scholar]
- Hieble J. P., Boyce A. J., Caine M. Comparison of the alpha-adrenoceptor characteristics in human and canine prostate. Fed Proc. 1986 Oct;45(11):2609–2614. [PubMed] [Google Scholar]
- Jacobsen S. J., Girman C. J., Guess H. A., Rhodes T., Oesterling J. E., Lieber M. M. Natural history of prostatism: longitudinal changes in voiding symptoms in community dwelling men. J Urol. 1996 Feb;155(2):595–600. doi: 10.1016/s0022-5347(01)66461-9. [DOI] [PubMed] [Google Scholar]
- Mebust W. K., Holtgrewe H. L., Cockett A. T., Peters P. C. Transurethral prostatectomy: immediate and postoperative complications. A cooperative study of 13 participating institutions evaluating 3,885 patients. J Urol. 1989 Feb;141(2):243–247. doi: 10.1016/s0022-5347(17)40731-2. [DOI] [PubMed] [Google Scholar]
- Stoner E. Maintenance of clinical efficacy with finasteride therapy for 24 months in patients with benign prostatic hyperplasia. The Finasteride Study Group. Arch Intern Med. 1994 Jan 10;154(1):83–88. [PubMed] [Google Scholar]
- Stoner E. Three-year safety and efficacy data on the use of finasteride in the treatment of benign prostatic hyperplasia. Urology. 1994 Mar;43(3):284–294. doi: 10.1016/0090-4295(94)90068-x. [DOI] [PubMed] [Google Scholar]
- Walsh P. C., Madden J. D., Harrod M. J., Goldstein J. L., MacDonald P. C., Wilson J. D. Familial incomplete male pseudohermaphroditism, type 2. Decreased dihydrotestosterone formation in pseudovaginal perineoscrotal hypospadias. N Engl J Med. 1974 Oct 31;291(18):944–949. doi: 10.1056/NEJM197410312911806. [DOI] [PubMed] [Google Scholar]
- Warwick R. T., Whiteside C. G., Arnold E. P., Bates C. P., Worth P. H., Milroy E. G., Webster J. R., Weir J. A urodynamic view of prostatic obstruction and the results of prostatectomy. Br J Urol. 1973 Dec;45(6):631–645. doi: 10.1111/j.1464-410x.1973.tb12234.x. [DOI] [PubMed] [Google Scholar]
- Wasson J. H., Reda D. J., Bruskewitz R. C., Elinson J., Keller A. M., Henderson W. G. A comparison of transurethral surgery with watchful waiting for moderate symptoms of benign prostatic hyperplasia. The Veterans Affairs Cooperative Study Group on Transurethral Resection of the Prostate. N Engl J Med. 1995 Jan 12;332(2):75–79. doi: 10.1056/NEJM199501123320202. [DOI] [PubMed] [Google Scholar]


