Abstract
The integrin family of cell adhesion receptors are important for a diverse set of biological responses during development. Although many integrins have been shown to engage a similar set of cytoplasmic effector proteins in vitro, the importance of these proteins in the biological events mediated by different integrin receptors and ligands is uncertain. We have examined the role of one of the best-characterized integrin effectors, the focal adhesion protein paxillin, by disruption of the paxillin gene in mice. Paxillin was found to be critically involved in regulating the development of mesodermally derived structures such as heart and somites. The phenotype of the paxillin−/− mice closely resembles that of fibronectin−/− mice, suggesting that paxillin is a critical transducer of signals from fibronectin receptors during early development. Paxillin was also found to play a critical role in fibronectin receptor biology ex vivo since cultured paxillin-null fibroblasts display abnormal focal adhesions, reduced cell migration, inefficient localization of focal adhesion kinase (FAK), and reduced fibronectin-induced phosphorylation of FAK, Cas, and mitogen-activated protein kinase. In addition, we found that paxillin-null fibroblasts show some defects in the cortical cytoskeleton and cell spreading on fibronectin, raising the possibility that paxillin could play a role in structures distinct from focal adhesions. Thus, paxillin and fibronectin regulate some common embryonic developmental events, possibly due to paxillin modulation of fibronectin-regulated focal adhesion dynamics and organization of the membrane cytoskeletal structures that regulate cell migration and spreading.
Regulation of cell-cell and cell-substratum interactions plays an important role in a variety of biological responses including cell migration, proliferation, and survival. Integrins, a group of heterodimeric receptors, mediate both cell-extracellular matrix (ECM) and cell-cell interactions and have been shown to be important in the regulation of these biological responses (20). Molecularly, integrin engagement induces a signaling cascade which modulates both the intracellular and extracellular environments including changes in protein and lipid phosphorylation, alterations in the cytoskeletal architecture, changes in gene expression, modulation of integrin affinity, and ECM organization (9). Their ability to participate in a range of signaling events is likely to be due to the complex set of effectors utilized by integrins (43). Some of these proteins have been implicated in signaling from the receptor to the cytoplasm (outside-in signaling), while others function in transducing signals from the cytoplasm to the extracellular milieu (inside-out signaling). Many of the effectors reside in focal adhesions and include enzymes such as focal adhesion kinase (FAK) and Src family kinases and scaffolding proteins such as p130Cas, paxillin, vinculin, and zyxin (22).
While integrin engagement results in the tyrosine, and in some cases, serine/threonine phosphorylation of many of these putative effectors, the requirement for these proteins in integrin biology or their function in cytoskeleton dynamics is unclear. Overexpression studies, use of dominant negatives, and gene disruption studies have helped to elucidate the functions of some of these components. For example, in vitro studies of FAK have revealed a role for this protein in focal adhesion dynamics and regulation of cell migration, and in vivo studies have shown that FAK is an important effector of fibronectin biology (16, 21, 39, 42, 46). In contrast, although the scaffolding protein Cas is tyrosine phosphorylated when cells are plated on fibronectin, the phenotype of Cas-deficient embryos does not support a critical biological role for Cas in fibronectin signaling (7, 18, 27) in vivo. Similarly, mice with a mutation in another focal adhesion protein, vinculin, also have a phenotype distinct from that of fibronectin-deficient mice; and cells from these animals show elevated levels of tyrosine phosphorylation when seeded on fibronectin (57). Thus, as in other signaling pathways, phosphorylation of a protein in response to receptor engagement suggests that a protein may participate in signaling downstream of that receptor, but it is not indicative of an absolute requirement in the signal transduction process. The scaffolding protein paxillin is another example of a protein that has been suggested to be an effector in integrin signaling as well as other receptor pathways, but its function and requirement in these pathways are uncertain.
Paxillin is a LIM domain protein originally identified as a substrate of the oncogenic tyrosine kinase v-src. It is also phosphorylated in response to engagement of a variety of receptors, including integrins, and its phosphorylation is regulated during development (50, 51). Two family members, Hic-5 and leupaxin, have also been identified, and at least three alternatively spliced variants of paxillin have been observed (30, 34, 45, 48).
In addition to the LIM domains, paxillin contains other motifs and domains which provide binding sites for a range of proteins including tyrosine kinases, other scaffolding and/or adapter proteins, and structural proteins (51). The motifs and domains present in paxillin include leucine-rich motifs (termed LD repeats), proline-rich sequences, and phosphotyrosine binding sites. The LD repeats are important for paxillin’s ability to interact with proteins such as FAK, vinculin, the papillomavirus E6 protein, and the ARF GAP, Git/PKL/CAT(4). Accordingly, many of these proteins can also bind to the paxillin family member Hic-5 due to the conservation of this region (48, 52). The LD motifs also play a role in localization of paxillin and Hic-5 to focal adhesions; however, the major determinant of focal adhesion localization resides in the C-terminal half of paxillin in the LIM domains. Paxillin has four LIM domains, and the second and third LIM domains appear to be critical for the localization of paxillin family proteins to focal adhesions (5).
While paxillin appears to function as a scaffolding protein, its precise role in mediating specific integrin signals is unclear. As indicated above, different integrins and numerous receptors induce phosphorylation of paxillin, suggesting that it could be a common effector of multiple pathways. Furthermore, many paxillin binding partners interact with multiple focal adhesion proteins, raising the possibility that their association with paxillin could be redundant. For example, the adapter protein crk can interact with paxillin as well as the focal adhesion protein CAS. The cytoplasmic tyrosine kinases Src and FAK can bind to paxillin but they can also interact with each other (17, 41, 55). The recent identification of two family members, leupaxin and Hic-5, also raises the possibility that paxillin function could be redundant in integrin biology. Finally, many paxillin binding partners appear to have opposing functions in integrin biology. For example, loss of FAK results in decreased migration, while loss of vinculin increases migration (21, 56). Thus, it is unclear whether and/or how this protein may function in regulation of an integrin pathway and responses such as cell migration and spreading. Therefore to gain insight into the function of this protein in vivo and its role in integrin signaling, a targeted disruption was generated in the paxillin locus. Analysis of paxillin-deficient embryos and cells derived from these animals demonstrates that paxillin is a critical mediator of fibronectin biology both in vivo and ex vivo, regulating focal adhesion dynamics, cell spreading, and tyrosine phosphorylation.
MATERIALS AND METHODS
Cells and culture.
AK7 embryonic stem cells and SNL fibroblasts were cultured and electroporated as described previously (28). Paxillin mutant and control cells were generated from littermate-matched embryos 7.5 days old (E7.5). Embryos were cultured in vitro individually for approximately 10 days, each in a single well of a gelatinized 24-well dish in embryonic stem cell media to allow fibroblastic cells to grow out. They were then trypsinized and replated in wells of the same size and gradually amplified. The passages of the cells used in these experiments were below passage 10, and thus far, the cells did not appear to have undergone a crisis. The rescued cells were generated by infecting paxillin-deficient cells with a retrovirus expressing myc-tagged, wild-type paxillin (48). The cells were selected in puromycin, the pooled puro-resistant population was expanded, and paxillin expression was confirmed by immunoblotting. Plating was done as described previously (37). Briefly, bacterial dishes were coated (10 μg/cm2) with fibronectin (Sigma) and incubated at room temperature for at least 1 h or overnight at 4°C. Following washing with distilled water, plates were blocked with 0.1% heat-inactivated bovine serum albumin for 1 h and washed with serum-free media (SFM). Serum-starved cells (≈80 to 90% confluence) were detached with 0.05% trypsin-0.35 mM EDTA and suspended in SFM containing 0.25 mg of soybean trypsin inhibitor per ml. After harvesting by centrifugation, cells were washed with SFM, centrifuged, resuspended in SFM, maintained at 37°C for 30 to 40 min, and then plated onto fibronectin-coated plates at 37°C for the times indicated.
Constructs and genotyping.
A 1.6-kb HindII/EcoRI fragment and a 4.4-kb HindIII fragment were cloned into the NotI and SalI sites, respectively, of the SAβgeolox2DTA vector (28). A mock vector containing a 3-kb EcoRI fragment cloned into the NotI site was also generated to optimize PCR conditions. G418r AK7 cells were screened by PCR using a paxillin-specific primer 5′ to the region of homology used in the targeting vector (CAAACCAGAATCCAGCCCAG) and a reverse primer in the SAβgeo cassette (TCGTAACCGTGCATCTGCC). The reaction was carried out at 95°C for 3 min and 82°C for 15 min followed by 40 cycles of 93°C for 30 s, 52°C for 45s, and 65°C for 3 min. This results in a 1.8-kb fragment specific for the targeted paxillin locus. Results were later confirmed by Southern blotting. Animals and embryos were genotyped by PCR using the above primers for detection of the mutant locus and the forward primer (CAGCCTCTCTACATGCCTTAGCTG) and wild-type-specific reverse primer (CCACTGATCTAAAGGGTCCAGGAC) for detection of the wild-type locus. Separate reactions were carried out since the sizes of the wild-type fragments were similar. The reaction conditions were identical, except that an annealing temperature of 54°C was used for the wild-type reaction. Because of the presence of the βgeo transgene, animals were routinely genotyped by 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) staining of tails as previously described (15). Sections were also genotyped by PCR after scraping and melting of the wax. The myc-tagged paxillin construct used for the rescue experiments has been described previously (48).
Histology and X-Gal staining.
Timed matings were performed between paxillin+/− animals. Mothers were sacrificed at various time points after fertilization, and embryos were dissected from the uterus. In some cases, X-gal staining was done, as previously described (15). For sections, embryos were fixed in 1× mirsky’s, and following dehydration they were embedded in paraffin and sectioned. Sections were counterstained with hematoxylin and eosin to better visualize the cells.
Spreading assay.
Spreading assays were done as previously described (37). Briefly, cells were starved for 16 to 24 h and then plated in SFM on fibronectin-coated bacterial dishes for the indicated time points. At least three independent fields were photographed under the 10× objective at the indicated time points. Initial assays were done on four different mutant cultures and two different control cultures, and they were repeated at least three times.
Cell migration.
Cells were grown to confluence and then wounded using a pipette tip. Three wounds were made for each sample, and all were photographed at the zero time point and at subsequent time points. Assays were initially done on four different mutant cultures and two different control cultures and were repeated at least three times.
Immunofluorescence.
Following starvation, cells were plated on fibronectin-coated coverslips overnight in SFM. Cortactin (4F11, generous gift of Thomas J. Parsons, University of Virginia), Hic-5 (HIM2), and vinculin (Sigma) staining were carried out as described previously (48, 49). For FAK staining, cells were fixed overnight in methanol and then stained with the FAK antibody, 2A7 (generous gift of Thomas J. Parsons, University of Virginia) and Hic-5 antibody followed by fluorescein isothiocyanate-conjugated anti-mouse and tetramethyl rhodamine isocyanate-conjugated anti-rabbit (Jackson Immunochemicals) antibodies. Cells were visualized on a Nikon fluorescence microscope using the 60× objective, and images were processed with a Photometrics digital camera and Phase 3 imaging software (Phase 3 Imaging).
Immunoprecipitations and Western analyses.
Following plating, cells were washed twice with phosphate-buffered saline and lysed (500 μl/10-cm-diameter plate) in radioimmunoprecipitation assay buffer containing protease and phosphatase inhibitors as described previously (49). Lysates were clarified by centrifugation for 15 min at 4°C. For immunoprecipitations, each sample (containing 200 to 500 μg of total protein) was incubated with the relevant antibody for 2 h at 4°C, followed by the addition of protein A-Sepharose beads (Sigma) and an additional 1-h incubation. Immune complexes were collected by centrifugation, washed four times with radioimmunoprecipitation buffer, resuspended in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer, boiled, and analyzed by SDS-8% PAGE. Proteins were electroblotted onto nitrocellulose (Bio-Rad) and probed with appropriate primary and peroxidase-conjugated secondary antibodies (Bio-Rad). Signals were detected by enhanced chemiluminescence (ECL; Amersham). Concentrations of primary antibodies used in immunoblots were 1:1,000 for anti-FAK and anti-Cas, 1:5,000 for 4G10 (generous gift of Debbie Morrison), 1:1,000 for phospho-mitogen-activated protein kinase (MAPK) (Cell Signaling), 1:1,000 for anti-paxillin (Transduction Labs), and 1:1,000 for Hic-5. For total cell lysates, 25 to 50 μg of lysate was used.
RESULTS
Generation of paxillin-deficient mice.
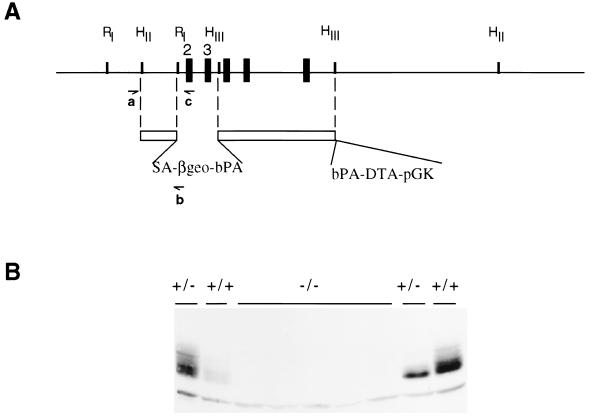
To begin to understand paxillin’s role in various pathways and in the regulation of the cytoskeletal architecture, a targeted disruption was generated in the paxillin gene. The paxillin locus was mutated by replacing 1.6 kb of genomic sequence with a promoterless β-galactosidase-neomycin resistance cassette (SAβgeo) (Fig. 1A) (15). The deleted sequence contains exons 2 and 3, which encode amino acids 5 to 74. Multiple targeted clones were isolated, and heterozygous mice were obtained in both hybrid (129/Sv;C57BL/6) and 129/Sv backgrounds. These animals are viable and breed normally. Proper targeting of the locus was confirmed by both Southern blot analysis and PCR (data not shown). As the construct contains a strong splice acceptor sequence and a bovine poly(A) which acts to trap any upstream message and as the next three exons are out of frame with the first exon, it was anticipated that this deletion would result in a loss of paxillin protein. Confirmation of a null mutation was obtained by Western blotting with two different paxillin antibodies (Fig. 1B and data not shown). No consistent decrease in paxillin levels was observed in lysates made from wild-type and heterozygous embryos, while a loss of paxillin reactivity was observed in lysates obtained from null embryos.
FIG. 1.
Targeting of the paxillin locus and generation of the null mutation. (A) The targeting vector used to generate the null mutation is diagrammed. SA, adenovirus splice acceptor; βgeo, β-galactosidase-neomycin resistance gene fusion; pGK, phosphoglucokinase promoter; DTA, diphtheria toxin A subunit; bPA, bovine(poly A). Primers used for genotyping are diagrammed (a, b, and c). Exons are represented by black boxes. Exons 2 and 3 were deleted and encode amino acids 5 to 74. Restriction sites used to generate the targeting vector are indicated. RI, EcoRI; HII, HindII; HIII, HindIII (see Materials and Methods for more detail). (B) Loss of paxillin expression in paxillin−/− cells. Lysates prepared from wild-type, paxillin+/−, or paxillin−/− embryos were subjected to SDS-PAGE, immobilized on nitrocellulose, and immunoblotted with an antibody to paxillin.
Paxillin expression during development.
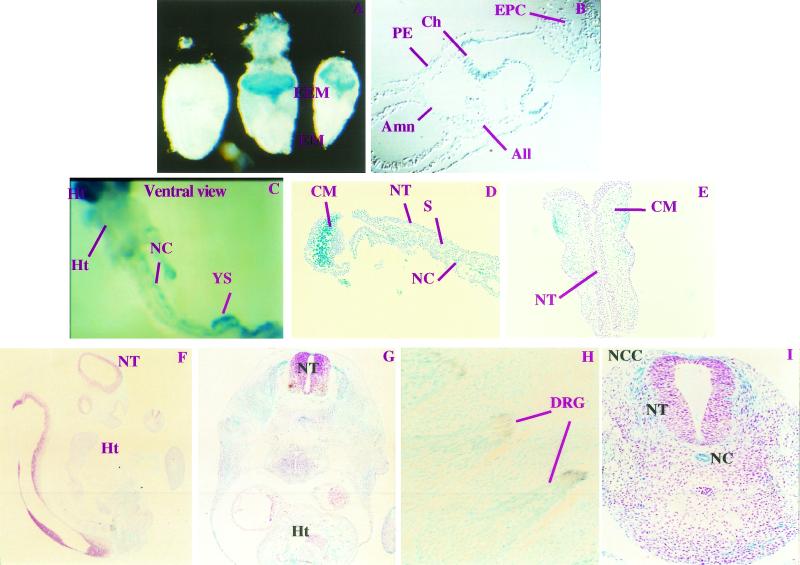
To determine in which developmental stages and tissues paxillin may function, we assayed expression of the paxillin gene in embryos. This was performed by X-Gal staining of staged embryos, which harbored one copy of the promoterless βgeo cassette within the paxillin locus. These data were confirmed by whole-mount antibody staining for paxillin (data not shown). Surprisingly, during the earliest stages of gastrulation (E6.5 and E7.5), paxillin expression is restricted to a subset of extraembryonic tissues: this includes parietal endoderm, ectoplacental cone, and three structures composed of extraembryonic mesoderm, the chorion (membrane to which the future umbilical chord will fuse), amnion (membrane separating embryonic from extraembryonic) and allantois (future umbilical chord) (Fig. 2A and B and data not shown) (24). Thus, paxillin is not likely to be required for patterning or growth of the embryo prior to gastrulation, except in extraembryonic structures. Whether two recently identified family members, Hic-5 and leupaxin (30, 33, 45, 48), are expressed in the embryonic region at E6.5 to E7.5 and could potentially compensate for the absence of paxillin in this region remains to be determined.
FIG. 2.
Paxillin expression between E7.5 and E10. Paxillin+/− or paxillin+/+ embryos were isolated between E6.5 and E10 and stained with X-Gal. In some cases they were counterstained with hematoxylin and eosin or immunostained with a neurofilament antibody. (A and B) Expression at E7.5 is seen only in the extraembryonic region (EEM) and not in the embryonic region (EM), and wild-type embryos show no staining. Sectioning of stained E7.5 embryos (B) indicates that the ectoplacental cone (EPC), chorion (Ch), allantois (All), and amnion (Amn) express paxillin. Expression at E8.5 to E10 (C to I) is detected in the embryo proper. At E8.5, paxillin expression is detected in many structures including mesodermally derived components such as the headfold (Hf), the notochord (NC), and the heart (Ht). Sectioning of E8.5 X-Gal-stained embryos (D and E) indicates that expression is observed in the underlying cephalic mesenchyme (CM) but is not detected in the neural tube (NT). Little staining is detected in the somites (S). At E9 and E10 (F to I), paxillin is expressed ubiquitously, with more intense staining observed in areas derived from neural crest cells including dorsal root ganglion (DRG). The brown staining represents neurofilament-positive structures (H). Sections of E10.5 embryos indicate strong staining of the notochord and the neural crest cells (NCC) emanating from the neural tube (I).
By midgastrulation (E8.5) paxillin expression was detected in the embryo proper, particularly in mesodermally and endodermally derived structures, and paxillin continued to be expressed in most structures at least through E10.5 (Fig. 2C to I). Positive mesodermal structures include the cephalic mesenchyme of the headfold, the endocardium, the dorsal aortae, and the yolk sac, which is the major site of hematopoiesis at this stage (Fig. 2C to G and data not shown) (24). Little expression is detected in the somites; however, paxillin is expressed in the notochord, a structure which regulates patterning of the neighboring paraxial mesoderm (e.g., somites) and the overlying floor plate of the neural tube (Fig. 2C, D, and I) (11, 38). This widespread mesodermal expression of paxillin suggests that it is likely to be involved in either the massive growth of mesodermally derived cells or the extensive morphogenetic movements associated with this stage.
In contrast, little expression is detected in neural ectoderm, specifically the neural tube. Interestingly, migrating neural crest cells, which emanate from the neural tube, do express paxillin (Fig. 2I). In addition, structures derived from these cells (dorsal root ganglia, trigeminal ganglia, and branchial arches [presumptive facial structures]) (Fig. 2H to I and data not shown) also express paxillin. Confirmation of the identity of the ganglion was obtained by costaining with neurofilament antibody (Fig. 2H). Thus, paxillin may play a role in regulating neural crest cell migration and/or function of these neural crest-derived tissues.
Phenotype of paxillin mutant mice.
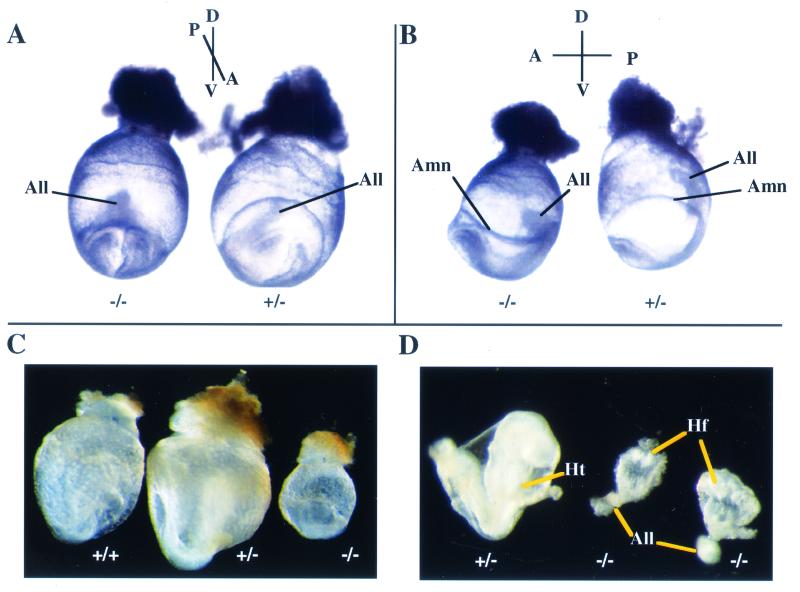
To address the role of paxillin in vivo, heterozygous mice were crossed, embryos were isolated at various stages, and genotypes were confirmed by PCR. Results were similar for 129/Sv and hybrid (C57BL/6;129/Sv) backgrounds. No paxillin−/− embryos were detected after E9.5, and any paxillin-null embryos detected at E9.5 were abnormal. Viable but abnormal paxillin-nullizygous embryos were detected at E7.5 to E8.5. Gross phenotypic analysis indicated that at E7.5 the overall sizes of paxillin-homozygous embryos, compared to those of their wild-type or heterozygous littermates, were not significantly different; however, two structures in which paxillin is expressed and which are derived, in part, from extraembryonic mesoderm were abnormal in paxillin−/− embryos. The amnion, a membrane separating the embryonic and extraembryonic regions, was collapsed on the embryo in the dorsal region (Fig. 3A and B). Defects were also observed in a second structure where paxillin is expressed, the allantois. The allantois is an important site of vasculogenesis and normally grows dorsally towards the chorion through a process of cavitation and eventually fuses with the chorion. In paxillin mutants, however, the allantois was misshapen and growing anteriorly towards the headfold structures. Whether the defects observed in the amnion and allantois are due to effects on adhesion, proliferation, and/or survival is uncertain. In addition, both the amnion and chorion play a role in proper development of the allantois, so whether paxillin is required in one or all of these structures for proper allantois development remains to be determined (12–14). Regardless, paxillin is clearly required for normal development of certain extraembryonic structures.
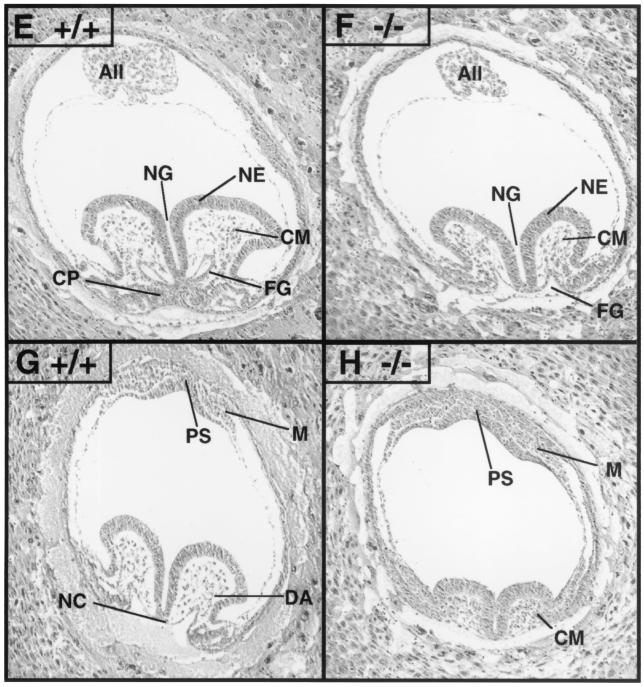
FIG. 3.
Paxillin-null embryos display defects in multiple mesodermally derived structures. Timed matings were performed with paxillin+/− mice and embryos isolated at E7.5 or E8.5. Genotypes of embryos were determined by PCR either with yolk sac DNA or from scraping of sections (data not shown). (A and B) At E7.5 in paxillin−/− embryos, the amnion (Amn) is collapsed on the embryo and the allantois (All) is misshapen and moving anteriorly rather than dorsally towards the chorion. (C and D) At E8.5, paxillin mutants are significantly smaller overall. No obvious heart structure is present (Ht), and mutants have an abnormal headfold (Hf) and allantois (All). (E to H) Histological sections of wild-type and paxillin-null embryos. The headfold region is smaller than normal, but expected cell populations are present adjacent to the neural groove (NG), including neural epithelium (NE) and cephalic mesenchyme (CM). In contrast, the mutant embryo lacks a cardiogenic plate (CP) and the endodermal lining of the foregut (FG) remains open. The allantois (All) also appears to be abnormal. (G) A more caudal section of a wild-type embryo, in which the notochord (NC) and rostral extension of dorsal aorta (DA) are visible. Adjacent to the primitive streak (PS), mesodermal cells (M) are visible. (H) More caudal section of a paxillin-null embryo, in which the discrepancy in overall headfold size, compared to that of the wild type, is more pronounced. Cephalic mesenchymal (CM) cells appear condensed. Mutant embryos also lack an organized notochord, and the dorsal aorta is absent. Paxillin-null embryos also lack organized somites (in more caudal sections; not shown). In contrast, the primitive streak (PS) and adjacent mesodermal (M) cells appear normal.
Gross analysis of E8 to E8.5 paxillin-null embryos revealed more dramatic defects. Paxillin−/− embryos were significantly smaller than their wild-type or heterozygous littermates, showed truncation of the anterior-posterior axis, and consistent with the pattern of expression, had small headfold structures and impaired development of the heart and somites (Fig. 3C and D). While little expression of paxillin was detected in somites at this stage (Fig. 2D), expression of paxillin was seen in the notochord, which sends critical signals involved in patterning of these structures. Histological analysis confirmed that there was poor development of the heart and somites, while the developing head, although small, had a distinct neurectoderm and underlying head mesenchyme (Fig. 3E to F). Although no gross heart structure was detected in the majority of the embryos, culturing of the embryos in vitro resulted in the development of beating cardiac myocytes (data not shown). In addition, a small percentage of embryos that had definitive but abnormal heart structures were found (data not shown). Thus, paxillin is not required for cardiocyte differentiation but may be important for the organization of these cells. Interestingly, the overall phenotype of E8 to E8.5 paxillin mutants closely resembles the phenotype observed in fibronectin-deficient embryos and is somewhat similar to the phenotype of FAK−/− embryos (16, 21). These results suggested that the phenotype of paxillin−/− embryos could be due to defects in fibronectin biology.
Effects of loss of paxillin on focal adhesions and lamellipodia.
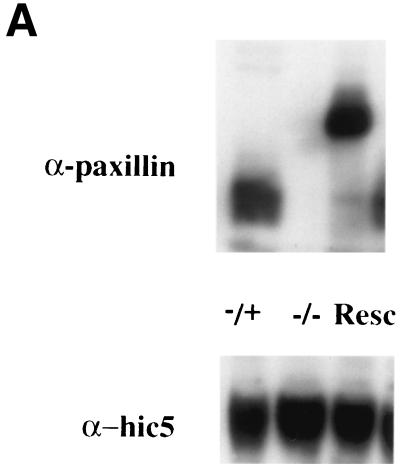
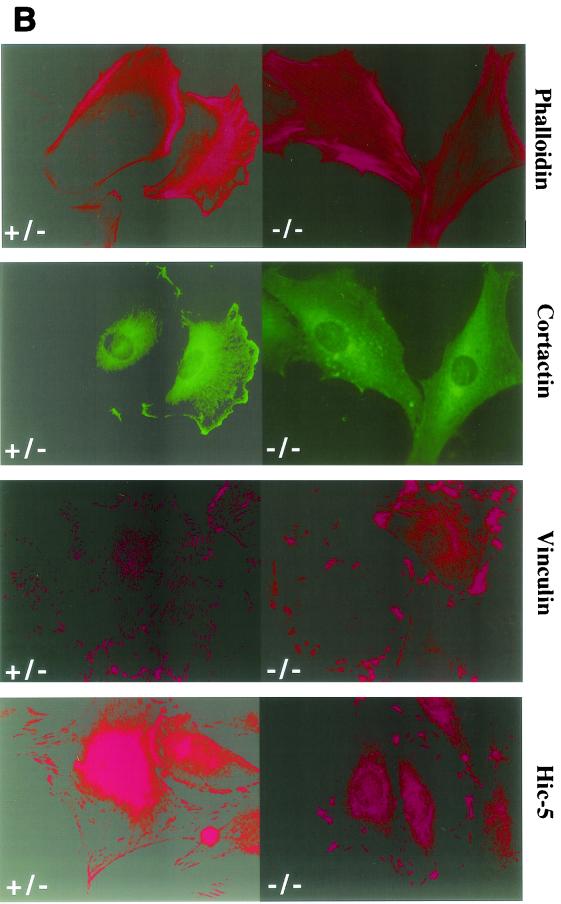
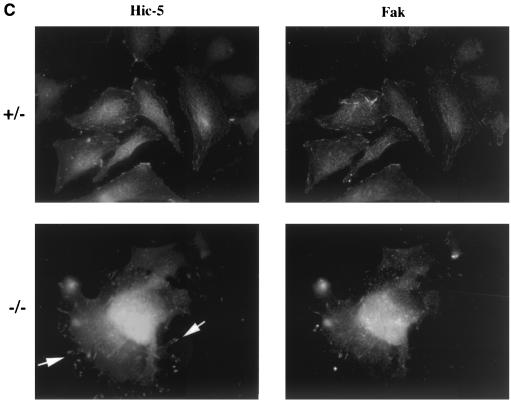
To determine whether paxillin may play a role in fibronectin receptor signaling, primary cells were cultured from mutant, heterozygous, or wild-type E7.5 embryos. Results are presented for two mutant primary cell cultures, but similar results have been obtained in five independently derived paxillin−/− primary cultures (data not shown). In addition, myc-tagged paxillin or untagged paxillin was re-expressed in the paxillin-deficient cells (Fig. 4A and data not shown) to confirm that any differences observed were due to loss of paxillin. Mutant or control cells were plated overnight in SFM on fibronectin-coated coverslips, and the ability to form focal adhesions was assessed. Staining of these cells with antibodies to two focal adhesion proteins, vinculin and the paxillin-related protein Hic-5, indicated that focal adhesions were present in paxillin mutant cells (Fig. 4B). However, while the control cells showed fine, filamentous focal adhesions, the focal adhesions of paxillin-null cells consisted of numerous short filaments. These results suggest that focal adhesions can form in the absence of paxillin, but paxillin may be important either for proper maintenance of these structures or for focal adhesion turnover. In addition, although vinculin is a paxillin binding partner and the region important for vinculin localization to the focal adhesions overlaps with its paxillin binding domain, paxillin is not required for vinculin localization to focal adhesions (53). Finally, although Hic-5, which can interact with a subset of paxillin binding partners, is present and expressed at similar levels in wild-type and paxillin-deficient fibroblasts (Fig. 4A), Hic-5 cannot compensate for loss of paxillin.
FIG. 4.
Paxillin mutant cells have abnormal focal adhesions and are defective in lamellipodium formation. (A) Paxillin and Hic-5 immunoblots of control cells, paxillin−/− cells, or paxillin−/− cells rescued with myc-tagged paxillin. (B) Cells were plated on fibronectin-coated coverslips overnight and stained with antibodies to vinculin or the paxillin-related protein Hic-5 to look at focal adhesions. Cells were stained with phalloidin or cortactin to look at stress fibers and the membrane cytoskeleton.
Analysis of actin organization indicated the presence of stress fibers in both control and paxillin-deficient cells. In contrast, the membrane cytoskeleton, as indicated by cortactin staining and phalloidin staining, was affected by loss of paxillin (Fig. 4B). Paxillin-deficient cells plated on fibronectin overnight had decreased cortical staining of cortactin, although cells plated for shorter periods of time were positive for cortical localization of cortactin. These studies suggest that loss of paxillin may affect cortactin localization and/or organization of the cortical cytoskeleton; however, whether these effects are direct or indirect remains to be determined. In any case, these studies suggest that paxillin plays a role in focal adhesion dynamics and could also be important for regulating the membrane cytoskeleton in response to fibronectin receptor engagement.
Paxillin’s role in cell spreading and migration.
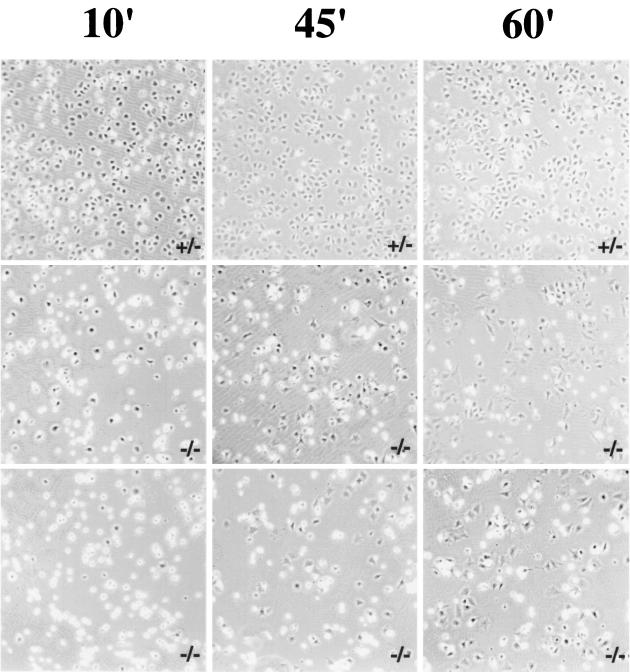
Given the effects of paxillin on focal adhesions and the cortical cytoskeleton, cells were assessed for their ability to spread and migrate. Paxillin−/− cells showed delayed spreading when plated on fibronectin (Fig. 5). While 70 to 85% of wild-type, heterozygous, or paxillin-deficient cells rescued with wild-type paxillin were spreading by 10 min, only 20 to 35% of paxillin-deficient cells had spread (Fig. 5, data not shown). By 60 min, however, the majority of paxillin-deficient cells had spread, suggesting that there was a delay but not a block in cell spreading.
FIG. 5.
Paxillin-deficient cells have a delayed rate of spreading. Control or paxillin-deficient cells were plated on fibronectin-coated bacterial dishes for the indicated periods of time and photographed by phase contrast. At least three independent fields were analyzed for each time point. Dark cells are considered to be spread, while bright cells are unspread. The photograph is representative of results obtained in four independent experiments. In the experiment shown at the 10-min time point, 76% of control cells were spread while only 34% (middle panel) and 20% (lower panel) of cells in the two different knockout cultures were spread at this time point. At 45 min almost 100% of the control cells were spread, while 55% of the knockout cells in one case (middle panel) and 39% of the knockout cells in the other case (lower panel) were spread. By 60 min, 60% of the cells were spread in all cultures.
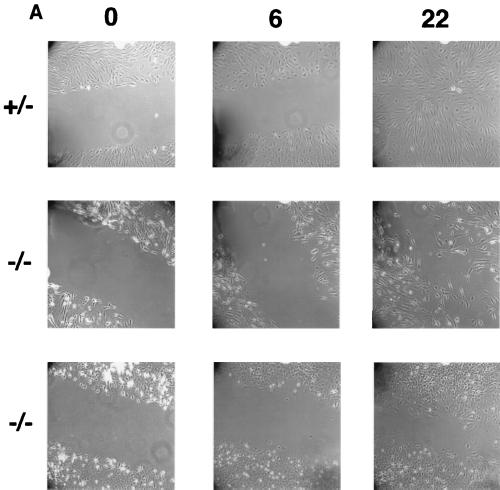
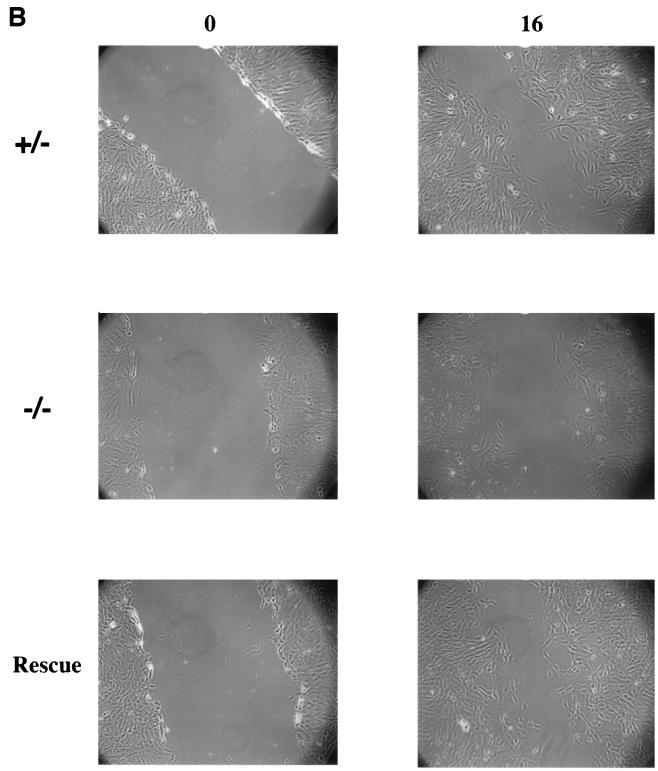
Cell migration was also affected by loss of paxillin, as measured by a wounding assay. Although paxillin-deficient cells could clearly migrate as measured by this assay, when compared to the control cells, a small but consistent delay in complete wound closure was observed (Fig. 6A). Re-expression of paxillin rescued this defect in migration (Fig. 6B), indicating that the defect was due to loss of paxillin. Although preliminary results suggest that the paxillin-deficient cells grow slower, cell division cannot account for the observed difference in this time frame. In addition, individual cells can be seen in the wound. Thus, these results suggest that paxillin plays a role in cell migration and raise the possibility that a defect in migration may contribute to the phenotype observed in vivo.
FIG. 6.
Paxillin-deficient cells migrate more slowly. (A) A confluent monolayer of control or paxillin-deficient cells was wounded with a yellow tip and photographed at the indicated time points (in hours). (B) A confluent monolayer of control, paxillin−/− cells, or paxillin−/− cells in which paxillin was re-expressed (rescue) was wounded with a yellow tip and photographed at the indicated time points (in hours). The pictures are representative of results obtained in four independent experiments.
Regulation of fibronectin-induced tyrosine phosphorylation by paxillin.
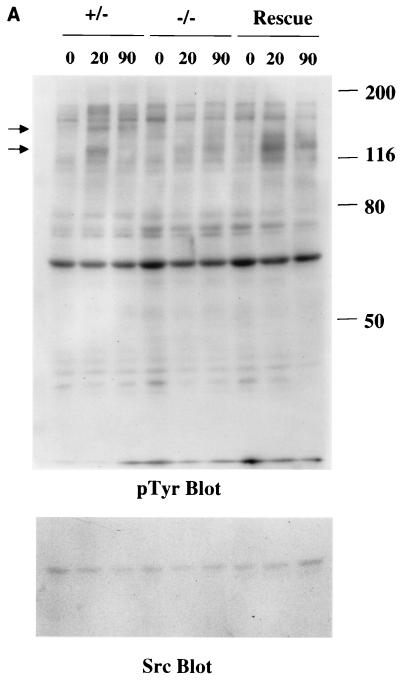
To begin to determine a potential molecular basis for these defects, fibronectin-induced tyrosine phosphorylation was examined in the paxillin−/− cells (Fig. 7A and B). Loss of paxillin did not appear to dramatically affect overall fibronectin-induced tyrosine phosphorylation. Surprisingly, some small but consistent changes in total cell phosphotyrosine were observed (Fig. 7A, arrows). While reprobing of the immunoblots with antibodies to src indicated that the lysates were normalized, because the identity of these proteins is unclear, whether these represent real changes in the extent of tyrosine phosphorylation or differences in the expression of these proteins remains to be determined. Interestingly, there was decreased tyrosine phosphorylation in the 120- to 130-kDa region, which could be rescued by re-expression of paxillin.
FIG. 7.
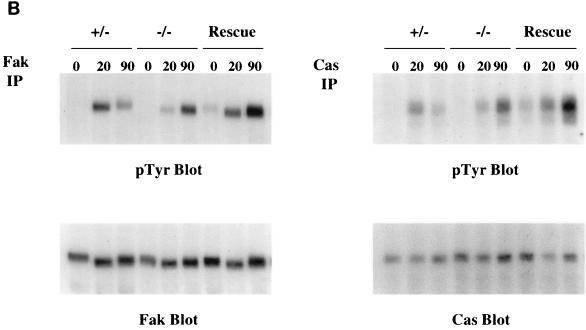
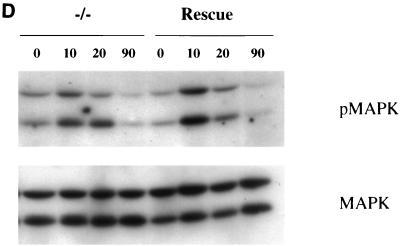
Fibronectin-induced phosphorylation of FAK, Cas, and MAPK and FAK localization are defective in paxillin-deficient cells. Cells were plated on fibronectin-coated plates for the indicated time points (in minutes). Lysates were prepared and analyzed for total cell phosphotyrosine (A) or tyrosine phosphorylation of specific substrates (B). The total cell lysate blot was probed with an antibody to Src to ensure equal loading. The pTyr blots of FAK and Cas immunoprecipitations were reprobed with an antibody to FAK or Cas to check for levels. (C) Cells were also costained with antibodies to FAK and Hic-5 to determine if loss of paxillin affects FAK localization. While Hic-5 and FAK colocalized to focal adhesions in control cells, a few Hic-5-positive focal adhesions which were negative for FAK in paxillin mutant cells were observed (arrows). (D) Total cell lysates were prepared from knockout or rescued cells plated on fibronectin for the indicated time points and analyzed with phospho-specific antibodies to MAPK. Blots were reprobed for MAPK to check for equal loading. In this experiment, comparison of the knockout cells to the rescued cells indicates that there is a twofold difference in MAPK activation at the 10- and 20-min time points.
The two prominent proteins in the 120- to 130-kDa range that are phosphorylated in fibroblasts in response to engagement of fibronectin receptors are FAK and p130Cas. FAK has been implicated in the regulation of cell spreading and migration; and as mentioned above, the phenotype of FAK-deficient embryos shows some similarities with that of paxillin−/− embryos (21). Therefore fibronectin-induced tyrosine phosphorylation of FAK was analyzed in the paxillin gene knockout cells (Fig. 7B). While the control cells also showed a difference in the kinetics of tyrosine phosphorylation of FAK compared to that observed with the knockout cells, this difference was not detected in the rescued cells; however, a small but consistent decrease in FAK tyrosine phosphorylation was observed in paxillin-null cells compared to control or rescued cells (Fig. 7B). Quantitation of several different experiments indicates that the difference in FAK phosphorylation in paxillin-null versus rescued cells ranged from two- to fourfold. Analysis of fibronectin-induced tyrosine phosphorylation of another focal adhesion protein, p130Cas, also revealed a small but consistent decrease in tyrosine phosphorylation. This difference ranged from 1.5- to 3-fold. The inability of the rescued cells to correct the difference in kinetics suggests that this difference is unlikely due to loss of paxillin and could represent subtle differences between the fibroblasts derived from the control versus mutant embryos. Regardless, these results further indicate that paxillin is important for at least some aspects of fibronectin signal transduction and suggest that paxillin is an important regulator of FAK and Cas tyrosine phosphorylation.
The effects on FAK tyrosine phosphorylation could be due to a role for paxillin in regulating FAK localization, FAK phosphorylation and/or dephosphorylation, or a more indirect mechanism. Previous studies have suggested that paxillin binding to FAK may play a role in FAK localization, while other studies have suggested that FAK localizes to focal adhesions independent of its interactions with paxillin (17, 47). To determine if loss of paxillin affects FAK localization to the focal adhesions, cells were costained with antibodies to FAK and the focal adhesion protein Hic-5 (Fig. 7C). The majority of focal adhesions showed complete costaining of FAK; however, a small but consistent number of focal adhesions were detected in paxillin−/− cells that were positive for Hic-5 but negative for FAK (Fig. 7C, arrows). Since in most cases colocalization is observed, paxillin is not absolutely required, but the results suggest that paxillin does play some role in efficient localization of FAK to focal adhesion structures.
Erk/MAPK is activated in response to engagement of fibronectin receptors (8, 26). Therefore, activation of these proteins in response to plating cells on fibronectin was analyzed with activation-specific antibodies. Showing results similar to those obtained for Cas and FAK, the phosphorylation and/or activation of p42/44 MAPK was decreased in the paxillin-deficient cells (Fig. 7D). Quantitation of various experiments indicated that the difference in MAPK activation between the knockout and rescued cells ranged from two- to fivefold. Since FAK plays some role in the activation of p42/44 MAPK, this difference could be due to paxillin’s regulation of FAK phosphorylation, although other mechanisms are possible.
DISCUSSION
The results presented here demonstrate a requirement for paxillin in embryonic development and identify paxillin as an important cytoplasmic effector of at least some fibronectin receptors. We have shown that (i) surprisingly, paxillin expression is restricted to extraembryonic structures at E6.5 to E7.5 but becomes more broadly expressed at later stages; (ii) correlating with its pattern of expression, loss of paxillin affects development of both extraembryonic and embryonic structures and the phenotype observed very closely resembles that of fibronectin-nullizygous embryos; (iii) paxillin mutant cells plated on fibronectin have abnormal focal adhesions, an altered cortical cytoskeleton, decreased tyrosine phosphorylation of FAK and Cas, decreased efficiency of FAK localization to focal adhesions, decreased activation of MAPK, a decreased rate of spreading, and decreased cell migration. These results suggest that paxillin is important both in vitro and in vivo for fibronectin signaling.
Analysis of paxillin expression indicated that paxillin expression is restricted early in gastrulation to extraembryonic structures but subsequently becomes more broadly expressed. At E6.5 and E7.5 paxillin expression is observed in extraembryonic structures which are derived in part from extraembryonic mesoderm (Fig. 2A and B and data not shown). Extraembryonic and embryonic mesoderm are derived from the same cells, but the extraembryonic mesoderm results from the cells moving dorsally (24). Thus, since we do not detect paxillin expression in the embryo proper at this stage, paxillin expression is induced only in the cells migrating dorsally. At later stages, however, paxillin is clearly expressed in structures derived from embryonic mesoderm and endoderm. Thus, paxillin may not play a role in the early movements or growth of the mesodermal layer, but clearly plays a role at later stages. This idea is supported by the phenotype of paxillin-nullizygous embryos at E8.5. Consistent with the expression data, paxillin−/− embryos show defects in the structures in which paxillin is expressed. It remains possible, however, that the embryonic defects observed could be due to a requirement for paxillin in the extraembryonic region. Detailed chimera and/or transplantation experiments may help address this issue. Chimera studies may also help to address the role of paxillin in neural crest migration and differentiation.
As indicated above, the phenotype observed shows striking similarities to that of fibronectin-null embryos (16). This includes defects in both the extraembryonic and embryonic regions, and the in vitro studies presented provide strong evidence for a critical role for paxillin in fibronectin outside-in signaling. Cells plated on fibronectin had altered focal adhesions, loss of lamellipodia, decreased rates of migration, decreased rates of spreading, effects on FAK, Cas, and MAPK phosphorylation, and inefficient FAK localization. Thus, paxillin is an important mediator of fibronectin outside-in signaling.
The precise mechanism by which paxillin functions to regulate events such as migration, spreading, and phosphorylation and activation of different integrin signaling components remains to be determined. Paxillin can interact directly or indirectly with a number of proteins which have been implicated in regulating the cytoskeletal architecture and processes such as spreading and migration. For example, paxillin can bind directly to FAK, and FAK has been shown to be important for focal adhesion turnover, spreading, migration, and survival (42). Paxillin can also be linked to Cas via the adapter protein Crk, and Cas plays a role in cell migration (3, 7, 27). Although the role of these interactions is uncertain, it is possible that binding of paxillin could affect the subcellular localization or function of these proteins. Paxillin may not be important for vinculin localization, but it appears to play a minor role in efficient FAK localization (Fig. 4 and 7C). Whether the presence of the paxillin family member Hic-5 accounts for this minor effect or paxillin family proteins are not the primary mediators of FAK localization to focal adhesions remains to be determined. With regards to FAK, paxillin could also be a critical effector of this protein either by relaying an important signal or acting as a scaffold to recruit a substrate of FAK. Paxillin is not likely to be a critical substrate of FAK, in that while FAK-deficient embryos and cells share similarities with the paxillin−/− embryos and cells, loss of FAK does not affect paxillin tyrosine phosphorylation. In addition, FAK−/− cells do not show changes in Cas phosphorylation, so paxillin’s regulation of FAK phosphorylation and localization is unlikely to account for these changes in phosphorylation. Regardless, loss of paxillin could alter FAK’s ability to modulate focal adhesion dynamics, migration, and possibly survival. Genetic and cell biological studies may help to address this issue. Similar models may also be postulated for Src and Cas.
On the other hand, paxillin can also interact with negative regulators such as Csk and PTP-PEST (10, 35, 40, 44). Here again, the consequence of these interactions are poorly understood. PTP-PEST has been shown to dephosphorylate Cas, and Csk negatively regulates Src PTKs (1). Paxillin may act to sequester these proteins away from their targets and thus allow positive signals to be sent via Cas and Src. Thus, loss of paxillin could result in Csk shutting down Src PTKs or PTP-PEST dephosphorylating Cas prematurely. Thus, this could account for the decrease in FAK and Cas phosphorylation. Studies are currently under way to address these issues.
Recent work has identified another paxillin complex which may also play a role in regulating migration. As seen in Fig. 4B, loss of paxillin results in poor cortactin localization to lamellipodia. While paxillin is localized primarily in focal adhesions, it has been detected in focal contacts associated with lamellipodia (29, 36). Lamellipodium formation is regulated by the small GTPase Rac, which also regulates cortactin localization (36, 54). Interestingly, recent data have suggested that paxillin can interact with a protein complex which includes an exchange factor for Rac, Pix/Cool, and the Rac effector, Pak (2, 32, 52). Consistent with these observations, expression of a mutant paxillin in a neuroblastoma line disrupts lamellipodium formation (52). Therefore, loss of paxillin may affect localization of this complex-altering Rac activity and subsequent fibronectin-induced lamellipodium formation. Thus, loss of regulation of one or all of these complexes could contribute to the defects observed in paxillin-null cells.
An alternate interpretation of these studies relates to the functional relationship between paxillin and a family member, Hic-5. Hic-5 localizes to focal adhesions and can bind to many of the proteins that have been identified for paxillin; however, two potential positive regulators, Src and crk, bind only to paxillin and not to Hic-5 (48, 53). In contrast, the negative regulator csk can bind to both Hic-5 and paxillin (48). These data and the association of Hic-5 with cell senescence and of paxillin with transformation have led to the suggestion that paxillin and Hic-5 could modulate each other’s functions. Therefore, the phenotype observed in the paxillin-deficient cells could be due to inhibitory effects of Hic-5 in the absence of paxillin. Genetic studies are under way to test this hypothesis.
Although these results indicate that paxillin is an important modulator of outside-in signaling by some fibronectin receptors, paxillin could also play a role in inside-out signaling. Preliminary results indicate a decrease in fibronectin staining in paxillin mutant embryos in vivo (unpublished data). While this could be a secondary effect, it remains possible that paxillin regulates the fibronectin matrix. Regulation could occur at the level of transcription, secretion of fibronectin, or matrix assembly. With regards to matrix assembly, not only do cytosolic proteins participate in outside-in signaling, but also some effectors play a role in inside-out signaling (19). For example, r-Ras and Rho regulate fibronectin matrix assembly by regulating receptor affinity and contractility, respectively (60, 61). Thus, it is possible that paxillin’s effect on the extracellular matrix could be due to a requirement for paxillin in inside-out signaling. Consistent with this hypothesis, studies by Brown and coworkers have suggested that phosphorylation of paxillin in the LIM domains regulates cell adhesion (6). Unfortunately, since results thus far indicate that the phenotypic effect on the fibronectin matrix may be lost when cells are placed in culture, it will be difficult to determine how paxillin regulates the fibronectin matrix. Genetic interactions between fibronectin and paxillin can, however, be tested.
While paxillin appears to be important for fibronectin biology in vivo, it is unclear which receptor(s) is being affected (54, 58). Two fibronectin receptors, α4 and α5, have overlapping patterns of expression with paxillin. For example, α4 is expressed in the chorion and both α4 and α5 are expressed in neural crest cells (23, 25). In addition, the phenotype of α5-deficient embryos shares some similarities with that of the paxillin−/− embryos (59). Although the phenotype of α4-deficient animals does not overlap with that of paxillin, recent experiments have shown that paxillin binds directly to the α4 cytoplasmic tail and regulates its ability to spread on a second ligand, VCAM (31). A third fibronectin receptor, αv, may also be functioning upstream of paxillin (57). Embryos lacking both αv and α5 die earlier than fibronectin-deficient embryos but share the amniotic defect seen in fibronectin- and paxillin-deficient embryos. On the other hand, α4/α5 double-mutant embryos have less severe phenotypes than fibronectin-deficient, and most likely, paxillin-deficient embryos. Additional genetic and cell biological studies may help determine the role of each of these receptors in the paxillin phenotype.
Finally, whether or not all of the defects observed in vivo are due to defects in fibronectin biology or involve other ECM or peptide growth factors remains to be determined. In addition, paxillin may function at later points in development in other receptor pathways. Regardless, generation of these mice and cells from these animals should help to further define the function of paxillin in vivo and delineate the molecular pathways in which paxillin is involved.
Acknowledgments
We thank Joan S. Brugge, Karen M. Downs, Karen Cichowski, Ben Neel, and members of the Thomas lab for helpful comments; P. Soriano and the Fred Hutchinson Cancer Research Center (Seattle, Wash.) for initial support for this work; and Tom Parsons, Amy Bouton, and Debbie Morrison for providing antibodies.
This work was supported by RO1CA75621, a Merck-sponsored Life Sciences Research Foundation Fellowship, and a Leukemia Society Scholar Award to S.M.T., by RO1GM47607 to C.E.T., and RO1GM57719 to E.L.G. C.E.T. is an Established Investigator of the American Heart Association.
REFERENCES
- 1.Angers, L. A., J. F. Cote, A. Charest, D. Dowbenko, S. Spencer, L. A. Lasky, and M. L. Tremblay. 1999. Protein tyrosine phosphatase-PEST regulates focal adhesion disassembly, migration, and cytokinesis in fibroblasts. J. Cell Biol. 144:1019–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagrodia, S., S. J. Taylor, K. A. Jordon, A. L. Van, and R. A. Cerione. 1998. A novel regulator of p21-activated kinases. J. Biol. Chem. 273:23633–23636. [DOI] [PubMed] [Google Scholar]
- 3.Birge, R. B., J. E. Fajardo, C. Reichman, S. E. Shoelson, Z. Songyang, L. C. Cantley, and H. Hanafusa. 1993. Identification and characterization of a high-affinity interaction between v-Crk and tyrosine-phosphorylated paxillin in CT10-transformed fibroblasts. Mol. Cell. Biol. 13:4648–4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown, M. C., M. S. Curtis, and C. E. Turner. 1998. Paxillin LD motifs may define a new family of protein recognition domains. Nat. Struct. Biol. 5:677–678. [DOI] [PubMed] [Google Scholar]
- 5.Brown, M. C., J. A. Perrotta, and C. E. Turner. 1996. Identification of LIM3 as the principal determinant of paxillin focal adhesion localization and characterization of a novel motif on paxillin directing vinculin and focal adhesion kinase binding. J. Cell Biol. 135:1109–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown, M. C., J. A. Perrotta, and C. E. Turner. 1998. Serine and threonine phosphorylation of the paxillin LIM domains regulates paxillin focal adhesion localization and cell adhesion to fibronectin. Mol. Biol. Cell 9:1803–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cary, L. A., D. C. Han, T. R. Polte, S. K. Hanks, and J. L. Guan. 1998. Identification of p130Cas as a mediator of focal adhesion kinase-promoted cell migration. J. Cell Biol. 140:211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, Q., M. S. Kinch, T. H. Lin, K. Burridge, and R. L. Juliano. 1994. Integrin-mediated cell adhesion activates mitogen-activated protein kinases. J. Biol. Chem. 269:26602–26605. [PubMed] [Google Scholar]
- 9.Clark, E. A., and J. S. Brugge. 1995. Integrins and signal transduction pathways: the road taken. Science 268:233–239. [DOI] [PubMed] [Google Scholar]
- 10.Cot, J. F., C. E. Turner, and M. L. Tremblay. 1999. Intact LIM 3 and LIM 4 domains of paxillin are required for the association to a novel polyproline region (Pro 2) of protein-tyrosine phosphatase-PEST. J. Biol. Chem. 274:20550–20560. [DOI] [PubMed] [Google Scholar]
- 11.Dodd, J., T. M. Jessell, and M. Placzek. 1998. The when and where of floor plate induction. Science 282:1654–1657. [DOI] [PubMed] [Google Scholar]
- 12.Downs, K. M. 1998. The murine allantois. Curr. Top. Dev. Biol. 39:1–33. [DOI] [PubMed] [Google Scholar]
- 13.Downs, K. M., and R. L. Gardner. 1995. An investigation into early placental ontogeny: allantoic attachment to the chorion is selective and developmentally regulated. Development 121:407–416. [DOI] [PubMed] [Google Scholar]
- 14.Downs, K. M., S. Gifford, M. Blahnik, and R. L. Gardner. 1998. Vascularization in the murine allantois occurs by vasculogenesis without accompanying erythropoiesis. Development 125:4507–4520. [DOI] [PubMed] [Google Scholar]
- 15.Friedrich, G., and P. Soriano. 1991. Promoter traps in embryonic stem cells: a genetic screen to identify and mutate developmental genes in mice. Genes Dev. 5:1513–1523. [DOI] [PubMed] [Google Scholar]
- 16.George, E. L., L. E. N. Georges, K. R. S. Patel, H. Rayburn, and R. O. Hynes. 1993. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development 119:1079–1091. [DOI] [PubMed] [Google Scholar]
- 17.Hildebrand, J. D., M. D. Schaller, and J. T. Parsons. 1995. Paxillin, a tyrosine phosphorylated focal adhesion-associated protein binds to the carboxyl terminal domain of focal adhesion kinase. Mol. Biol. Cell 6:637–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Honda, H., H. Oda, T. Nakamoto, Z. Honda, R. Sakai, T. Suzuki, T. Saito, K. Nakamura, K. Nakao, T. Ishikawa, M. Katsuki, Y. Yazaki, and H. Hirai. 1998. Cardiovascular anomaly, impaired actin bundling and resistance to Src-induced transformation in mice lacking p130Cas. Nat. Genet. 19:361–365. [DOI] [PubMed] [Google Scholar]
- 19.Hughes, P. E., and M. Pfaff. 1998. Integrin affinity modulation. Trends Cell Biol. 8:359–364. [DOI] [PubMed] [Google Scholar]
- 20.Hynes, R. O. 1992. Integrins: versatility, modulation, and signaling in cell adhesion. Cell 69:11–25. [DOI] [PubMed] [Google Scholar]
- 21.Ilic, D., Y. Furuta, S. Kanazawa, N. Takeda, K. Sobue, N. Nakatsuji, S. Nomura, J. Fujimoto, M. Okada, and T. Yamamoto. 1995. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature 377:539–544. [DOI] [PubMed] [Google Scholar]
- 22.Jockusch, B. M., P. Bubeck, K. Giehl, M. Kroemker, J. Moschner, M. Rothkegel, M. Rudiger, K. Schluter, G. Stanke, and J. Winkler. 1995. The molecular architecture of focal adhesions. Annu. Rev. Cell Dev. Biol. 11:379–416. [DOI] [PubMed] [Google Scholar]
- 23.Joos, T. O., C. A. Whittaker, F. Meng, D. W. DeSimone, V. Gnau, and P. Hausen. 1995. Integrin alpha 5 during early development of Xenopus laevis. Mech. Dev. 50:187–199. [DOI] [PubMed] [Google Scholar]
- 24.Kaufman, M. H. 1995. The atlas of mouse development. Academic Press, Inc., New York, N.Y.
- 25.Kil, S. H., C. E. Krull, G. Cann, D. Clegg, and F. M. Bronner. 1998. The alpha4 subunit of integrin is important for neural crest cell migration. Dev. Biol. 202:29–42. [DOI] [PubMed] [Google Scholar]
- 26.King, W. G., M. D. Mattaliano, T. O. Chan, P. N. Tsichlis, and J. S. Brugge. 1997. Phosphatidylinositol 3-kinase is required for integrin-stimulated AKT and Raf-1/mitogen-activated protein kinase pathway activation. Mol. Cell. Biol. 17:4406–4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klemke, R. L., J. Leng, R. Molander, P. C. Brooks, K. Vuori, and D. A. Cheresh. 1998. CAS/Crk coupling serves as a “molecular switch” for induction of cell migration. J. Cell Biol. 140:961–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lanier, L. M., M. A. Gates, W. Witke, A. S. Menzies, A. M. Wehman, J. D. Macklis, D. Kwiatkowski, P. Soriano, and F. B. Gertler. 1999. Mena is required for neurulation and commissure formation. Neuron 22:313–325. [DOI] [PubMed] [Google Scholar]
- 29.Leventhal, P. S., E. A. Shelden, B. Kim, and E. L. Feldman. 1997. Tyrosine phosphorylation of paxillin and focal adhesion kinase during insulin-like growth factor-I-stimulated lamellipodial advance. J. Biol. Chem. 272:5214–5218. [DOI] [PubMed] [Google Scholar]
- 30.Lipsky, B. P., C. R. Beals, and D. E. Staunton. 1998. Leupaxin is a novel LIM domain protein that forms a complex with PYK2. J. Biol. Chem. 273:11709–11713. [DOI] [PubMed] [Google Scholar]
- 31.Liu, S., S. M. Thomas, D. G. Woodside, D. M. Rose, W. B. Kiosses, M. Pfaff, and M. H. Ginsberg. 1999. Binding of paxillin to alpha4 integrins modifies integrin-dependent biological responses. Nature 402:676–681. [DOI] [PubMed] [Google Scholar]
- 32.Manser, E., T. H. Loo, C. G. Koh, Z. S. Zhao, X. Q. Chen, L. Tan, I. Tan, T. Leung, and L. Lim. 1998. PAK kinases are directly coupled to the PIX family of nucleotide exchange factors. Mol. Cell 1:183–192. [DOI] [PubMed] [Google Scholar]
- 33.Matsuya, M., H. Sasaki, H. Aoto, T. Mitaka, K. Nagura, T. Ohba, M. Ishino, S. Takahashi, R. Suzuki, and T. Sasaki. 1998. Cell adhesion kinase beta forms a complex with a new member, Hic-5, of proteins localized at focal adhesions. J. Biol. Chem. 273:1003–1014. [DOI] [PubMed] [Google Scholar]
- 34.Mazaki, Y., S. Hashimoto, and H. Sabe. 1997. Monocyte cells and cancer cells express novel paxillin isoforms with different binding properties to focal adhesion proteins. J. Biol. Chem. 272:7437–7444. [DOI] [PubMed] [Google Scholar]
- 35.Nishiya, N., Y. Iwabuchi, M. Shibanuma, J. F. Cote, M. L. Tremblay, and K. Nose. 1999. Hic-5, a paxillin homologue, binds to the protein-tyrosine phosphatase PEST (PTP-PEST) through its LIM 3 domain. J. Biol. Chem. 274:9847–9853. [DOI] [PubMed] [Google Scholar]
- 36.Nobes, C. D., and A. Hall. 1995. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 81:53–62. [DOI] [PubMed] [Google Scholar]
- 37.Oh, E. S., H. Gu, T. M. Saxton, J. F. Timms, S. Hausdorff, E. U. Frevert, B. B. Kahn, T. Pawson, B. G. Neel, and S. M. Thomas. 1999. Regulation of early events in integrin signaling by protein tyrosine phosphatase SHP-2. Mol. Cell. Biol. 19:3205–3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Placzek, M. 1995. The role of the notochord and floor plate in inductive interactions. Curr. Opin. Genet. Dev. 5:499–506. [DOI] [PubMed] [Google Scholar]
- 39.Richardson, A., and T. Parsons. 1996. A mechanism for regulation of the adhesion-associated protein tyrosine kinase pp125FAK. Nature 380:538–540. [DOI] [PubMed] [Google Scholar]
- 40.Sabe, H., A. Hata, M. Okada, H. Nakagawa, and H. Hanafusa. 1994. Analysis of the binding of the Src homology 2 domain of Csk to tyrosine-phosphorylated proteins in the suppression and mitotic activation of c-Src. Proc. Natl. Acad. Sci. USA 91:3984–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schaller, M. D., J. D. Hildebrand, J. D. Shannon, J. W. Fox, R. R. Vines, and J. T. Parsons. 1994. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol. Cell. Biol. 14:1680–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schlaepfer, D. D., C. R. Hauck, and D. J. Sieg. 1999. Signaling through focal adhesion kinase. Prog. Biophys. Mol. Biol. 71:435–478. [DOI] [PubMed] [Google Scholar]
- 43.Schwartz, M. A., M. D. Schaller, and M. H. Ginsberg. 1995. Integrins: emerging paradigms of signal transduction. Annu. Rev. Cell. Dev. Biol. 11:549–599. [DOI] [PubMed] [Google Scholar]
- 44.Shen, Y., G. Schneider, J. F. Cloutier, A. Veillette, and M. D. Schaller. 1998. Direct association of protein-tyrosine phosphatase PTP-PEST with paxillin. J. Biol. Chem. 273:6474–6481. [DOI] [PubMed] [Google Scholar]
- 45.Shibanuma, M., J. Mashimo, T. Kuroki, and K. Nose. 1994. Characterization of the TGF beta 1-inducible hic-5 gene that encodes a putative novel zinc finger protein and its possible involvement in cellular senescence. J. Biol. Chem. 269:26767–26774. [PubMed] [Google Scholar]
- 46.Sieg, D. J., D. Ilic, K. C. Jones, C. H. Damsky, T. Hunter, and D. D. Schlaepfer. 1998. Pyk2 and Src-family protein-tyrosine kinases compensate for the loss of FAK in fibronectin-stimulated signaling events but Pyk2 does not fully function to enhance FAK-cell migration. EMBO J. 17:5933–5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tachibana, K., T. Sato, N. D’Avirro, and C. Morimoto. 1995. Direct association of pp125FAK with paxillin, the focal adhesion-targeting mechanism of pp125FAK. J. Exp. Med. 182:1089–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomas, S. M., M. Hagel, and C. E. Turner. 1999. Characterization of a focal adhesion protein, Hic-5, that shares extensive homology with paxillin. J. Cell Sci. 112:181–190. [DOI] [PubMed] [Google Scholar]
- 49.Thomas, S. M., P. Soriano, and A. Imamoto. 1995. Specific and redundant roles of Src and Fyn in organizing the cytoskeleton. Nature 376:267–271. [DOI] [PubMed] [Google Scholar]
- 50.Turner, C. E. 1991. Paxillin is a major phosphotyrosine-containing protein during embryonic development. J. Cell Biol. 115:201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Turner, C. E. 1994. Paxillin: a cytoskeletal target for tyrosine kinases. Bioessays 16:47–52. [DOI] [PubMed] [Google Scholar]
- 52.Turner, C. E., M. C. Brown, J. A. Perrotta, M. C. Riedy, S. N. Nikolopoulos, A. R. McDonald, S. Bagrodia, S. Thomas, and P. S. Leventhal. 1999. Paxillin LD4 motif binds PAK and PIX through a novel 95-kD ankyrin repeat, ARF-GAP protein: a role in cytoskeletal remodeling. J. Cell Biol. 145:851–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Turner, C. E., J. R. J. Glenney, and K. Burridge. 1990. Paxillin: a new vinculin-binding protein present in focal adhesions. J. Cell Biol. 111:1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weed, S. A., Y. Du, and J. T. Parsons. 1998. Translocation of cortactin to the cell periphery is mediated by the small GTPase Rac1. J. Cell Sci. 111:2433–2443. [DOI] [PubMed] [Google Scholar]
- 55.Weng, Z., J. A. Taylor, C. E. Turner, J. S. Brugge, and D. C. Seidel. 1993. Detection of Src homology 3-binding proteins, including paxillin, in normal and v-Src-transformed BALB/c 3T3 cells. J. Biol. Chem. 268:14956–14963. [PubMed] [Google Scholar]
- 56.Xu, W., H. Baribault, and E. D. Adamson. 1998. Vinculin knockout results in heart and brain defects during embryonic development. Development 125:327–337. [DOI] [PubMed] [Google Scholar]
- 57.Yang, J. T., B. L. Bader, J. A. Kreidberg, C. M. Ullman, J. E. Trevithick, and R. O. Hynes. 1999. Overlapping and independent functions of fibronectin receptor integrins in early mesodermal development. Dev. Biol. 215:264–277. [DOI] [PubMed] [Google Scholar]
- 58.Yang, J. T., H. Rayburn, and R. O. Hynes. 1993. Embryonic mesodermal defects in alpha 5 integrin-deficient mice. Development 119:1093–1105. [DOI] [PubMed] [Google Scholar]
- 59.Yang, J. T., H. Rayburn, and R. O. Hynes. 1995. Cell adhesion events mediated by alpha 4 integrins are essential in placental and cardiac development. Development 121:549–560. [DOI] [PubMed] [Google Scholar]
- 60.Zhang, Z., K. Vuori, H. Wang, J. C. Reed, and E. Ruoslahti. 1996. Integrin activation by R-ras. Cell 85:61–69. [DOI] [PubMed] [Google Scholar]
- 61.Zhong, C., W. M. Chrzanowska, J. Brown, A. Shaub, A. M. Belkin, and K. Burridge. 1998. Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. J. Cell Biol. 141:539–551. [DOI] [PMC free article] [PubMed] [Google Scholar]