Abstract
Mitogen-activated protein kinase (MAPK) pathways coordinate critical cellular responses to mitogens, stresses, and developmental cues. The coupling of MAPK kinase kinase (MAP3K) → MAPK kinase (MEK) → MAPK core pathways to cell surface receptors remains poorly understood. Recombinant forms of MAP3K MEK kinase 1 (MEKK1) interact in vivo and in vitro with the STE20 protein homologue germinal center kinase (GCK), and both GCK and MEKK1 associate in vivo with the adapter protein tumor necrosis factor (TNF) receptor-associated factor 2 (TRAF2). These interactions may couple TNF receptors to the SAPK/JNK family of MAPKs; however, a molecular mechanism by which these proteins might collaborate to recruit the SAPKs/JNKs has remained elusive. Here we show that endogenous GCK and MEKK1 associate in vivo. In addition, we have developed an in vitro assay system with which we demonstrate that purified, active GCK and TRAF2 activate MEKK1. The RING domain of TRAF2 is necessary for optimal in vitro activation of MEKK1, but the kinase domain of GCK is not. Autophosphorylation within the MEKK1 kinase domain activation loop is required for activation. Forced oligomerization also activates MEKK1, and GCK elicits enhanced oligomerization of coexpressed MEKK1 in vivo. These results represent the first activation of MEKK1 in vitro using purified proteins and suggest a mechanism for MEKK1 activation involving induced oligomerization and consequent autophosphorylation mediated by upstream proteins.
Cells are continuously and often simultaneously exposed to a variety of extracellular signals to which they must mount rapid and appropriate responses. Protein Ser/Thr kinases of the mitogen-activated protein kinase (MAPK) family are critically important to the coordinated responses of cells to mitogenic, developmental, and stress stimuli. All MAPKs are regulated as part of three-tiered core signaling modules wherein MAPKs are activated by Tyr/Thr phosphorylation catalyzed by MAPK/extracellular signal-regulated kinase (ERK) kinases (MEKs). MEKs, in turn, are activated by Ser/Thr phosphorylation catalyzed by MAPK kinase kinases (MAP3Ks). Multiple parallel MAPK core pathways allow the cell to respond contemporaneously and appropriately to multiple divergent inputs (21, 22).
The stress-activated protein kinases/c-Jun NH2-terminal kinases (SAPKs/JNKs) and the p38 MAPKs are two MAPK groups activated by a wide variety of stresses and proinflammatory stimuli. Of particular importance, the SAPKs/JNKs and p38s are activated by cytokines of the tumor necrosis factor (TNF) family. The SAPKs/JNKs and p38s are potent upstream activators of the activator protein 1 (AP-1) transcription factor. AP-1 is a heterodimer that consists typically of c-Jun complexed with a member of the c-fos or activating transcription factor family. The SAPKs/JNKs regulate AP-1 by phosphorylating and activating the trans-activating function of c-Jun and activating transcription factor 2 and by phosphorylating and activating Elk1, a component of the serum response factor transcription factor that trans activates c-fos. Once activated, AP-1 trans activates numerous stress- and mitogen-regulated genes (14, 21, 22). Consistent with their central role in the regulation of gene expression, genetic studies indicate that mammalian SAPKs/JNKs are important in neuronal development, CD4+ T-helper-cell differentiation, T-cell activation, and the proapoptotic response to genotoxins (6, 7, 19, 31, 32, 38, 45).
Two MEKs are known to lie upstream of the SAPKs/JNKs: SAPK/ERK kinase 1/MAPK kinase 4 (SEK1/MKK4) and MKK7. Genetic and biochemical studies indicate that MKK7 is activated by all stimuli that recruit the SAPKs/JNKs, while SEK1/MKK4 is activated by a more restricted spectrum of agonists that includes certain environmental stresses and, to a lesser extent, inflammatory cytokines (21, 22).
At least a dozen Ser/Thr kinases falling into several protein kinase families have been implicated as MAP3Ks upstream of the SAPKs/JNKs. These include the MEK kinases (MEKKs) and the mixed-lineage kinases (MLKs) (21, 22). MAP3Ks are the most proximal elements of MAPK core pathways. Accordingly, MAP3Ks represent conduits through which specific cell surface stimuli signal to specific MAPK groups. The sheer number of MAP3Ks identified thus far is daunting, and a central unresolved issue is the molecular basis of MAP3K regulation. Such an understanding would clarify the molecular basis by which extracellular signals couple to MAPK core pathways.
MEKK1 is a 190-kDa MAP3K (23, 42, 44). Genetic and biochemical studies indicate that MEKK1 is strikingly selective for the SAPKs/JNKs (42, 44, 49). These studies also indicate that MEKK1 is an effector for TNF (2, 41). TNF recruitment of SAPK/JNK requires the adapter protein TNF receptor-associated factor 2 (TRAF2), which couples indirectly to the type 1 TNF receptor through the platform adapter protein TNF receptor-associated death domain protein (1). Coimmunoprecipitation studies indicate that MEKK1 and TRAF2 can interact in vivo in a TNF-dependent manner. Moreover, coexpression with TRAF2 activates MEKK1 in vivo. Oligomerization may mediate TRAF2 activation of MEKK1 (2); however, the molecular mechanism by which TRAF2 activates MEKK1 has heretofore not been established.
Germinal center kinase (GCK) is the founding member of the GCKs, a group of Ser/Thr kinases homologous to the Saccharomyces cerevisiae Ste20p (20). Studies of the S. cerevisiae pheromone response pathway indicate that Ste20p is a direct upstream activator of the MAP3K Ste11p (39). In keeping with the placement of Ste20p homologues as proximal activators of MAPK pathways, several members of the GCK group are potent and selective activators of the SAPKs/JNKs in vivo. Like MEKK1, GCK is activated by TNF and associates in vivo with TRAF2 (29, 48). In addition, recombinant forms of GCK and MEKK1 can associate in vivo and in vitro (48). Recombinant constructs of at least two other members of the GCK group, hematopoietic progenitor kinase 1 (HPK1) and Nck-interacting kinase (NIK), also activate the SAPKs/JNKs in vivo and can also bind MEKK1 in vivo (12, 36). However, a regulatory relationship between these STE20 protein homologues and MEKK1 has not been established.
Taken together, these findings imply a linear pathway wherein GCK couples TRAF2 to MEKK1. Still, the exact function of TRAF2 and GCK with regard to MEKK1 regulation is unclear. Accordingly, we used in vitro methods and purified proteins to begin to investigate the molecular basis of MEKK1 regulation by GCK and TRAF2. We find that endogenous GCK and MEKK1 associate in vivo. Moreover, as with TRAF2, coexpression with GCK activates MEKK1 in vivo. Coincident with MEKK1 activation, GCK stimulates MEKK1 autophosphorylation in vivo and in vitro but does not itself significantly phosphorylate the full-length MEKK1 polypeptide. We also show that purified GCK, as well as a purified construct consisting of the noncatalytic carboxyl-terminal regulatory domain of GCK, can activate MEKK1 in vitro. Activation of MEKK1 by GCK likely requires phosphorylation at two autophosphorylation sites, T1381 and T1393 (4, 34), in the MEKK1 kinase activation loop. We also show that purified TRAF2 can activate MEKK1 in vitro in a reaction that requires the TRAF2 RING effector domain. TRAF2 and GCK activation of MEKK1 is not additive in vitro. We find that GCK can enhance MEKK1 oligomerization in vivo and that forced oligomerization of MEKK1 in vivo triggers MEKK1 autophosphorylation and is sufficient for activation. We also observe that GCK, but not TRAF2, can activate the MAP3K MLK3, a member of the mixed-lineage family of Ser/Thr kinases. These findings represent the first in vitro activation of MEKK1 using purified components. Taken together, our results indicate that TRAF2 and GCK can directly and probably independently activate MEKK1 and that MEKK1 activation likely involves induced proximity and/or oligomerization and coincides with autophosphorylation. GCK may also recruit other MAP3Ks, including members of the MLK family.
MATERIALS AND METHODS
Plasmids and constructs.
The following vectors were used: pEBG for expression of glutathione S-transferase (GST)-tagged proteins [GST, GST-tagged human full-length GCK and GST-tagged carboxyl-terminal regulatory domain [CTD] of GCK (48), mouse SEK1(K129R) and rat SAPK-p54α1 [JNK2β2]) (21, 22), pCMV1, for expression of untagged polypeptides (human GCK), pCMV5 for expression of M2 FLAG- or Myc-tagged polypeptides (FLAG-human GCK, FLAG-rat MEKK1, FLAG-human MLK3, and Myc-human MEKK3), pMT3 for expression of hemagglutinin (HA)-tagged polypeptides (HA- rat MEKK1 mutants) and pFPK3E(HA) for expression of constructs tagged at the amino terminus with an HA epitope plus three copies of the FK506 binding domain of FKBP12 (FKBP-HA-rat MEKK1). pCEP4-HA-rat wild-type MEKK1 was kindly provided by Melanie Cobb. FKBP-HA-MEKK1 and FLAG-MEKK1 constructs were prepared by PCR amplification and cloning according to standard methods. Mutagenesis of MEKK1 was performed with a transformer site-directed mutagenesis kit (Clontech), and all mutants were confirmed by DNA sequencing.
Cells and transfections.
Human embryonic kidney 293 cells were cultured in Dulbecco’s modified Eagle medium supplemented with 10% fetal bovine serum. Cells were transfected with 0.05 to 3 μg of plasmid DNA by using Lipofectamine (Gibco-BRL) according to the manufacturer’s instructions. Transfected DNA levels were balanced with empty plasmid, and cells were harvested 16 to 20 h after transfection. Coimmunoprecipitation of endogenous or GCK and MEKK1 as well as recombinant GCK and MLK3 was performed as described previously (48).
Treatment of cells with AP1510.
Cells were transfected with 50 ng of FKBP-HA-MEKK1 or FKBP-HA empty vector and 1 μg of GST-SAPK plasmid DNA per 10-cm dish. Sixteen hours later, the medium was replaced with medium containing ethanol vehicle or 100 nM AP1510 (Ariad Pharmaceuticals), and the cells were incubated at 30°C for 3 h. TNF treatment was carried out for 5 min at 30°C at a final concentration in the medium of 100 ng/ml. Cells were lysed, GST-SAPK was purified on glutathione agarose beads, and SAPK activity was assayed by using GST-c-Jun(1–135) as a substrate (48). For phosphopeptide analysis, cells were transfected with 0.5 to 3 μg of HA-MEKK1, HA-MEKK1, and FLAG-GCK and labeled with 32PO4 in phosphate-free Dulbecco’s modified Eagle medium for 2 h. The MEKK1 bands were visualized by autoradiography and excised from the gel for further analysis by two-dimensional tryptic phosphopeptide mapping (3).
In vitro activation of MEKK1 by GCK, GCK CTD, TRAF2, or TRAF2ΔRING.
The method for purification from transfected 293 cells of the GST, GST-GCK, GST-GCK CTD, GST-TRAF2, GST-TRAF2(ΔRING), and GST-SEK1(K129R) proteins was described previously (48). For these assays, HA-MEKK1 was immunoprecipitated from three to five 10-cm dishes of 293 cells expressing 1 μg of the HA-MEKK1 plasmid DNA per dish. The procedure for lysis and immunoprecipitation has been described (48). MEKK1 beads (20 μl, estimated to contain ∼0.5 pmol of MEKK1) were resuspended in 40 μl of assay buffer (20 mM MOPS [morpholinepropanesulfonic acid, pH 7.2], 2 mM EGTA, 10 mM MgCl2, 0.1% [wt/vol] Triton X-100, 1 mM dithiothreitol). Assay buffer (20 μl) containing 25 pmol of either GST or GST-GCK (wild type or CTD) and/or 70 pmol of GST-TRAF2 (wild type or ΔRING) and 2 μg of GST-SEK1(K129R) were added to the beads. The reactions were started with the addition of 15 μl of [γ-32P]ATP-MgCl2 mixture to give final concentrations of 10 μM [γ-32P]ATP and 10 mM MgCl2. The reactions were allowed to proceed for 5 min at 30°C and then stopped with the addition of Laemmli sample buffer. Proteins were resolved on 15% sodium dodecyl sulfate (SDS)-polyacrylamide gels, transferred to polyvinylidene difluoride membranes, and detected with anti-FLAG (Kodak), anti-GST (Upstate Biotechnology, Inc.), or anti-HA antibodies.
For assays of activation of eluted, soluble MEKK1, three to five 10-cm dishes of 293 cells were transfected with 2 μg of HA-MEKK1 or the different FLAG-tagged GCK or TRAF2 constructs or with empty FLAG vector. Proteins were immunoprecipitated with the relevant antibodies and eluted with 500 μM cognate FLAG or HA antigen peptide (Sigma) for 1 h at 25°C. Glycerol was added to 50%, and the proteins were stored at −20°C until use. For assays, 20 μl of purified MEKK1 or storage buffer was mixed with 20 μl of purified FLAG-tagged protein or storage buffer. Reactions were started with the addition of GST-SEK1(K129R) and [32P]ATP-Mg2+ as described above. Reaction products were resolved by SDS-polyacrylamide gel electrophoresis (PAGE) and analyzed as above.
Sucrose density gradient centrifugation.
293 cells were transfected with HA-MEKK1 and either vector or FLAG-GCK. Extracts were prepared as described above except that detergent was omitted, and the extracts were layered onto sucrose density gradients (5 to 60% sucrose) in detergent-free cell lysis buffer. Molecular weight standards (Amersham) were included for calibration purposes. Samples were subjected to centrifugation (34,000 rpm in a Beckman SW41 Ti rotor) for 16 h at 4°C. Fractions (150 μl) were collected and assayed for SEK1 phosphorylation or HA immunoreactivity.
RESULTS
Endogenous MEKK1 and GCK interact in vivo: in vivo activation of MEKK1 by GCK.
TNF activates both MEKK1 and GCK (2, 29), and our previous studies indicated that GCK could interact in vivo and in vitro with TRAF2 and recombinant MEKK1 (48). However, the significance of GCK for the regulation of MEKK1 was unclear. Accordingly, we wished to determine if the endogenous forms of GCK and MEKK1 could interact in vivo. RAMOS is a Burkitt lymphoma cell line that expresses a comparatively large amount of endogenous GCK (15). GCK is substantially activated by TNF in RAMOS cells (29). We find that endogenous GCK and MEKK1 associate constitutively in these cells (Fig. 1A). Treatment with TNF does not increase the binding of GCK and MEKK1 (data not shown). To determine if MEKK1 could be activated in vivo by GCK, we expressed full-length MEKK1 with GCK, immunoprecipitated the MEKK1, and assayed it for autophosphorylation and for phosphorylation of the MEKK1 substrate SEK-1/MKK4(K129R) (44). From Fig. 1, it is clear that coexpression with GCK activates MEKK1 autophosphorylating and SEK1/MKK4 kinase activity ∼3-fold in vivo.
FIG. 1.
In vivo binding of endogenous GCK and MEKK1: activation of MEKK1 by coexpressed GCK in vivo. (A) Binding of endogenous GCK and MEKK1. RAMOS cells were lysed and subjected to immunoprecipitation (IP) with anti-GCK and immunoblotting (IB) with anti-MEKK1 or immunoprecipitation with anti-MEKK1 and immunoblotting with anti-GCK. Crude lysates were immunoblotted with the GCK and MEKK1 antibodies as well. (B) GCK activates MEKK1 in vivo. 293 cells were transfected with a low dose of HA-MEKK1 plasmid and with either vector or FLAG-GCK as described in Materials and Methods. MEKK1 was immunoprecipitated with anti-HA and assayed for SEK1(K129R) phosphorylation and autophosphorylation (left panel). The right panel documents expression of the transfected plasmids and the level of GST-SEK1(K129R) used in the MEKK1 assays.
MEKK1 activation in vivo coincides with increased MEKK1 phosphorylation that resembles autophosphorylation.
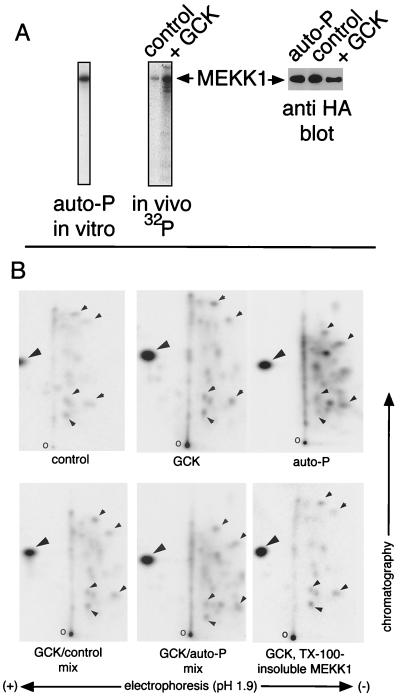
We have observed that deletion or mutation of GCK’s kinase domain impairs but does not eliminate the ability of recombinant GCK to activate the SAPK/JNK pathway in vivo (29, 48). Given that the kinase activity of GCK is necessary for optimal recruitment of the SAPKs/JNKs, we wished to determine if coexpression with GCK enhanced MEKK1 phosphorylation in vivo. 293 cells were transfected with GCK and MEKK1 and labeled metabolically with 32PO4. It is clear that coexpression with GCK results in a dramatic increase in MEKK1 phosphorylation in vivo (Fig. 2A). We subjected MEKK1 phosphorylated in vivo to two-dimensional tryptic phosphopeptide mapping and compared the maps to that of MEKK1 expressed without GCK and autophosphorylated in vitro. From Fig. 2B it is evident that the increase in MEKK1 phosphorylation that occurs in vivo upon expression with GCK does not coincide with the appearance of de novo phosphopeptides. Instead, there is a general increase in phosphorylation of all detectable tryptic phosphopeptides. Moreover, mixing experiments indicate that the MEKK1 phosphorylation pattern in vivo is essentially identical to that obtained in vitro upon autophosphorylation (Fig. 2B). This is true whether or not the MEKK1 phosphorylated in vivo is isolated from the Triton X-100-soluble or -insoluble (cytoskeleton-associated) fraction (Fig. 2B). We conclude from this that GCK either triggers MEKK1 autophosphorylation or phosphorylates sites on the MEKK1 polypeptide that also undergo autophosphorylation.
FIG. 2.
GCK stimulates MEKK1 phosphorylation in vivo: similarity to MEKK1 autophosphorylation. 293 cells were transfected with HA-MEKK1 and either vector or FLAG-GCK. A portion of the cells expressing MEKK1 alone and all of the cells expressing MEKK1 and GCK were metabolically labeled with 32PO4. The remaining cells expressing MEKK1 alone were left unlabeled. MEKK1 was immunoprecipitated with anti-HA. (A) MEKK1 phosphorylation. (Left) In vitro autophosphorylation of MEKK1 immunoprecipitated from unlabeled cells that did not coexpress GCK; (middle) in vivo phosphorylation of MEKK1 in cells expressing vector or GCK; (right) expression of the MEKK1 polypeptides. (B) Two-dimensional tryptic phosphopeptide mapping of MEKK1 in vivo phosphorylation and in vitro autophosphorylation (auto-P). Expression with GCK or in vitro autophosphorylation is indicated underneath each map. The large arrowheads indicate a prominent labeled spot, probably free phosphate, detected in each map. Smaller arrowheads indicate some of labeled phosphopeptides that are common to MEKK1 in vivo phosphorylation and in vitro autophosphorylation. o, origins.
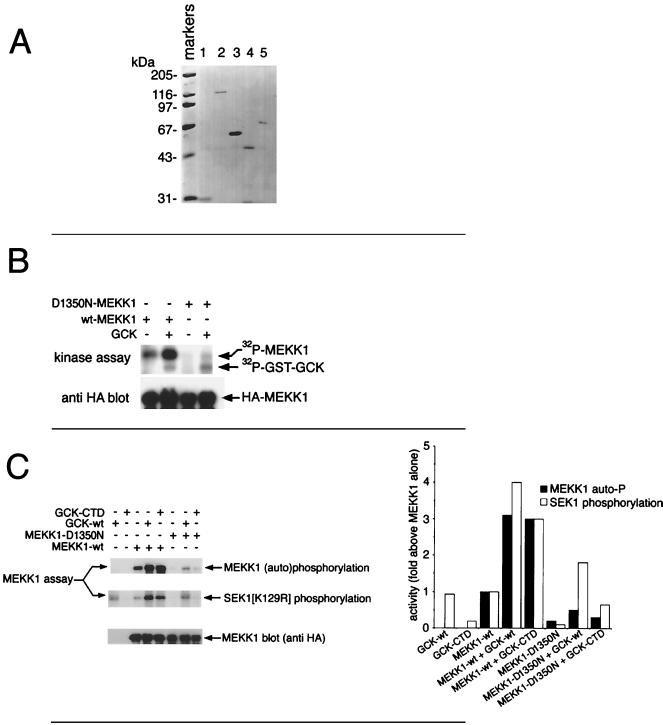
Support for the idea that GCK stimulates MEKK1 autophosphorylation comes from the in vitro experiment whose results are shown in Fig. 3B. D1350 is a residue in the phosphotransfer region of the MEKK1 kinase domain (42). Mutation of D1350 to N renders MEKK1 inactive. For Fig. 3B, either wild-type or D1350N MEKK1 (HA tagged) was immunoprecipitated and incubated in vitro with purified wild-type GST-GCK (purification shown in Fig. 3A). It is clear that GCK can trigger dramatic phosphorylation of wild-type MEKK1 in vitro. The D1350N mutation abolishes in vitro MEKK1 phosphorylation mediated by GCK, suggesting that the MEKK1 phosphorylation stimulated by GCK and revealed in the complex pattern of tryptic phosphopeptides in Fig. 2B is largely self catalyzed. This finding differs from the results of a previous study (48) in which it was observed that GCK could phosphorylate a fragment of MEKK1 consisting of residues 817 to 1221 in vitro. This difference suggests that residues 817 to 1221 of intact, full-length MEKK1 are not available for GCK-catalyzed phosphorylation in vitro.
FIG. 3.
In vitro activation of MEKK1 by purified GCK, GCK CTD, and TRAF2. (A) Purification of GST (lane 1), GST-GCK (lane 2), GST-GCK CTD (lane 3), GST-TRAF2ΔRING (lane 4), and GST-TRAF2 (lane 5). These preparations were used for the in vitro studies described herein (Fig. 3 to 5 and 7). Proteins were purified from transfected 293 cells as described previously (48), and 10% of each preparation was subjected to SDS-PAGE and Coomassie blue staining. (B) GCK stimulates MEKK1 autophosphorylation in vitro but does not itself significantly phosphorylate full-length MEKK1. HA-tagged wild type (wt) or kinase-dead (D1350N) MEKK1 was immunoprecipitated from transfected 293 cells and incubated with soluble GST-GCK and purified as described above, with [γ-32P]ATP. Incubations in the absence of GCK were performed with GST. Phosphorylation of the MEKK1 polypeptides was analyzed after SDS-PAGE and autoradiography (top). Expression of the MEKK1 polypeptides was determined by immunoblotting with anti-HA (bottom). (C) GCK activates MEKK1 in vitro. The kinase domain of GCK is dispensable for MEKK1 activation. Wild-type or D1350N HA-MEKK1 was immunoprecipitated as for panel B and incubated with an equimolar amount of purified GST-GCK or the GCK CTD (see above) plus [γ-32P]ATP. Incubations in the absence of GST-GCK or GST-GCK CTD were performed with GST. After a brief incubation, GST-SEK1(K129R) was added. The reaction was stopped with SDS-EDTA, and the samples were subjected to SDS-PAGE and autoradiography (top two panels, left) or immunoblotting with anti-HA. Kinase activity was assessed as MEKK1 autophosphorylation and phosphorylation of GST-SEK1(K129R). The bar graph on the right represent phosphorimager quantitation of the kinase assay results. Quantitation of MEKK1 autophosphorylation for samples not containing MEKK1 represents phosphorimager counts of mock anti-FLAG immunoprecipitations in the region of the gel corresponding to the migration position of MEKK1.
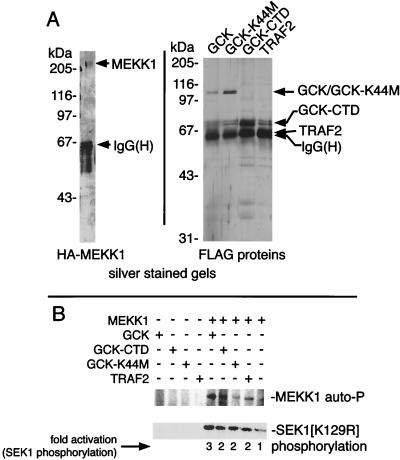
Activation of MEKK1 by GCK in vitro: activation does not require the kinase activity of GCK.
We next asked if in vitro incubation with GCK could activate the MAP3K function of MEKK1. The GCK polypeptide consists of an amino-terminal kinase domain (aa 1 to 278) and a carboxyl-terminal regulatory region, the CTD (amino acids [aa] 279 to 819) (15, 20). Given the previous finding that free GCK CTD could interact with MEKK1 in vivo and in vitro and, upon overexpression, could activate the SAPKs/JNKs (29, 48), we compared the ability of full-length GCK and free GCK CTD to activate MEKK1 in vitro. Accordingly, GST-GCK and GST-GCK CTD were purified by glutathione agarose chromatography and eluted with free glutathione as described previously (48). The purified proteins are shown in Fig. 3A. Wild-type and mutant MEKK1 were expressed as HA-tagged constructs and immunoprecipitated with anti-HA, and the immunoprecipitates were washed at high stringency (48). The immobilized MEKK1 polypeptides were incubated in vitro with purified GCK or the GCK CTD and with [γ-32P]ATP. MEKK1 substrate [GST-SEK1(K129R)] was then added. Wild-type GST-GCK substantially activated wild-type MEKK1’s SEK1 kinase activity in vitro. The GST-GCK CTD, which was devoid of SEK1 phosphorylating activity, also activated MEKK1 (Fig. 3C). Enhanced phosphorylation of the MEKK1 polypeptide was also observed upon incubation with either intact, wild-type GCK or GCK CTD (Fig. 3C), again supporting the idea that GCK fosters MEKK1 autophosphorylation coincident with activation.
Kinase-inactive MEKK1(D1350N) had little or no basal autophosphorylating or SEK1 kinase activity, and incubation of this construct with GCK resulted in no significant increase in overall SEK1 kinase activity above the small amount contributed by GCK itself. Incubation of MEKK1(D1350N) with GCK CTD was also without effect, stimulating neither an increase in MEKK1 phosphorylation nor a significant increase in SEK1 phosphorylation (Fig. 3C). This is consistent with the idea that neither the GCK nor GCK CTD preparation contained a contaminating MAP3K activity or a kinase that activated MEKK1 and, instead, that GCK directly fosters MEKK1 autophosphorylation-dependent activation.
MEKK1 activation by GCK requires MEKK1 autophosphorylation within the kinase domain activation loop.
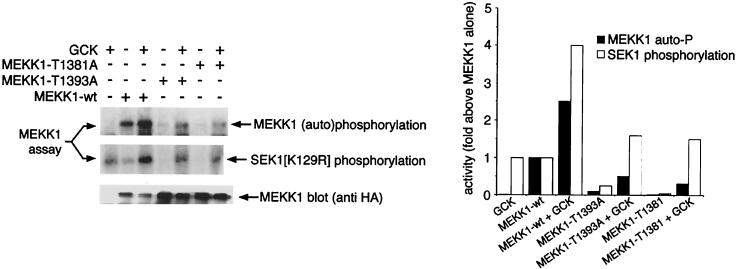
T1381 and T1393 are residues in the MEKK1 kinase domain activation loop that, in assays of amino-terminally truncated, constitutively active MEKK1 mutant constructs, have been shown to be autophosphorylation phosphoacceptor sites (4, 34). Autophosphorylation at T1381 and T1393 appears to be necessary for the kinase activity of these truncated MEKK1 mutants (4, 34). However, the relationship of phosphorylation of T1381 and T1393 to activation of full-length MEKK1 by upstream components is unclear. MEKK1(T1381A) and MEKK1(T1393A) had no basal activity and were not activated in vitro by GCK (Fig. 4). Thus, mutagenesis of T1381 and T1393 to alanine completely abolished detectable basal MEKK1 autophosphorylation and almost completely abolished GCK-stimulated MEKK1 autophosphorylation in our in vitro system, reducing MEKK1 autophosphorylation to levels too low for tryptic phosphopeptide mapping. Moreover, as with MEKK1(D1350N), incubation of MEKK1(T1381A) or MEKK1(T1393A) with GCK produced little or no increase in SEK1 phosphorylating activity in vitro, above the low level of activity contributed by GCK itself. From Fig. 3 and 4, we conclude that GCK can activate MEKK1 autophosphorylation and SEK1 kinase functions and that the kinase activity of GCK is largely dispensable for this process. We also conclude that mutagenesis of the critical autophosphorylation sites T1381 and T1393 abolishes in vitro MEKK1 activation by GCK, even if the remainder of the MEKK1 kinase domain and amino-terminal regulatory region are intact. Thus, in spite of the apparently complex pattern of MEKK1 autophosphorylation in vivo and in vitro (Fig. 2B), autophosphorylation within the MEKK1 kinase activation loop is necessary for activation by GCK.
FIG. 4.
Activation of MEKK1 by GCK requires the autophosphorylation phosphoacceptor sites T1381 and T1393. Wild-type HA-MEKK1, MEKK1(T1381A), and MEKK1(T1393A) were immunoprecipitated from transfected 293 cells as for Fig. 3B and incubated with purified GST-GCK (Fig. 3A). Control incubations (minus GCK) were performed with GST. GST-SEK1(K129R) was added, and the results were analyzed as for Fig. 3B. The graph shows a phosphorimager quantitation of the results in the autoradiograms.
Activation of MEKK1 by TRAF2 in vitro: requirement for the TRAF2 RING domain.
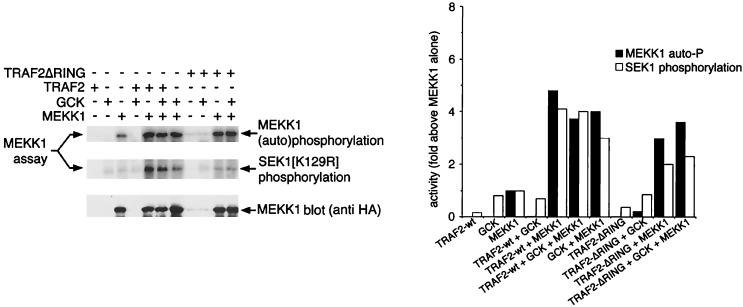
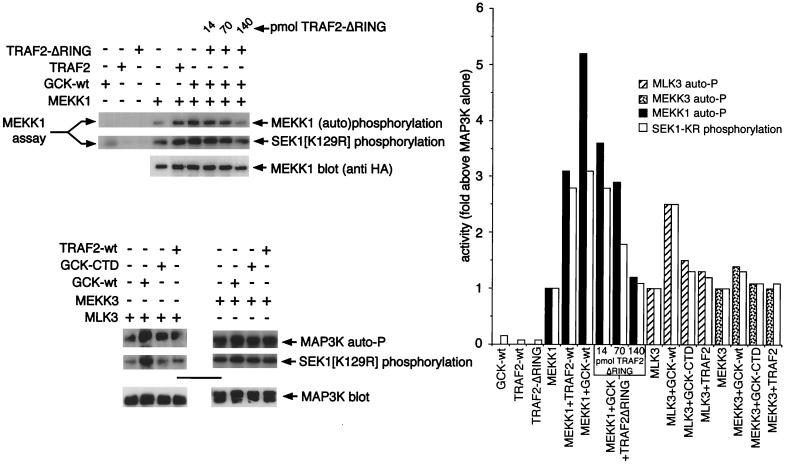
Recent studies have shown that TRAF2 can associate with both GCK and MEKK1 in vivo (2, 48). Moreover, TRAF2 activates coexpressed MEKK1 in vivo, and both GCK and MEKK1 are activated by TNF (2, 29, 48). Consistent with these observations, we observe that incubation of immobilized MEKK1 in vitro with purified, soluble TRAF2 (see Fig. 3A for purification) results in MEKK1 activation, measured as either (auto)phosphorylation of the MEKK1 polypeptide or phosphorylation of SEK1(K129R) (Fig. 5). As shown in Fig. 3B and 4, GCK also activates MEKK1 in vitro. However, the combination of TRAF2 and GCK does not activate MEKK1 in vitro to a greater degree than either TRAF2 or GCK alone (Fig. 5). Similar results are achieved when the GCK CTD is used to activate MEKK1 (data not shown). It is possible that maximum MEKK1 activation was achieved in these in vitro assays. However, it is also plausible, based on these results, that GCK and TRAF2 act independently, rather than coordinately, to activate MEKK1.
FIG. 5.
Activation of MEKK1 by TRAF2 and GCK is not additive; GCK also activates MLK3. (A) TRAF2 activation of MEKK1 in vitro: requirement for the TRAF2 RING domain. HA-MEKK1 (wild-type) was immunoprecipitated from 293 cells as for Fig. 3 and incubated with equimolar concentrations of purified (Fig. 3A) GST-TRAF2, GST-GCK, or GST-TRAF2ΔRING as described for Fig. 3B. Controls were incubated with GST alone. GST-SEK1 was added as for Fig. 3B, and the incubation was continued. Samples were subjected to SDS-PAGE and either autoradiography or immunoblotting as for Fig. 3 and 4. The bar graph shows a phosphorimager quantitation of the kinase assays.
TRAF2 consists of an amino-terminal RING effector domain, a central zinc finger region, and a carboxyl-terminal TRAF domain (1). Activation of MEKK1 by TRAF2 in vivo requires the RING domain (2). Consistent with this, we find that a purified TRAF2 construct in which the RING domain has been deleted (TRAF2ΔRING; see Fig. 3A for purification) fails to activate MEKK1 in vitro to a degree comparable to that achieved in vitro by an equimolar amount of wild-type TRAF2. Moreover, TRAF2ΔRING may in fact suppress in vitro GCK activation of MEKK1 when the two proteins are employed together as potential MEKK1 activators (Fig. 5; see also below and Fig. 7). TRAF2ΔRING can stimulate MEKK1 autophosphorylation, albeit to a lesser degree than wild-type TRAF2 or GCK (Fig. 5), coincident with the reduced ability of TRAF2ΔRING to activate MEKK1’s SEK1 kinase activity. Thus, the TRAF2 RING domain is necessary for optimal activation of MEKK1 in vitro.
FIG. 7.
Increasing concentrations of TRAF2ΔRING suppresses GCK activation of MEKK1 in vitro. GCK can also activate MLK3 but not MEKK3. (Top, left) Titration of increasing concentrations of purified (Fig. 3A) TRAF2ΔRING suppresses in vitro activation of MEKK1 by GCK. Immobilized MEKK1 was incubated with GCK in the presence or absence of the indicated amounts of TRAF2ΔRING. (Bottom, left) GCK also activates MLK3 but fails to activate MEKK3 in vitro. Immobilized FLAG-MLK3 or Myc-MEKK3 was incubated with the purified GCK or TRAF2 preparation (Fig. 3A) used in the titration experiment; however, samples were run on separate SDS-PAGE gels. The method was as described for Fig. 3 to 5. Activity was measured as phosphorylation of substrate protein [GST-SEK1(K129R)] and autophosphorylation (auto-P). At right is a quantitation of the results.
GST can spontaneously oligomerize. Thus, it could be argued that our findings that GCK and TRAF2 could activate MEKK1 in vitro might reflect the ability of GST-tagged proteins to spontaneously oligomerize. Alternatively, use of immobilized MEKK1 in the assays shown in Fig. 1 to 5 might not reflect in vivo conditions. Accordingly, we determined if soluble, purified preparations of MEKK1, various FLAG-GCK constructs, and FLAG-TRAF2 behaved similarly to immobilized MEKK1 and GST-GCK or TRAF2. Thus, GCK, GCK(K44M) (kinase-dead), GCK CTD, and TRAF2 were expressed as FLAG-tagged polypeptides and immunoprecipitated with anti-FLAG. The polypeptides were eluted with free FLAG peptide (Fig. 6A, right). HA-MEKK1 was similarly expressed, immunoprecipitated, and eluted with HA peptide (Fig. 6A, left). The soluble MEKK1 was diluted in order to minimize spontaneous activation and then tested for activation by the various GCK constructs and by TRAF2. Control MEKK1 (minus GCK or TRAF2) was incubated with material prepared from FLAG vector-transfected cells and processed in a manner identical to the GCK and TRAF2 preparations. Consistent with the results in Fig. 1 to 5, we observed that the SEK1-phosphorylating activity of soluble MEKK1 was activated by soluble FLAG-GCK in a manner analogous to that incurred by GST-GCK when assayed against immobilized MEKK1. As with GST-GCK, this activation did not require the FLAG-GCK’s kinase activity inasmuch as FLAG-tagged K44M GCK and the GCK CTD were each capable of activating the MEKK1 in vitro (Fig. 6B). Thus, use of immobilized MEKK1 and/or the presence of GST tags on the GCK or TRAF2 constructs does not artificially trigger GCK or TRAF2 activation of MEKK1.
FIG. 6.
Soluble, purified MEKK1 is activated by soluble, purified GCK or TRAF2 in vitro. HA-MEKK1 and FLAG-GCK, GCK(K44M), GCK CTD, and TRAF2 were immunoprecipitated from transfected 293 cells and eluted with the cognate antigen peptide. (A) Silver-stained gels of the purified HA-MEKK1 and FLAG-GCK and TRAF2 constructs. Note that the immunoglobulin G heavy chain [IgG(H)], from the immunoprecipitations, is still present in the eluted protein preparations; however, the free-peptide concentration used in the elutions (100 μM) is far in excess of the IgG concentrations employed in the immunoprecipitation. FLAG-TRAF2 comigrates with the IgG and is not visible in the preparations. (B) Assays for activation of purified MEKK1 by the purified GCK and TRAF2 constructs.
We have shown that both the TRAF2 TRAF domain and MEKK1 interact with an overlapping region of the GCK CTD (48). Thus, in vitro coincubation of TRAF2ΔRING, GCK, and MEKK1 (Fig. 5) may result in competition between TRAF2 and MEKK1 for binding to GCK, thereby hindering the ability of GCK to activate MEKK1. Consistent with this, we find that titration of purified TRAF2ΔRING into an in vitro incubation of GCK and MEKK1 progressively suppresses GCK-mediated activation of MEKK1 (Fig. 7).
GCK can also activate MLK3 in vitro.
The results presented in Fig. 1 to 6 indicate that MEKK1 is an effector for GCK. We wished next to determine if other MAP3Ks might be GCK effectors. MLK3 is a member of the MLK family of MAP3Ks and is a specific and potent activator of the MEKs MKK4 and MKK7. As with all MLKs, MLK3 contains an amino-terminal kinase domain that is structurally similar to both Ser/Thr and Tyr kinases but exhibits only Ser/Thr kinase activity in vivo and in vitro. The kinase domain is followed by a leucine zipper and a Cdc42/Rac interaction and binding domain that interacts with the Rho family GTPases Cdc42 and Rac. At the MLK3 carboxyl terminus is a proline-rich region containing potential binding sites for polypeptides with Src homology 3 (SH3) domains. MLK3 and the related MLK2 also possess SH3 domains immediately amino terminal to the kinase domains (21, 22). The GCK group kinase HPK1 not only interacts with MEKK1 in vivo (12) but also interacts in vivo with MLK3 (16, 37). In this instance, the interaction requires the MLK3 SH3 domain and the carboxyl-terminal two of four SH3 binding motifs in the HPK1 polypeptide (16). Thus, GCK-like kinases may regulate MLK enzymes.
From Fig. 7, it is clear that in vitro incubation of immobilized MLK3 with GCK activates MLK3. Interestingly, in vitro incubation of MLK3 with the GCK CTD or with TRAF2 produced little or no increase in MLK3-catalyzed SEK1 phosphorylating activity above the low level of activity contributed by GCK itself. Thus, the kinase activity of GCK may be more relevant to MLK3 regulation than to MEKK1 regulation. SEK1 phosphorylation catalyzed by MEKK3, a promiscuous MAP3K that can recruit all known mammalian MAPK pathways (22), is not activated in vitro by GCK, and coincubation in vitro of MEKK3 and purified GCK fails to increase SEK1 phosphorylation beyond the small amount of SEK1 phosphorylation contributed by GCK itself, nor is MEKK3 directly activated by TRAF2 in vitro (Fig. 7).
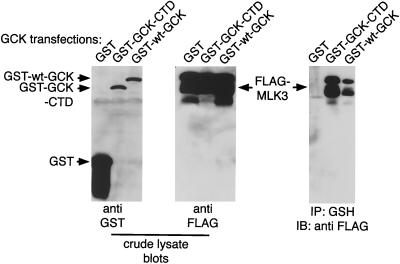
Consistent with the ability of GCK to activate MLK3 in vitro, we find that GCK, through its CTD, can interact in vivo with MLK3 (Fig. 8)—an interaction similar to that between HPK1 and MLK3 (16). Thus, GCK may have a broader spectrum of effectors that includes MEKK1 and MLK3 but not MEKK3. Our findings do not rule out MEKK3 as an effector for TRAF2. However, our results would suggest that if MEKK3 is recruited by TRAF2, the activation is not direct and does not proceed through GCK.
FIG. 8.
In vivo association of GCK and MLK3. 293 cells were transfected with GST-GCK or GCK CTD and FLAG-MLK3. GCK constructs were purified on glutathione agarose and subjected to SDS-PAGE and immunoblotting with anti-FLAG to detect associated MLK3.
Oligomerization is sufficient to activate MEKK1: GCK prompts MEKK1 oligomerization in vivo.
We next wished to determine how GCK and TRAF2 might foster MEKK1 autophosphorylation and activation. Several MAP3Ks undergo activation upon forced oligomerization. Thus, fusion proteins consisting of the stress-regulated MAP3K apoptosis signal-regulating kinase-1 (ASK1) and DNA gyrase can be forced to oligomerize in the presence of the binary drug coumermycin. Expression of ASK1-DNA gyrase allows the coumermycin-dependent activation of coexpressed SAPK/JNK (11). Similarly, expression of DNA-gyrase fused to the mitogenic MAP3K Raf-1 permits coumermycin-dependent activation of ERK1, while expression of FK506 binding protein 12 (FKBP12) fused to Raf1 allows activation of ERK1 in response to the binary drug FK1012 (10, 26, 35). Furthermore, TRAF2 signaling apparently involves oligomerization. Thus, expression of a construct consisting of FKBP12 fused to the RING and zinc fingers of TRAF2 (FKBP-TRAF2) allows FK1012-dependent activation of SAPK/JNK (2). FK1012 treatment of cells expressing FKBP-TRAF2 also results in the formation of insoluble FKBP-TRAF2 aggregates. If MEKK1 is coexpressed with FKBP-TRAF2, MEKK1 appears in these aggregates in an FK1012-dependent manner (2). While this phenomenon may merely reflect a carrier protein effect, it is equally possible that TRAF2 oligomerization can, in turn, promote MEKK1 binding, oligomerization, and activation.
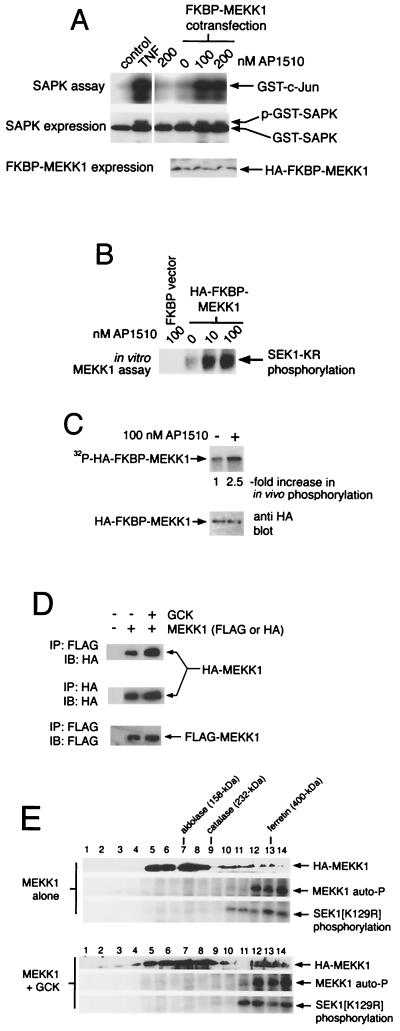
Both MEKK1 and GCK are constitutively active when expressed at high levels (29, 42), suggesting that increasing the concentration of these enzymes in the cell results in spontaneous, stimulus-independent oligomerization and activation. Consistent with this, we find that forced oligomerization of MEKK1 expressed at low levels results in vigorous activation of coexpressed SAPK/JNK. We generated a construct consisting of FKBP12 fused to the amino terminus of full-length MEKK1 (FKBP-MEKK1). Expression of this construct alone, at low levels (50 ng/10-cm plate), failed to activate coexpressed SAPK/JNK (Fig. 9A). Similarly, addition of AP1510, an improved derivative of FK1012, did not activate SAPK/JNK in the absence of coexpressed FKBP-MEKK1. By contrast, addition of AP1510 to cells coexpressing SAPK/JNK and FKBP-MEKK1 resulted in robust SAPK/JNK activation (Fig. 9A). FKBP-MEKK1 can also be immunoprecipitated from transfected-cell extracts and activated in vitro with AP1510 (Fig. 9B). Labeling cells with 32PO4 revealed that in vivo AP1510-dependent oligomerization stimulated enhanced FKBP-MEKK1 phosphorylation (Fig. 9C).
FIG. 9.
Oligomerization is sufficient to trigger MEKK1 activation and phosphorylation in vivo. GCK promotes MEKK1 oligomerization. (A) 293 cells were transfected with GST-SAPK and either empty FKBP12-HA vector or FKBP12-HA-MEKK1. Cells were then treated with vehicle or the indicated concentrations of AP1510 for 1 h. Control cells were treated with vehicle or TNF (100 ng/ml). GST-SAPK was purified on glutathione agarose and assayed. (B) Oligomerization of immunoprecipitated MEKK1 results in activation. FKBP-HA-MEKK1 was expressed in 293 cells and immunoprecipitated with anti-HA. Immunoprecipitates were treated with vehicle or the indicated concentration of AP1510 plus 100 μM [32P]ATP. Alternatively, a blank immunoprecipitate was identically treated with 100 nM AP1510. SEK1(K129R) was added as a substrate for MEKK1, and the activity of MEKK1 towards the SEK1 polypeptide is shown. (C) Forced oligomerization of MEKK1 enhances its in vivo phosphorylation. 293 cells expressing FKBP-HA-MEKK1 were labeled with 32PO4 and treated with vehicle or AP1510 (200 nM). FKBP-HA-MEKK1 was recovered by immunoprecipitation with anti-HA and subjected to SDS-PAGE and autoradiography or to anti-HA immunoblotting. (D) Expression with GCK enhances spontaneous MEKK1 oligomerization in vivo. 293 cells were transfected with FLAG- and HA-MEKK1 plus either vector or untagged GCK. FLAG-MEKK1 was immunoprecipitated (IP) and subjected to SDS-PAGE and immunoblotting (IB) with anti-HA to detect the presence of HA-MEKK1 in FLAG-MEKK1 immunoprecipitates, an indication of MEKK1 oligomerization. FLAG- and HA-MEKK1 immunoprecipitates were also immunoblotted with cognate antibodies to document even expression of the MEKK1 constructs. (E) Sucrose density gradient centrifugation of MEKK1 expressed alone or coexpressed with GCK. Active MEKK1 exists as a high-molecular-weight oligomer, and GCK shifts MEKK1 into this oligomer. Cells were transfected with HA-MEKK1 alone or with FLAG-GCK. Cell extracts were subjected to density gradient centrifugation through sucrose (5 to 60%). Fractions (50 μl) were collected and assayed for HA-MEKK1 immunoreactivity and SEK1 phosphorylating activity. The positions of molecular weight standards are shown.
We also observe that expression of GCK at levels that ensure its activation in vivo can enhance oligomerization of coexpressed MEKK1. We coexpressed untagged GCK, at high enough concentrations to trigger spontaneous activation (29, 48), with two heterologously tagged (HA or FLAG) full-length MEKK1 constructs. MEKK1 oligomerization was defined as the presence of HA-MEKK1 immunoreactivity in FLAG-MEKK1 immunoprecipitates. From Fig. 9D it is clear that expression of GCK increases the amount of HA-MEKK1 recovered in FLAG-MEKK1 immunoprecipitates, suggesting that active GCK fosters enhanced MEKK1 oligomerization.
As further confirmation that GCK enhances MEKK1 oligomerization, we performed sucrose density gradient fractionation of extracts from cells expressing MEKK1 alone or MEKK1 and GCK (Fig. 9E). In cells expressing MEKK1 alone, the MEKK1 polypeptide split into two peaks. An apparently monomeric peak, migrating between the aldolase (150-kDa) and catalase (232-kDa) standards, contained most HA-MEKK1 immunoreactivity but no detectable MEKK1 SEK1 kinase or autophosphorylating activity. A second peak, migrating with ferritin (∼400-kDa), contained substantially less HA-MEKK1 immunoreactivity and all detectable MEKK1 SEK1 kinase and autophosphorylating activity (Fig. 9E). MEKK1 expressed along with GCK also split into two peaks upon sucrose density gradient centrifugation (Fig. 9E). However, in comparison with extracts expressing MEKK1 alone, there was significantly more HA-MEKK1 immunoreactivity in the high-molecular-weight peak. Coincident with this, the high-molecular-weight peak of MEKK1 immunoreactivity also contained more SEK1 phosphorylating and autophosphorylating activity when the MEKK1 was expressed with GCK (Fig. 9E). From these results we draw the following conclusions. Inactive MEKK1 is monomeric. Active MEKK1 exists as a oligomer (≥400 kDa) larger than the combined molecular masses of individual MEKK1 and GCK polypeptides, and GCK shifts MEKK1 into this high-molecular-weight oligomer. These results are consistent with the idea that GCK activates MEKK1 coincident with enhancing its oligomerization.
DISCUSSION
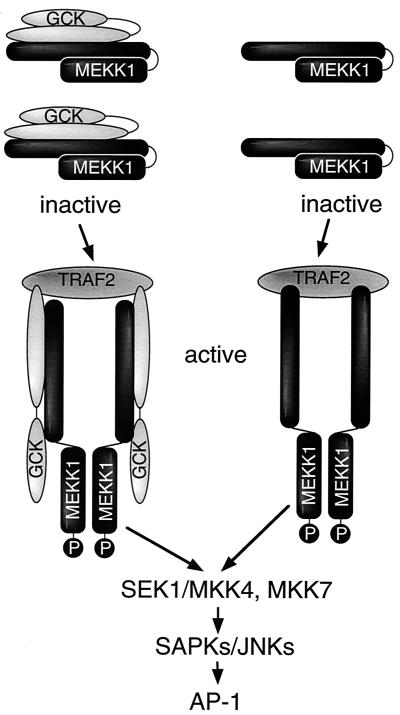
We have established an in vitro assay system in which the regulation of stress-activated MAP3Ks can be assayed. Our results point to a comparatively simple model for the regulation of MEKK1 by elements coupled to the TNFRs. In this model, GCK and MEKK1 exist as an inactive complex in resting cells, and within this complex MEKK1 is either monomeric or at a stoichiometry inconsistent with activation. Recruitment to the membrane by upstream activators such as TRAF2 creates a higher-order aggregate that activates GCK and alters the conformation of the components in the complex, forcing MEKK1 into an oligomeric state that fosters its activation. Inasmuch as autophosphorylation within the kinase activation loop at T1381 and T1393 is required for MEKK1 activity, and forced oligomerization or coincubation with GCK enhances MEKK1 autophosphorylation, it is reasonable to hypothesize that autophosphorylation at T1381 and T1393 is a consequence of MEKK1 oligomerization and a prerequisite for activation. This model is illustrated in Fig. 10. In an overexpression context, GCK spontaneously oligomerizes and activates, resulting in oligomerization and activation of any associated MEKK1 or other immediate downstream components.
FIG. 10.
Model for TNF and agonist activation of MEKK1 by GCK and TRAF2. Details are discussed in the text. P, phosphorylation of the MEKK1 polypeptide.
The position of some GCK group kinases as effectors for TRAFs is becoming clear. Thus, GCK-related enzyme (GCKR), which is closely similar to GCK, also binds TRAF2 and is activated in vivo by TRAF2 (33). Moreover, two hybrid screens that employed the Drosophila GCK group kinase Misshapen identified a Drosophila TRAF as a Misshapen interactor (25). Inasmuch as purified TRAF2 can activate MEKK1 in vivo and in vitro in the absence of GCK, it is possible that TRAF2 can activate MEKK1 in vivo either indirectly through GCK or directly, independently of GCK. It will be important to determine the contribution of this direct MEKK1 activation relative to indirect activation. Although TRAF2 and GCK interact in vivo, we have thus far been unable to generate assay conditions in which activation of GCK by TRAF2 can be detected in vitro. Further work will be necessary to establish whether TRAF2 can directly activate GCK, stimulating its ability to activate MEKK1. It is also likely that stimuli other than TRAF2 could recruit the GCK-MEKK1 complex and trigger MEKK1 activation. Of note, the structure of the GCK CTD includes several putative SH3 binding motifs (aa 428 to 434 and 462 to 466) (15) suggesting that GCK may be regulated by SH3 containing adapter proteins.
In support of the model in Fig. 10, we find that endogenous GCK and MEKK1 exist as a preformed complex in cells, and recombinant TRAF2 and GCK (2, 48; this study) can bind and activate MEKK1 in vivo. MEKK1 activation by TRAF2 or GCK is direct inasmuch as this activation can be reproduced in vitro with purified proteins. In vivo and in vitro, GCK activation of MEKK1 coincides with an increase in MEKK1 phosphate incorporation that is essentially indistinguishable from that incurred upon autophosphorylation in vitro. Kinase-inactive mutants of MEKK1 do not undergo appreciable phosphorylation upon incubation with GCK in vitro; however, incubation of wild-type MEKK1 with GCK, catalytically inactive GCK(K44M), or the free GCK CTD in vitro stimulates MEKK1 activation. Thus, GCK’s kinase function is dispensable for MEKK1 activation. T1381 and T1393, residues in the MEKK1 kinase domain activation loop, undergo autophosphorylation in vitro and in vivo, when truncated constitutively active MEKK1 constructs are assayed (4, 34). We find that for full-length MEKK1, stimulation of autophosphorylation and activation in vitro by GCK is drastically reduced in T1381A or T1393A MEKK1, suggesting that autophosphorylation at these residues is a prerequisite for MEKK1 activation. It should be noted, however, that while MEKK1 activation is always accompanied by an increase in autophosphorylation (Fig. 1 to 6), there is not always a 1:1 correspondence between the degree of MEKK1 autophosphorylation and the degree of activation by GCK of MEKK1’s SEK1 kinase activity. Examination of the tryptic phosphopeptide maps of MEKK1 phosphorylated in vivo and in vitro reveal a highly complex pattern of phosphopeptides. Although it is evident that T1381 and T1393 are important for maintenance of MEKK1 activity, it is likely that MEKK1 undergoes autophosphorylation at many sites, a substantial portion of which may be trivial, undergoing phosphorylation in a manner that does not correlate with MEKK1 activity. These trivial autophosphorylation events may obscure more pronounced changes in MEKK1 autophosphorylation at sites such as T1381 and T1393 that are relevant to activation.
Consistent with previous results indicating that TRAF2 oligomerization can activate SAPK/JNK (2), we find that oligomerization of MEKK1 also activates SAPK/JNK and that coexpression with MEKK1 of GCK, which is constitutively active when overexpressed, increases MEKK1 oligomerization in vivo. Finally, GCK’s effectors are not limited to MEKK1. We also observe that GCK can bind MLK3 in vivo and activate MLK3 in vitro.
GCK is a member of the STE20 family of protein kinases (20). An emerging body of evidence indicates that Ste20p-like kinases and other upstream components activate MAP3Ks either by direct phosphorylation or by fostering MAP3K activating autophosphorylation. In the mating pheromone pathway of S. cerevisiae, Ste20p contributes to activation of the MAP3K Ste11p by direct phosphorylation at S302 and/or Ser306 within the Ste11p kinase activation loop, residues that correspond to T1381 and T1393 of MEKK1 (39). In mammals, p21-activated kinases phosphorylate the mitogenic MAP3K Raf1 at S338/339 at the amino-terminal end of the kinase domain (17). However, although MEKK1 requires regulatory phosphorylation for activation in response to an upstream signal from GCK, this phosphorylation is self catalyzed and the kinase activity of GCK is dispensable for MEKK1 activation. This finding is similar to results for the transforming growth factor β- and interleukin 1-activated MAP3K transforming growth factor β-activated kinase-1 (TAK1) and for the MLK family MAP3K dual leucine zipper kinase (DLK) (18, 28). Upon ectopic expression with its activating subunit TAK1 binding protein 1, TAK1 is activated in vivo by autophosphorylation at S192 within the kinase activation loop. Dimerization of DLK, mediated by the DLK leucine zipper, also coincides with autophosphorylation within the kinase domain activation loop. This autophosphorylation appears to be necessary for activation (28).
As mentioned above, MEKK1 autophosphorylation is complex and does not tightly correlate with the degree of MEKK1 activation. We do not know if additional (auto)phosphorylation events are critical to MEKK1 activation. MEKK1 binds proteins of the 14-3-3 family (14-3-3ɛ). Binding of 14-3-3 proteins has been mapped to the amino-terminal 393 aa of MEKK1 (9). 14-3-3 proteins bind to the consensus sequence R-S-X-Sp-X-P (X = any amino acid, Sp = phosphoserine) (27). S242 of MEKK1 lies within a rough consensus 14-3-3 binding motif—S242 is, in fact, the only residue in the MEKK1 polypeptide that lies within a potential 14-3-3 binding motif. Mutagenesis of S242 to Ala has no effect on MEKK1 activity, GCK binding, or regulation (data not shown). Thus, either 14-3-3 proteins bind to structural motifs on MEKK1 yet to be identified, or 14-3-3 proteins do not regulate MEKK1 catalytic activity but may instead regulate MEKK1 localization, turnover, etc.
Oligomerization is emerging as a theme in MAP3K regulation. Thus, ASK1, Raf1, and the MLKs are activated, in part, by oligomerization (10, 11, 24, 26). Our findings (Fig. 9) suggest that active MEKK1 exists as an oligomer and that forced oligomerization of MEKK1 is sufficient to engender activation in vivo and in vitro. We find that GCK enhances MEKK1 oligomerization, as determined by coimmunoprecipitation of heterologously tagged MEKK1 constructs and by sucrose density gradient centrifugation. MEKK1 oligomerization may relieve autoinhibition mediated by the MEKK1 amino-terminal domain insofar as full-length MEKK1, when expressed transiently, is less active in vivo than the free MEKK1 catalytic domain (42). Oligomerization also increases MEKK1 phosphorylation in vivo; thus, oligomerization may trigger MEKK1, activating autophosphorylation.
Given that the kinase domain of GCK is dispensable for MEKK1 activation, what is the function of the GCK kinase domain? Data from our previous studies (48) indicate that, in vivo, wild-type GCK is the most potent activator of coexpressed SAPK/JNK, while kinase-dead GCK(K44M) and the free GCK CTD are comparatively modest activators of coexpressed SAPK/JNK (29, 48). By contrast, the free GCK CTD interacts with MEKK1 more stably than wild-type GCK or GCK(K44M) (48), supporting the idea that activation of GCK’s kinase activity enables the GCK CTD to trigger MEKK1 oligomerization, autophosphorylation, and activation, as well as, possibly, turnover of activated MEKK1. On the other hand, we find (Fig. 3 and 6) that in vitro GCK, GCK(K44M), and the free GCK CTD activate MEKK1 to comparable extents, highlighting the lack of a role for the GCK kinase domain in the actual process of activation of GCK effectors but not ruling out the possibility that activation of GCK may regulate access to GCK effectors. We also observe that endogenous GCK and MEKK1 interact constitutively in cells (Fig. 1A). It is possible that GCK’s kinase function is evolutionarily vestigial. However, our in vitro studies employed GCK from transfected cells, conditions under which even catalytically inactive forms of the enzyme may display substantial basal activity. Thus, while our data clearly indicate that GCK’s kinase activity is not necessary for activation of MEKK1, further studies will be necessary to establish if the ability of GCK to trigger MEKK1 autoactivation can be linked to activation GCK’s kinase function.
The comparatively simple mechanism of MEKK1 activation described herein belies the size and complexity of the MEKK1 polypeptide. MEKK1 possesses plekstrin homology domains (aa 439 to 455 and 643 to 750) and proline-rich motifs (aa 74 to 149 and 233 to 291) that may mediate binding to proteins that possess SH3 domains (23, 42). Accordingly, MEKK1 is probably regulated by multiple upstream signals, in addition to those from TRAFs and GCKs, and MEKK1 may, in turn, have diverse functions over and above activation of the SAPKs/JNKs. Consistent with these ideas, MEKK1 interacts with Ras superfamily GTPases (Ras, Rac, and Cdc42) in a GTP-dependent manner. These interactions are mediated by the MEKK1 kinase domain (8, 30). In addition, the extreme amino terminus of MEKK1 binds SAPKs/JNKs and Raf-1 (aa 30 to 221 and 221 to 370, respectively) (13, 43). A small segment of the MEKK1 amino-terminal domain (aa 30 to 200) also associates with the HTLV1 Tax protein, coupling Tax to activation of NF-κB (47). Thus, MEKK1 may perform a scaffold function regulating or at least nucleating additional pathways, besides the SAPK/JNK pathway, including NF-κB and, through Raf-1, the ERK pathway. GCK, on the other hand, is apparently a quite selective regulator of the SAPKs/JNKs and does not activate ERK or NF-κB (20, 29, 33). It will be important to determine if, in addition to activating MEKK1, GCK sequesters MEKK1 from other signaling pathways, directing it to specific activation of SAPK/JNK.
With regard to the putative SH3 binding domains in the GCK CTD, it is noteworthy that MLK3, which contains an SH3 domain, is also activated by GCK but not by TRAF2 (at least not directly, as is the case for MEKK1). It is plausible to speculate that the GCK-MLK3 interaction involves one or more of GCK’s SH3 binding domains and the SH3 domain of MLK3. In support of this idea, the MLK3 SH3 domain interacts with the carboxyl-terminal two of four polyproline SH3 binding motifs in the GCK group kinase HPK (16, 37), and a similar interaction may mediate the observed association between MLK3 and another GCK group kinase, GCKR (40). The structures of MLK3 and other members of the MLK family are also suggestive of complex regulation and function. Accordingly, MLK3 can associate in vivo with the SAPK/JNK scaffold proteins JNK-interacting protein 1 (JIP-1) and JIP-2 through the JIP SH3 domains. This interaction may serve to nucleate a signaling complex containing MKK7 and SAPK/JNK (5, 40, 46). The interaction between GCKR and MLK3 through the MLK3 SH3 domain allows the incorporation of GCKR into the MLK3-MKK7-SAPK/JNK-JIP complex (40). It will be important to determine if the binding of MLK3 to JIP proteins or other scaffolds also directs GCK-mediated activation of MLK3 by upstream components other than TRAFs.
Thus, GCK may integrate multiple signaling pathways to recruit and activate at least two MAP3Ks, likely through induced proximity and/or oligomerization-dependent autophosphorylation. In addition, TRAF2 can directly activate MEKK1, probably in a similar manner (Fig. 9). Future work will identify the pathways that couple GCK to MLK3 as well as the kinetics and relative contributions of GCK-dependent and -independent activation of MEKK1 and the SAPKs by TNF.
Acknowledgments
This work was supported by National Institutes of Health grant GM46577, a Basic Science Grant from the Arthritis Foundation (both to J.M.K), and a postdoctoral fellowship from the Canadian Institute of Health Research (D.N.C).
We thank Melanie Cobb for full-length wild-type-MEKK1, Ulrich Siebenlist for MEKK3, and Árpád Molnár for advice on cloning MEKK1 mutants.
REFERENCES
- 1.Arch, R. H., R. W. Gedrich, and C. B. Thompson. 1998. Tumor necrosis factor receptor-associated factors (TRAFs)—a family of adapter proteins that regulates life and death. Genes Dev. 12:2821–2830. [DOI] [PubMed] [Google Scholar]
- 2.Baud, V., Z.-G. Liu, B. Bennett, N. Suzuki, Y. Xia, and M. Karin. 1999. Signaling by proinflammatory cytokines: oligomerization of TRAF2 and TRAF6 is sufficient for JNK and IKK activation and target gene induction via an amino terminal effector domain. Genes Dev. 13:1297–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyle, W. J., P. van der Geer, and T. Hunter. 1991. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 201:110–152. [DOI] [PubMed] [Google Scholar]
- 4.Deak, J. C., and D. J. Templeton. 1997. Regulation of the activity of MEK kinase 1 (MEKK1) by autophosphorylation within the kinase activation domain. Biochem. J. 322:185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dickens, M., J. S. Rogers, J. Cavanagh, A. Raitano, Z. Xia, J. R. Halpern, M. E. Greenberg, C. L. Sawyers, and R. J. Davis. 1997. A cytoplasmic inhibitor of the JNK signal transduction pathway. Science 277:693–696. [DOI] [PubMed] [Google Scholar]
- 6.Dong, C., D. D. Yang, M. Wysk, A. J. Whitmarsh, R. J. Davis, and R. A. Flavell. 1998. Defective T cell differentiation in the absence of Jnk1. Science 282:2092–2095. [DOI] [PubMed] [Google Scholar]
- 7.Dong, C., D. D. Yang, C. Tournier, A. J. Whitmarsh, J. Xu, R. J. Davis, and R. A. Flavell. 2000. JNK is required for effector T-cell function but not for T cell activation. Nature 405:91–94. [DOI] [PubMed] [Google Scholar]
- 8.Fanger, G. R., N. L. Johnson, and G. L. Johnson. 1997. MEK kinases are regulated by EGF and selectively interact with Rac/Cdc42. EMBO J. 16:4961–4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fanger, G. R., C. Widmann, A. C. Porter, S. Sather, G. L. Johnson, and R. R. Vaillancourt. 1998. 14-3-3 proteins interact with specific MEK kinases. J. Biol. Chem. 273:3476–3483. [DOI] [PubMed] [Google Scholar]
- 10.Farrar, M. A., J. Alberol-Ila, and R. M. Perlmutter. 1996. Activation of the Raf-1 kinase cascade by coumermycin-induced dimerization. Nature 383:178–181. [DOI] [PubMed] [Google Scholar]
- 11.Gotoh, Y., and J. A. Cooper. 1998. Reactive oxygen species- and dimerization-induced activation of apoptosis signal-regulating kinase 1 in tumor necrosis factor-α signal transduction. J. Biol. Chem. 273:17477–17482. [DOI] [PubMed] [Google Scholar]
- 12.Hu, M. C.-T., W. R. Qiu, X. Wang, C. F. Meyer, and T.-H. Tan. 1996. Human HPK1, a novel human hematopoietic progenitor kinase that activates the JNK/SAPK kinase cascade. Genes Dev. 10:2251–2264. [DOI] [PubMed] [Google Scholar]
- 13.Karandikar, M., S. Xu, and M. H. Cobb. 2000. MEKK1 binds Raf-1 and the ERK2 cascade components. J. Biol. Chem. 275:40120–40127. [DOI] [PubMed] [Google Scholar]
- 14.Karin, M., Z.-G. Liu, and E. Zandi. 1997. AP-1 function and regulation. Curr. Opin. Cell Biol. 9:240–246. [DOI] [PubMed] [Google Scholar]
- 15.Katz, P., G. Whalen, and J. H. Kehrl. 1994. Differential expression of a novel protein kinase in human B lymphocytes: preferential localization in the germinal center. J. Biol. Chem. 269:16802–16809. [PubMed] [Google Scholar]
- 16.Kiefer, F., L. A. Tibbles, M. Anafi, A. Janssen, B. W. Zanke, N. Lassam, T. Pawson, J. R. Woodgett, and N. R. Iscove. 1996. HPK1, a hematopoietic protein kinase activating the SAPK/JNK pathway. EMBO J. 15:7013–7025. [PMC free article] [PubMed] [Google Scholar]
- 17.King, A. J., H. Sun, B. Diaz, D. Barnard, W. Miao, S. Bagrodia, and M. S. Marshall. 1998. The protein kinase Pak3 positively regulates Raf-1 through phosphorylation of serine 338. Nature 396:180–183. [DOI] [PubMed] [Google Scholar]
- 18.Kishimoto, K., K. Matsumoto, and J. Ninomiya-Tsuji. 2000. TAK1 mitogen-activated protein kinase kinase kinase is activated by autophosphorylation within its activation loop. J. Biol. Chem. 275:7359–7364. [DOI] [PubMed] [Google Scholar]
- 19.Kuan, C.-Y., D. Yang, D. R. Samanta Roy, R. J. Davis, P. Rakic, and R. A. Flavell. 1999. The Jnk1 and Jnk2 protein kinases are required for regional specific apoptosis during early brain development. Neuron 22:667–676. [DOI] [PubMed] [Google Scholar]
- 20.Kyriakis, J. M. 1999. Signaling by the germinal center kinase family of protein kinases. J. Biol. Chem. 274:5259–5262. [DOI] [PubMed] [Google Scholar]
- 21.Kyriakis, J. M. 2000. Mammalian MAP kinase pathways, p.40–156. In J. Woodgett (ed.), Protein kinase functions. Oxford University Press, Oxford, United Kingdom.
- 22.Kyriakis, J. M., and J. Avruch. 2001. Mammalian mitogen-activated protein kinase pathways activated by stress and inflammation. Physiol. Rev. 81:807–869. [DOI] [PubMed] [Google Scholar]
- 23.Lange-Carter, C. A., C. M. Pleiman, A. M. Gardner, K. J. Blumer, and G. L. Johnson. 1993. A divergence in the MAP kinase regulatory network defined by MEK kinase and Raf. Science 260:315–319. [DOI] [PubMed] [Google Scholar]
- 24.Leung, I. W.-L., and N. Lassam. 1998. Dimerization via tandem leucine zippers is essential for the activation of the mitogen-activated protein kinase kinase kinase, MLK-3. J. Biol. Chem. 273:32408–32415. [DOI] [PubMed] [Google Scholar]
- 25.Liu, H., Y.-C. Su, E. Becker, J. Treisman, and E. Y. Skolnik. 1999. A Drosophila TNF-receptor-associated factor (TRAF) binds the Ste20 kinase Misshapen and activates Jun kinase. Curr. Biol. 9:101–104. [DOI] [PubMed] [Google Scholar]
- 26.Luo, Z., G. Tzivion, P. J. Belshaw, D. Vavvas, M. Marshall, and J. Avruch. 1996. Oligomerization activates c-Raf-1 through a Ras-dependent mechanism. Nature 383:181–184. [DOI] [PubMed] [Google Scholar]
- 27.Muslin, A. J., J. W. Tanner, P. M. Allen, and A. S. Shaw. 1996. Interaction of 14-3-3 with signaling proteins is mediated by the recognition of phosphoserine. Cell 84:889–897. [DOI] [PubMed] [Google Scholar]
- 28.Nihalani, D., S. Merritt, and L. B. Holzman. 2000. Identification of structural and functional domains in mixed lineage kinase dual leucine zipper-bearing kinase required for complex formation and stress-activated protein kinase activation. J. Biol. Chem. 275:7273–7279. [DOI] [PubMed] [Google Scholar]
- 29.Pombo, C. M., J. H. Kehrl, I. Sánchez, P. Katz, J. Avruch, L. I. Zon, J. R. Woodgett, T. Force, and J. M. Kyriakis. 1995. Activation of the SAPK pathway by the human STE20 homologue germinal centre kinase. Nature 377:750–754. [DOI] [PubMed] [Google Scholar]
- 30.Russell, M., C. A. Lange-Carter, and G. L. Johnson. 1995. Direct interaction between Ras and the kinase domain of mitogen-activated protein kinase kinase kinase (MEKK1). J. Biol. Chem. 270:11757–11760. [DOI] [PubMed] [Google Scholar]
- 31.Sabapathy, K., Y. Hu, T. Kallunki, M. Schreiber, J.-P. David, W. Jochum, E. F. Wagner, and M. Karin. 1999. JNK2 is required for efficient T-cell activation and apoptosis but not for normal lymphocyte development. Curr. Biol. 9:116–125. [DOI] [PubMed] [Google Scholar]
- 32.Sabapathy, K., W. Jochum, K. Hochedlinger, L. Chang, M. Karin, and E. F. Wagner. 1999. Defective neural tube morphogenesis and altered apoptosis in the absence of both JNK1 and JNK2. Mech. Dev. 89:115–124. [DOI] [PubMed] [Google Scholar]
- 33.Shi, C.-S., and J. H. Kehrl. 1997. Activation of stress-activated protein kinase/c-Jun N-terminal kinase, but not NF-κB, by the tumor necrosis factor (TNF) receptor 1 through a TNF receptor-associated factor 2- and germinal center kinase related-dependent pathway. J. Biol. Chem. 272:32102–32107. [DOI] [PubMed] [Google Scholar]
- 34.Siow, T. L., G. B. Kalmar, J. S. Sanghera, G. Tai, S. S. Oh, and S. L. Pelech. 1997. Identification of two essential phosphorylated threonine residues in the catalytic domain of MEKK1. J. Biol. Chem. 272:7586–7594. [DOI] [PubMed] [Google Scholar]
- 35.Spencer, D. M., T. J. Wandless, S. L. Schreiber, and G. R. Crabtree. 1993. Controlling signal transduction with synthetic ligands. Science 262:1019–1024. [DOI] [PubMed] [Google Scholar]
- 36.Su, Y.-C., J. Han, S. Xu, M. Cobb, and E. Y. Skolnik. 1997. NIK is a new Ste20-related kinase that binds NCK and MEKK1 and activates the SAPK/JNK cascade via a conserved regulatory domain. EMBO J. 16:1279–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tibbles, L. A., Y. L. Ing, F. Kiefer, J. Chan, N. Iscove, J. R. Woodgett, and N. J. Lassam. 1996. MLK-3 activates the SAPK/JNK and p38/RK pathways via SEK1 and MKK3/6. EMBO J. 15:7026–7035. [PMC free article] [PubMed] [Google Scholar]
- 38.Tournier, C., P. Hess, D. D. Yang, J. Xu, T. K. Turner, A. Nimnual, D. Bar-Sagi, S. N. Jones, R. A. Flavell, and R. J. Davis. 2000. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science 288:870–874. [DOI] [PubMed] [Google Scholar]
- 39.van Drogen, F., S. M. O’Rourke, V. M. Stucke, M. Jaquenoud, A. Neiman, and M. Peter. 2000. Phosphorylation of the MEKK Ste11p by the PAK-like kinase Ste20p is required for MAP kinase signaling in vivo. Curr. Biol. 10:630–639. [DOI] [PubMed] [Google Scholar]
- 40.Whitmarsh, A. J., J. Cavanagh, C. Tournier, J. Yasuda, and R. J. Davis. 1998. A mammalian scaffold complex that selectively mediates MAP kinase activation. Science 281:1671–1674. [DOI] [PubMed] [Google Scholar]
- 41.Xia, Y., C. Makris, B. Su, E. Li, J. Yang, G. R. Nemerow, and M. Karin. 2000. MEK kinase 1 is critically required for c-Jun N-terminal kinase activation by proinflammatory stimuli and growth factor-induced cell migration. Proc. Natl. Acad. Sci. USA 97:5243–5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu, S., D. J. Robbins, L. B. Christerson, J. M. English, C. Vanderbilt, and M. H. Cobb. 1996. Cloning of Rat MEK kinase 1 cDNA reveals an endogenous membrane-associated 195-kDa protein with a large regulatory domain. Proc. Natl. Acad. Sci. USA 93:5291–5295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu, S., and M. H. Cobb. 1997. MEKK1 binds directly to the c-Jun N-terminal kinases/stress-activated protein kinases. J. Biol. Chem. 272:32056–32060. [DOI] [PubMed] [Google Scholar]
- 44.Yan, M., T. Dai, J. C. Deak, J. M. Kyriakis, L. I. Zon, J. R. Woodgett, and D. J. Templeton. 1994. Activation of stress-activated protein kinase by MEKK1 phosphorylation of its activator SEK1. Nature 372:798–800. [DOI] [PubMed] [Google Scholar]
- 45.Yang, D. D., D. Conze, A. J. Whitmarsh, T. Barrett, R. J. Davis, M. Rincon, and R. A. Flavell. 1998. Differentiation of CD4+ T cells to Th1 cells requires MAP kinase JNK2. Immunity 9:575–585. [DOI] [PubMed] [Google Scholar]
- 46.Yasuda, J., A. J. Whitmarsh, J. Cavanagh, M. Sharma, and R. J. Davis. 1999. The JIP group of mitogen-activated protein kinase scaffold proteins. Mol. Cell. Biol. 19:7245–7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yin, M.-J., L. B. Christerson, Y. Yamamoto, Y.-T. Kwak, S. Xu, F. Mercurio, M. Barbosa, M. H. Cobb, and R. B. Gaynor. 1998. HTLV-I Tax protein binds to MEKK1 to stimulate IκB kinase activity and NF-κB activation. Cell 93:875–884. [DOI] [PubMed] [Google Scholar]
- 48.Yuasa, T., S. Ohno, J. H. Kehrl, and J. M. Kyriakis. 1998. Tumor necrosis factor signaling to stress-activated protein kinase (SAPK)/Jun NH2-terminal kinase (JNK) and p38. J. Biol. Chem. 273:22681–22692. [DOI] [PubMed] [Google Scholar]
- 49.Yujiri, T., S. Sather, G. R. Fanger, and G. L. Johnson. 1998. Role of MEKK1 in cell survival and activation of JNK and ERK pathways defined by targeted gene disruption. Science 282:1911–1914. [DOI] [PubMed] [Google Scholar]