Abstract
The Tup1-Ssn6 complex regulates diverse classes of genes in Saccharomyces cerevisiae and serves as a model for corepressor functions in many organisms. Tup1-Ssn6 does not directly bind DNA but is brought to target genes through interactions with sequence-specific DNA binding factors. Full repression by Tup1-Ssn6 appears to require interactions with both the histone tails and components of the general transcription machinery, although the relative contribution of these two pathways is not clear. Here, we map Tup1 locations on two classes of Tup1-Ssn6-regulated genes in vivo via chromatin immunoprecipitations. Distinct profiles of Tup1 are observed on a cell-specific genes and DNA damage-inducible genes, suggesting that alternate repressive architectures may be created on different classes of repressed genes. In both cases, decreases in acetylation of histone H3 colocalize with Tup1. Strikingly, although loss of the Srb10 mediator protein had no effect on Tup1 localization, both histone tail mutations and histone deacetylase mutations crippled the association of Tup1 with target loci. Together with previous findings that Tup1-Ssn6 physically associates with histone deacetylase activities, these results indicate that the repressor complex alters histone modification states to facilitate interactions with histones and that these interactions are required to maintain a stable repressive state.
Proper regulation of gene expression requires not only gene activation but also active gene repression. As with activation, mechanisms of repression appear varied and may be multistep. The yeast Tup1-Ssn6 corepressor complex has provided a useful model for dissecting possible modes of repression. Tup1-Ssn6 mediates the repression of diverse classes of genes, including those controlled by mating type, DNA damage, glucose, and anaerobic stress (10, 48). The Tup1-Ssn6 complex does not directly bind DNA but is brought to target genes by interactions with specific DNA binding factors such as α2 (22, 41) and Crt1 (19), regulators of a cell-specific genes and DNA damage-inducible genes, respectively. Once recruited, Tup1-Ssn6 appears to interact with the transcription machinery. Mutations in several components of the RNA polymerase II holoenzyme, including Sin4 (7), Srb10/11 (25, 47), Med3 (35), and Srb7 (13), decrease repression by Tup1-Ssn6. Moreover, direct physical interactions have been observed between Tup1-Ssn6 and Med3 (35), Srb7 (13), and Srb10/11 (55). Additionally, a modest amount of repression can be established on a nonnucleosomal template in vitro (36), indicating that Tup1-Ssn6 may directly target multiple aspects of the transcription machinery for repression.
In eukaryotes, gene regulation occurs within the context of chromatin, and repression mediated by the Tup1-Ssn6 complex clearly has a chromatin component. Chromatin is composed of nucleosomal subunits, which can be folded into progressively higher-order structures. Each nucleosome consists of an octamer of histone proteins and two turns (147 bp) of DNA spooled around the exterior of the histone octamer (32, 51). Individual nucleosomes as well as more compacted structures are refractory to transcription, and chromatin remodeling is now recognized as a central component of gene regulation. One major mechanism of chromatin remodeling involves alterations in the levels, sites, and types of posttranslational histone modifications (42). Increased histone acetylation, for example, is associated with increased transcription whereas decreased acetylation is associated with gene repression (23, 26, 44, 52).
The repression domain of Tup1 binds preferentially to underacetylated isoforms of histones H3 and H4 in vitro (9), and histones associated with sites of Tup1 recruitment are underacetylated in vivo (2). Moreover, specific combined mutations in the H3 and H4 histone tail domains that are subject to acetylation cripple Tup1-Ssn6-mediated repression (9, 18). Tup1-Ssn6 interact physically with at least three histone deacetylases (49, 53), and a combination of mutations in the deacetylase genes RPD3, HOS1, and HOS2 derepresses all Tup1-Ssn6-regulated genes examined (49). These data lead to a model where repression is mediated through an organization of chromatin directed by interactions of the Tup1-Ssn6 complex with H3 and H4.
The chromatin- and the transcription machinery-mediated models for Tup1-Ssn6 function are not exclusive and may represent different steps in the repression process. For example, Tup1-Ssn6 may halt transcription via interactions with the holoenzyme and then organize chromatin to maintain the repressed state. Alternatively, interactions with histones may stabilize Tup-Ssn6 at the promoter, where the complex can then interact with holoenzyme components.
Understanding how the repressor complex interacts with target promoters in vivo and which factors alter these interactions would greatly enhance our understanding of the repression process. Here, we examine the physical location of Tup1 on two members of each of two different classes of repressed genes by using chromatin immunoprecipitation. We find differences in the extent of Tup1 localization at different promoters, suggesting some flexibility in the types of repressive structures created. Further, we demonstrate that changes in histone H3 acetylation patterns track Tup1 recruitment profiles at each promoter. Strikingly, our data indicate that stable interaction of Tup1 with repressed promoters requires interactions with histones, but not Srb10, a holoenzyme component. This work suggests that the interaction of Tup1 with underacetylated histones is self-reinforcing in nature and provides a unique example of a reciprocal relationship between the modification of chromatin by a repressor complex and the stable recruitment of that complex via interactions with the histones.
MATERIALS AND METHODS
Yeast strains and methods.
All yeast strains used in this study are listed in Table 1. All strains except MSY590 and MSY577 are isogenic to W303 (R. Rothstein). Standard conditions of yeast culture and transformation were used (3, 16).
TABLE 1.
Yeast strains used in this study
| Strain | Genotype |
|---|---|
| DY150a | MATaade2-1 can1-100 ura3 leu2-3,112 his3-11,15 trp1 |
| DY151a | MATα ade2-1 can1-100 ura3 leu2-3,112 his3-11,15 trp1 |
| JDY10 | As DY150 except TUP1::3×HA |
| JDY11 | As DY151 except TUP1::3×HA |
| JDY12 | As JD10 except ssn6Δ::Kanr |
| JDY13 | As JDy11 except ssn6Δ::Kanr |
| JDY14 | As JDy10 except srb10Δ::Kanr |
| JDY15 | As JDy11 except srb10Δ::Kanr |
| Y577b | MATaade2-1 can1-100 ura3 leu2-3,112 his3-11,15 trp1 crt1Δ::LEU2 |
| JDY18 | As Y577 except TUP1::3×HA |
| MSY590c | MATα ura3-52 lys2-Δ201 leu2-3 Δhht2-hhf2 |
| MSY577c | MSY590, except hht1–3 (Δ1–28) and hhf1–327 (H4 K12Q, K16Q) |
| DY4565a | As DY151 except rpd3Δ::LEU2 hos1Δ::HIS3 hos2Δ::TRP1 |
Obtained from D. Stillman.
Obtained from S. Elledge.
Obtained from M. Smith.
The HA-tagged Tup1 was constructed by a previously described method (38). Briefly, a PCR strategy was used to insert a 3×HA tag-URA3-3×HA tag cassette into the C terminus of TUP1 in the chromosome. Selection on 5-fluoroorotic acid medium was then used to identify colonies that recombined the direct repeats of the HA tag, excising URA3 and leaving only one copy of the 3× HA tag. Insertion of the tag was confirmed by PCR analysis of the TUP1 locus.
SSN6 and SRB10 were deleted in the HA-Tup1 strains by replacement of the entire coding region with the Kanr gene as previously described (15). Disruptions were confirmed by PCR analysis with primers corresponding to both wild-type and disruption alleles.
Chromatin immunoprecipitation.
Chromatin-containing extracts were prepared as previously described (3, 43) with minor modifications. Extracts were prepared from 200-ml cultures at a density of 107 cells/ml. The chromatin-containing extract was sonicated to yield an average DNA size of 500 bp (the majority of the fragments were approximately 500 bp long, but very small numbers of the fragments were as small as 100 bp or as large as 1 kb). Sonication conditions were 30% output, 100% duty cycle, six 12-s cycles with a Branson Sonifier 250. The chromatin size was confirmed for each input sample by running 10% of the DNA on a 1.5% agarose gel. The sonicated extract was subsequently clarified by centrifugation. For HA-Tup1 immunoprecipitations, 700 μl of extract was immunoprecipitated with 10 μl of anti-HA 16B12 monoclonal antibody (Babco/Covance). For Tup1 immunoprecipitation, 500 μl of extract was immunoprecipitated with 10 μl of affinity-purified Tup1 antibody (50). For the histone immunoprecipitations, 200 μl of extract was immunoprecipitated with antibodies specific to either H3 Ac9,18 (15 μl) (9) or unacetylated H3 (20 μl) (9). For the Crt1 chromatin immunoprecipitation, an inducible N-terminally tagged 3XMYC-CRT1 under the control of the GAL1 promoter (PMH190) was used. Plasmid PMH190 was a gift from S. Elledge (19). Cells were grown in dextrose media selective for the plasmid, transferred to selective media containing galactose, and allowed to double twice before harvesting. Expression of myc-Crt1 was confirmed by Western analysis. For the immunoprecipitation, 500 μl of extract was immunoprecipitated with 5 μl of anti-c-myc antibody (clone 9E10; Roche).
Quantitative PCR.
All primers were designed to be 19- to 25-mers, with a Tm of approximately 60°C. Primer sequences are available on request. The PCR conditions were 94°C for 1 min, 55°C for 30 s, and 72°C for 1 min for 25 cycles. A 2-min 94°C step prior to the cycles and a 4-min 72°C extension following completion of the cycles were added. Three dilutions of each sample were used for PCR. If two of the dilutions were not in the linear range, PCR of additional dilutions was performed. For the chromatin immunoprecipitation (ChIP) experiments using antibodies against Tup1 and unacetylated H3, the initial dilution series was 1/100, 1/50, and 1/30. For the ChIP experiments using antibodies against acetylated H3, the dilution series was 1/500, 1/250, and 1/100. For the ChIP experiments with myc-Crt1, the PCR cycles were increased to 28 and the dilution series was 1/25, 1/50, and 1/75. PCR amplification products were resolved on 7% polyacrylamide gels and stained with SYBR green nucleic acid dye (Molecular Probes). Stained gels were scanned using a Storm 840 system (Molecular Dynamics).
RESULTS
The localization of Tup1 differs at mating type-specific and DNA damage-inducible genes.
We examined the location of Tup1 at two classes of Tup1-Ssn6-regulated genes by using a chromatin immunoprecipitation assay with an antibody directed against Tup1. The genes chosen for this analysis were the a cell-specific genes STE6 and STE2 and the DNA damage-response genes RNR2 and RNR3. Following cross-linking of proteins and DNA within living cells with formaldehyde, chromatin was extracted, fragmented by sonication, and subjected to immunoprecipitation. After reversal of the cross-links, the immunoprecipitated DNA was purified, and amounts of specific sequences were measured by quantitative PCR.
For these experiments, an anti-HA antibody was used to immunoprecipitate a derivative of Tup1 (HA-Tup1) carrying a C-terminal 3×HA tag. Sequences encoding the 3×HA were inserted into the TUP1 native chromosomal location. HA-Tup1 was assayed in several ways to confirm the wild-type function of the protein (data not shown). First, it showed equivalent levels of repression of Tup1-responsive reporter genes to the native Tup1. Second, the anti-HA antibody immunoprecipitated both HA-Tup1 and native Ssn6, indicating that the tagged protein was assembled properly into the repressor complex. Lastly, HA-Tup1 fully reversed the flocculation phenotype (31) of tup1 strains.
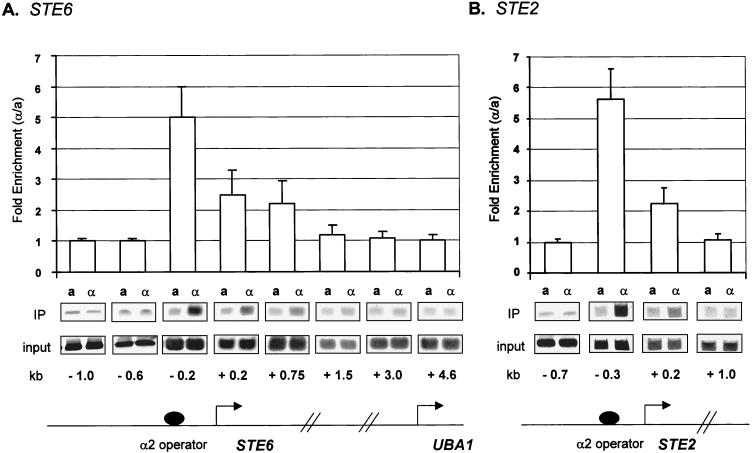
HA-Tup1 formed cross-links with highest efficiency to the site of recruitment by the DNA binding factor (α2 or Crt1) at all genes we examined (Fig. 1 and 2). No cross-linking was observed at sequences upstream of the operator binding site for either class of genes (examples are the −1.0 and −0.6 probes in Fig. 1A). However, the extent of Tup1 association with downstream sequences differed for the a cell-specific genes and the DNA damage-inducible genes. At both STE6 and STE2, Tup1-Ssn6 was recruited by interactions with the α2/Mcm1 DNA binding complex in α cells but not in a cells. HA-Tup1 exhibited a fivefold enhancement in association with sequences adjacent to the α2/Mcm1 operator in α cells, where these genes are repressed, relative to a cells, where the genes are expressed (Fig. 1A, −0.2 probe, and Fig. 1B, −0.3 probe). Smaller but significant amounts of HA-Tup1 were associated with the first 0.75 to 1 kb of each of the coding regions of these two genes, whereas equivalent amounts of HA-Tup1 were immunoprecipitated from a and α cells throughout the remainder of the genes. Sequences immediately adjacent to the end of the coding region of STE6 (+3.8 probe) were also examined (data not shown), and again, equivalent amounts of HA-Tup1 were precipitated from a and α cells. To confirm that the association of HA-Tup1 across STE2 and STE6 faithfully mimicked that of the native protein, we repeated our experiments using a Tup1-specific antibody previously used to map the association of Tup1 with STE6 (8). The Tup1 association profiles obtained with both antibodies were identical (data not shown) but differed from that previously reported in that no Tup1 association was observed past the first 1 kb of the coding region of STE6 or the first 200 bp of the coding region of STE2. These results also differ from those of another recent report (53), where Tup1 was associated only with the site of recruitment at STE6. The reasons for these experimental differences are unclear (see Discussion).
FIG. 1.
Tup1 spreads into the coding region of the a cell-specific genes STE6 and STE2. Chromatin immunoprecipitations (IP) were preformed on cell extracts from a and α HA-TUP1 strains by using an antibody against HA. The location of the PCR primer sets is given in kilobases with the starting ATG as a reference (0.0 kb). These positions are also shown on gene diagrams for STE6 (A) and STE2 (B). All PCR primer sets were designed to generate ∼100-bp products. At STE6 and STE2, the α2 operator spans positions −212 to −182 and positions −228 to −200, respectively. The bar graph represents the ratio between the signals from α cells (repressed) and a cells (derepressed). For the graph, four independent experiments were averaged and the error bars are shown. Quantitative PCR products from one representative experiment are shown.
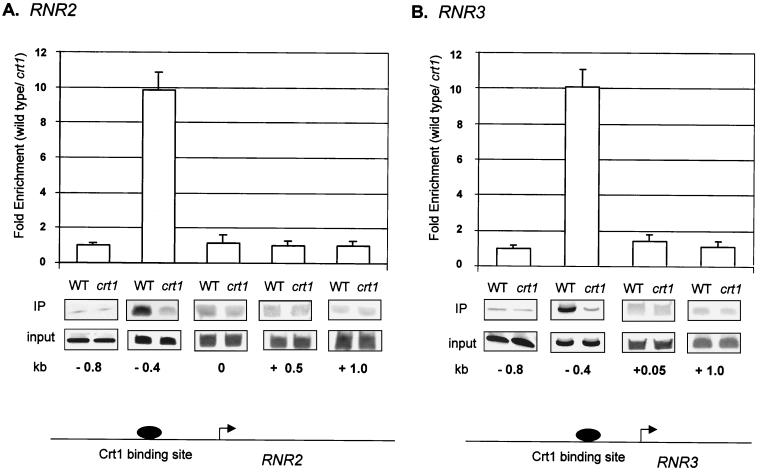
FIG. 2.
Tup1 is present only near the Crt1 binding site at RNR2 and RNR3. Chromatin immunoprecipitations (IP) were performed on cell extracts from wild-type (WT) and crt1 HA-TUP1 strains by using an antibody against HA. The location of the PCR primer sets is given in kilobases with the starting ATG as a reference (0.0 kb). These positions are also indicated on gene diagrams for RNR2 (A) and RNR3 (B). PCR fragments were ∼100 bp in length. The Crt1 binding sites are X-box-related sequences, located at positions −436 and −363 for RNR2 and −383, −321, and −272 for RNR3 (19). The bar graph shows the ratio between the signals from wild-type cells (repressed) and crt1 cells (derepressed). For the graph, four independent experiments were averaged and the error bars are shown. Quantitative PCR products from one representative experiment are shown.
At the DNA damage response genes RNR2 and RNR3, Tup1-Ssn6 is recruited by interaction with the DNA binding protein Crt1 (19). The association with Tup1 was strongest in the region immediately adjacent to the Crt1 binding site (Fig. 2, −0.4 probes). A 10-fold-greater signal was observed in Tup1 immunoprecipitates from extracts of wild-type strains than from crt1 strains. However, in contrast to the a cell-specific genes, no Tup1 association was observed with downstream coding sequences of either RNR2 or RNR3 (Fig. 2). These differences in association may reflect differences in the amounts or conformations of the repressor complex recruited by the different DNA binding factors, i.e., α2 and Crt1, as suggested previously by others (40) (see Discussion).
Ssn6 is required for Tup1 recruitment in vivo.
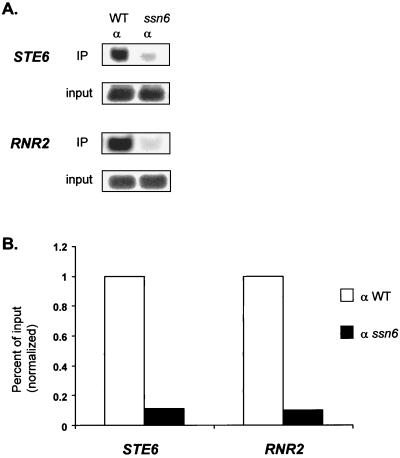
Both Tup1 and Ssn6 are normally required for repressor function. However, Tup1 can function in the absence of Ssn6 if it is targeted to a LexA operator sequence via fusion to a LexA DNA binding domain (46). Since Ssn6 is required for repression in vivo, it has been suggested that Ssn6 is required for recruitment of the repressor complex. Also, Ssn6 interacts directly with both α2 (41) and Crt1 (19), again indicating that it is involved in recruitment. Interestingly, Tup1 can also interact with α2, raising the possibility that it might be recruited independently to the a cell-specific genes (22). To test directly the requirement of Ssn6 for Tup1 recruitment, we repeated the chromatin immunoprecipitation assay by using HA antibodies and HA-Tup1 in an ssn6 strain (Fig. 3). Western analysis confirmed that HA-Tup1 was expressed at equivalent levels in both the wild-type and ssn6 strains (data not shown). In the absence of Ssn6, however, neither class of target gene was coimmunoprecipitated with HA-Tup1, confirming that Ssn6 is required for stable recruitment of the repressor complex to its target genes.
FIG. 3.
Ssn6 is required for Tup1 recruitment to target loci. (A) Quantitative PCR products from chromatin immunoprecipitations (IP) performed on cell extracts from wild-type (WT) and ssn6 HA-TUP1 strains by using an antibody against HA. PCR primer sets correspond to −0.2 kb for STE6 (as in Fig. 1A) and −0.4 kb for RNR2 (as in Fig. 2A). (B) Bar graph representing the percentage of input DNA immunoprecipitated from wild-type and ssn6 extracts. The wild-type value was set at 1 for each gene to allow comparison between genes. The standard deviation of the SSN6+/ssn6 ratios was less than 0.01. Note that for both classes of genes, the reductions in immunoprecipitation efficiency were almost identical.
Mutation in SRB10 has no effect on Tup1 recruitment, but mutations in the histone tails severely cripple Tup1 recruitment.
Tup1 repression is compromised both by mutations in members of the general transcription machinery and by mutations that affect chromatin structure. To gain insight into how these two components contribute to repression, we performed Tup1-specific chromatin immunoprecipitations from strains lacking the mediator protein Srb10 and from strains containing a combination of histone mutations shown to compromise both repression in vivo and Tup1-histone binding in vitro (9).
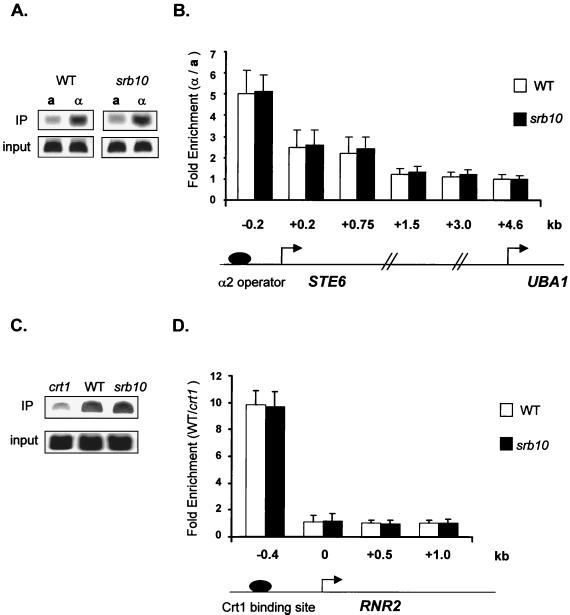
We found normal association and distribution of HA-Tup1 at both STE6 and RNR2 in the srb10 mutant strain (Fig. 4). In no instance did we observe diminution of Tup1 association in the absence of Srb10. These data support a role for this factor in repression that is downstream of repressor recruitment.
FIG. 4.
Srb10 is not required for Tup1 recruitment to target loci. Chromatin immunoprecipitations (IP) were performed on cell extracts from wild-type (WT) and srb10 strains containing HA-TUP1 by using an antibody against HA. Primer sets spanning both STE6 and RNR2 were used. The location of the PCR primer sets is as in Fig. 1A for STE6 and Fig. 2B for RNR2. Quantitative PCR products from one representative experiment are shown for position −0.2 kb of STE6 (A) and position −0.4 kb of RNR2 (C). Data for all primer sets are graphed for STE6 (B) and RNR2 (D). For STE6, the bar graph represents the ratio between the signals from α cells and a cells as well as the ratio between the signals from srb10 α cells and srb10 a cells. For RNR2, the bar graph represents the ratio between the signals from WT cells and crt1 cells as well as the ratio between signals from srb10 cells and crt1 cells. For the graph, three independent experiments were averaged and the error bars are shown.
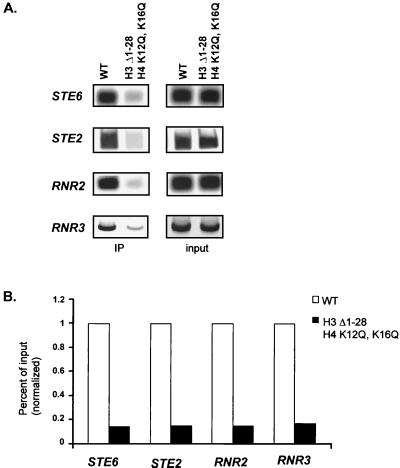
Genetic experiments indicate that the H3 and H4 amino-terminal tail domains serve redundant functions in Tup1-Ssn6-mediated repression. Whereas mutation of either tail individually affects repression only modestly, simultaneous mutations in both histones have synergistic effects (9, 18). These previous studies also established that Tup1 and Ssn6 levels are not altered in these histone mutant strains. To determine whether this compromised repression reflects a decrease in Tup1 association, we repeated our chromatin immunoprecipitation experiments in a yeast strain carrying a deletion (Δ1–28) of the H3 tail and a double point mutation in the H4 tail (K12Q, K16Q) that was previously shown to cause a loss of repression of both a cell-specific and DNA damage-inducible reporter genes. Immunoprecipitation of STE6, STE2, RNR2, and RNR3 sequences was severely reduced in the mutant strain relative to that observed in an isogenic strain containing wild-type histones (Fig. 5). While the lack of cross-linking in this assay does not necessarily indicate that Tup1 is absent at this promoter, this result clearly demonstrates that the physical association of Tup1 at its site of recruitment is significantly altered in the histone mutant strain. These results support a central role for the H3 and H4 amino-terminal tail domains in the repression mechanism by facilitating the stable association of Tup1 with its target genes.
FIG. 5.
Histone mutations severely cripple the association of Tup1 with target loci. (A) Quantitative PCR products from chromatin immunoprecipitations (IP) preformed on cell extracts from wild-type (WT) (MSY590) and H3 Δ1–28 H4 K12QK16Q (MSY 577) strains by using an antibody against Tup1. PCR primer sets correspond to −0.2 kb for STE6 (as in Fig. 1A), −0.3 kb for STE2 (as in Fig. 1B), −0.4 kb for RNR2 (as in Fig. 2A), and −0.4 kb for RNR3 (as in Fig. 2B). (B) Bar graph representing the percentage of input DNA immunoprecipitated from MSY590 and MSY577 extracts. The wild-type value was set at 1 to allow comparison between genes. For the graph, four independent experiments were averaged. The standard deviation of the MSY590/MSY577 ratios was less than 0.01. Note that for each gene, the reductions in immunoprecipitation efficiency were almost identical.
Changes in H3 acetylation colocalize with Tup1.
Previously we demonstrated that Tup1 interacts best with underacetylated isoforms of histones H3 and H4 in vitro (9) and that it is physically associated with histone deacetylase activities that are required for repression in vivo (49). These data suggest that Tup1 may induce and/or stabilize a hypoacetylated state for histones associated with repressed promoters. Indeed, Tup1-dependent loss of histone acetylation is observed for a minichromosome construct bearing an α2/Mcm1 operator sequence and at the endogenous SUC2 gene, which is also repressed by Ssn6-Tup1 (2). To confirm that such changes occur at additional endogenous Tup1-Ssn6-regulated genes and to determine how changes in histone acetylation levels might correlate with the location of Tup1, we mapped the acetylation state of histone H3 at STE6, STE2, RNR2, and RNR3 by using antibodies specific for either underacetylated H3 or H3 isoforms acetylated at lysines 9 and/or 18.
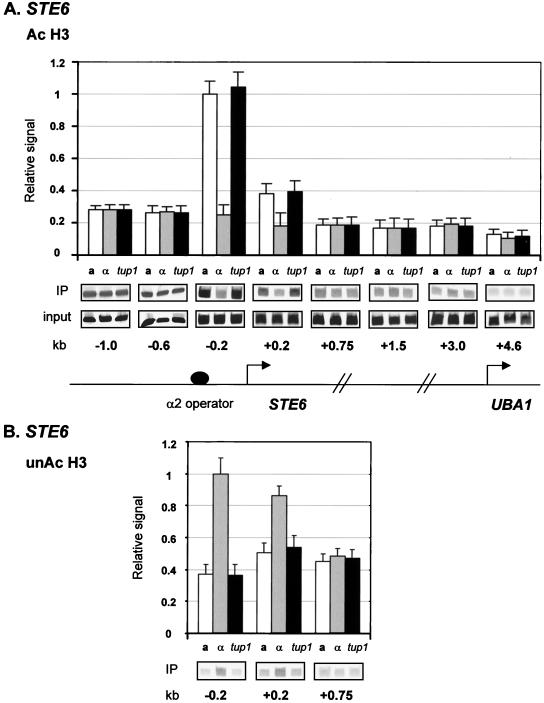
Sequences near the α2/Mcm1 operator (−0.2 probe) through the first 200 bp (+0.2 probe) of STE6 exhibited decreased association with acetylated H3 (Fig. 6A) and a concomitant increase in association with underacetylated H3 (Fig. 6B) in α cells relative to a cells. Notably, α cells containing a tup1 deletion show a level of acetylation equal to that of a cells, confirming the Tup1 dependence of the decreased acetylation in α cells. Both sequences further downstream and sequences upstream of the operator binding site exhibited equal levels of acetylation in a, α, and tup1 cells. Strikingly, these changes in H3 acetylation correlate well with the location of Tup1 at STE6 (Fig. 1A). Similar profiles of H3 acetylation levels were obtained at STE2 (data not shown).
FIG. 6.
Decreases in H3 acetylation colocalize with Tup1 at STE6. Chromatin immunoprecipitations (IP) using antibodies against acetylated H3 (Ac H3) (A) and unacetylated H3 (unAc H3) (B) were performed on cell extracts from a, α, and α tup1 strains. The location of the primer sets is as in Fig. 1A for STE6. The data are presented as signal normalized to both PCR amplification of ACT1 from each sample (to control for sample variation) and input DNA levels (to allow comparison of relative acetylation levels along the gene). Three experiments were averaged for the graphs, and error bars are shown. Quantitative PCR products from one representative experiment are shown.
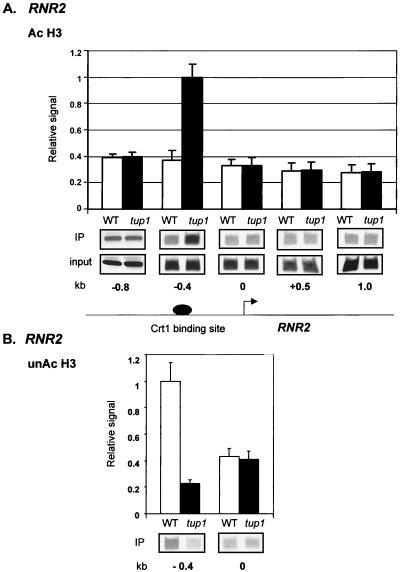
Decreased H3 acetylation also correlated with the Tup1 location at RNR2. Here, low levels of acetylated H3 were observed near the Crt1 binding site (−0.4 probe) in wild-type cells relative to cells lacking TUP1 (Fig. 7A) whereas increased levels of unacetylated H3 (Fig. 7B) were associated with these same sequences in the wild-type cells. Equal levels of H3 acetylation were observed at both downstream sequences in RNR2 and sequences upstream of the Crt1 binding site in both types of cells. A similar H3 acetylation profile was observed at RNR3 (data not shown). As above, the chromatin domain exhibiting decreased H3 acetylation corresponds to the region of Tup1 location.
FIG. 7.
Decreases in H3 acetylation colocalize with Tup1 at RNR2. Chromatin immunoprecipitations (IP) using antibodies against acetylated H3 (Ac H3) (A) and unacetylated H3 (unAc H3) (B) were performed on cell extracts from wild-type (WT) and tup1 strains. The location of the primer sets is as in Fig. 2A for RNR2. The data are presented as signal normalized to both PCR amplification of ACT1 from each sample (to control for sample variation) and input DNA levels (to allow comparison of relative acetylation levels along the gene). Three experiments were averaged for the graphs, and error bars are shown. Quantitative PCR products from one representative experiment are shown.
Mutations in three specific histone deacetylases also cripple Tup1 recruitment.
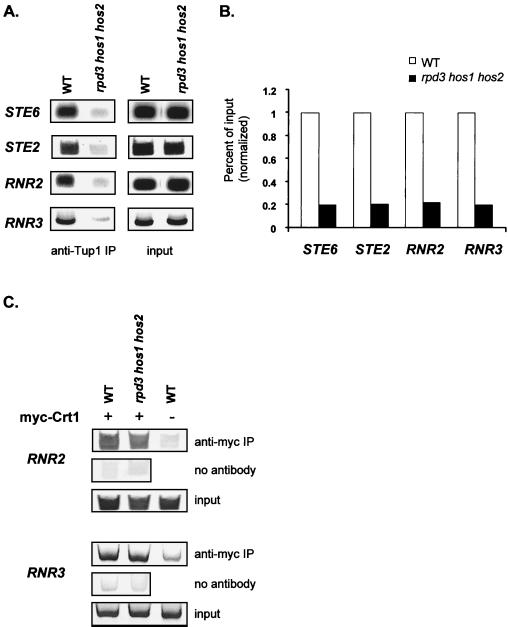
Given that the histone tail domains are essential for the association of Tup1 with repressed genes and that decreases in histone acetylation track Tup1 profiles at repressed genes, it seemed reasonable that loss of histone deacetylase activities important for repression might affect Tup1 recruitment or stability. Previously we demonstrated that a yeast strain with three histone deacetylase genes, RPD3, HOS1, and HOS2, deleted specifically affected Tup1-Ssn6 repression but did not change Tup1 or Ssn6 protein levels (49). Increased acetylation of H3 and H4 was also observed at the affected promoters in the rpd3 hos1 hos2 cells. Therefore, we compared the association of Tup1 at STE2, STE6, RNR2, and RNR3 in this triple histone deacetylase mutant and in isogenic wild-type cells. Chromatin immunoprecipitations with anti-Tup1 antibodies revealed a dramatic loss of Tup1 association at all of these target promoters in this triple histone deacetylase mutant strain (Fig. 8A). Interestingly, the fold effects on Tup1 association at both a cell-specific and DNA damage-inducible genes were almost identical in these experiments and fit well with the high degree of derepression previously observed in the presence of these mutations (49). The lack of Tup1 cross-linking in this assay could reflect a loss of the DNA binding factor, α2 or Crt1. However, we confirmed in our previous work that α2 expression levels are unchanged in the presence of the histone deacetylase mutations. Here, we examined the presence of Crt1 at the operator regions of both RNR2 and RNR3 in the triple histone deacetylase mutant strain (Fig. 8C). We found no alteration in Crt1 binding in the presence of these mutations, again confirming that the lack of Tup1 cross-linking observed above reflects a change in the association of Tup1 with these promoters, not a change in the association of the DNA binding factor with the DNA. Taken together with our other experiments, these data indicate not only that Tup1 induces alterations in histone acetylation levels that are conducive to interactions with the repressor but also that Tup1-histone interactions are required for the stable association of Tup1 at its target sites.
FIG. 8.
Mutations in histone deacetylases severely cripple the association of Tup1 with target loci. (A) Quantitative PCR products from chromatin immunoprecipitations (IP) performed on cell extracts from wild-type (WT) (DY151) and rpd3 hos1 hos2 (DY4565) strains using an antibody against Tup1. PCR primer sets correspond to −0.2 kb for STE6 (as in Fig. 1A), −0.3 kb for STE2 (as in Fig. 1B), −0.4 kb for RNR2 (as in Fig. 2A), and −0.4 kb for RNR3 (as in Fig. 2B). (B) Bar graph representing the percentage of input DNA immunoprecipitated from DY151 and DY4565 extracts. The wild-type value was set at 1 to allow comparison between genes. For the graph, four independent experiments were averaged. The standard deviation between the DY151/DY4565 ratios was less than 0.01. (C) Quantitative PCR products from chromatin immunoprecipitations performed on cell extracts from both wild-type cells (DY151) and rpd3 hos1 hos2 (DY4565) cells containing a plasmid expressing myc-Crt1 (PMH190), using an antibody against the c-myc epitope. Cell extract from DY151 without PMH190 was also used as a control. PCR primer sets correspond to −0.4 kb for RNR2 (as in Fig. 2A) and −0.4 kb for RNR3 (as in Fig. 2B).
DISCUSSION
We have mapped the location of Tup1 on two different classes of Tup1-Ssn6-regulated genes and found that Tup1 localizes strongly to the site of recruitment by DNA binding factors. Strikingly, Tup1 recruitment correlates with a decreases in histone acetylation levels, and both the integrity and the modification state of the histones are important for maintaining Tup1 association with its target genes. This reciprocity provides an important paradigm for the way in which a repressor can both influence and be influenced by changes in histone modifications.
Notably, we observed a difference between the pattern of Tup1 at a cell-specific genes (STE2 and STE6) and DNA damage response genes (RNR2 and RNR3). At STE6 and STE2, we observed a peak of Tup1 association with sequences immediately adjacent to the site of Tup1 recruitment, with a reduced but significant association with downstream sequences encompassing the 5′ end of the coding region. In contrast, at RNR2 and RNR3, Tup1 does not extend beyond sequences adjacent to the recruitment site and is not associated with the downstream coding region.
The results at STE6 differ from those in a previous report showing that Tup1 association spread across the entire coding region of STE6 (8), and they are also in contrast to those in a recent report showing Tup1 association only at the site of recruitment (53). The reasons for these discrepancies are unclear, although the exact method of the chromatin immunoprecipitation assay may underlie these differences. Variations in the degree of formaldehyde fixation or chromatin shearing could easily produce alternate results. However, within our own experiments, these parameters cannot explain the differences we observe between the a cell-specific genes and the DNA damage response genes since our analyses of these two classes of genes were performed simultaneously, using the same cell extracts. That is, STE6, STE2, RNR3, and RNR2 sequences were all amplified from the same Tup1 immunoprecipitates and the differences we observed between the gene classes were reproducible in multiple experiments.
The differences in Tup1 localization at different gene classes suggest that the Tup1-Ssn6 repressor complex may adopt different conformations at different classes of genes. This hypothesis has been previously suggested (40) based on the apparent flexibility of the structures of the Tup1 and Ssn6 proteins. For example, different TPR repeats of Ssn6 are required for repression of different classes of genes (45), and the WD repeats of Tup1 are required for repression of some but not all of these genes (22, 46). The use of different domains was proposed to create specific architectures at individual promoters that would accommodate differences in the spacing of regulatory DNA elements. In this model, the conformation of the Tup1-Ssn6 complex is determined largely by the nature of its interactions with the DNA-bound, sequence-specific repressor (such as α2 or Crt1) that recruits the corepressor. This same flexibility in Tup1-Ssn6 conformation might allow different degrees of multimerization of the corepressor complex at different promoters and, ultimately, the creation of alternative chromatin structures. Indeed, certain Tup1-regulated classes (such as the a cell-specific genes) show positioned nucleosomes under conditions of repression (37, 39), while other classes (such as haploid-specific genes) do not (18).
The prominence of Tup1 near its site of recruitment, in the promoter regions of the repressed genes, strongly indicates that this corepressor complex inhibits gene expression by interfering with transcription initiation. These observations are consistent with studies by others that have shown that Tup1-Ssn6 blocks TATA binding protein (TBP) binding in vivo (27). This interference might occur through direct negative interactions with components of the transcription machinery or through the organization of chromatin, or both.
Interestingly, deletion of SRB10 had no discernible effect on the stability of the association of Tup1 with either of the two classes of genes examined here. Mutations in SRB10 and SRB11 (identified as ssn3 and ssn8, respectively) were originally recovered in the same genetic screen that identified MIG1 (SSN1) and SSN6 as mutations affecting SUC2 repression (4, 25). Moreover, SRB10 was also identified as ARE1 in a screen for mutations causing loss of repression of an a cell-specific reporter gene (47, 48). Three additional, independent studies using reporter constructs also show significant effects of loss of the Srb10/11 cyclin kinase pair on Tup1-Ssn6 repression (24, 25, 47, 55), consistent with the finding that Tup1 physically interacts with Srb10 and Srb11 (55). However, a separate study found little or no detectable effect of srb10, srb11, or srb10 srb11 mutations on the repression of endogenous Tup1-responsive genes (29). While the role of Srb10/11 in Tup1-Ssn6 repression remains to be determined, our experiments indicate that they act downstream of Tup1 recruitment. The chromatin immunoprecipitation assay will be a useful approach to extending these conclusions to additional mediator components required for efficient repression by Tup1-Ssn6.
In contrast to our findings with Srb10, we found that interactions of Tup1 with the histone tails are crucial for the establishment of a stable repressor complex at the promoters of repressed genes. These results are consistent with our previous observations that combined mutations in H3 and H4 compromise repression (9, 18). The importance of chromatin to the repression mechanism is further highlighted by the correspondence between the localization of Tup1 and the profile of decreased H3 acetylation associated with the repressed genes. The functional connection between Tup1 recruitment and decreased histone acetylation is provided by our previous discovery that the Tup1-Ssn6 complex interacts directly with at least two histone deacetylase activities, Rpd3 and Hos2, and that specific histone deacetylase mutations cause a loss of repression of vivo (49). Recently, another group has reported interactions between Tup1 and a third histone deacetylase, Hda1 (53), again supporting a central role for chromatin modification in the repression process. Taken together, the results from our laboratory and others support a multistep mechanism for Tup1-Ssn6 repression (Fig. 9). The Tup1-Ssn6 complex recruits one or more histone deacetylases to induce changes in the acetylation state of the histone tails which facilitate interactions between Tup1 and histones H3 and H4. These interactions not only set up a chromatin architecture that is refractory to transcription but also stabilize Tup1-Ssn6 interactions with their target genes. In some ways this self-reinforcing situation is similar to that described recently for heterochromatin proteins that recruit histone-methylating activities necessary for their stable association with condensed chromatin structures (1, 28, 33).
FIG. 9.
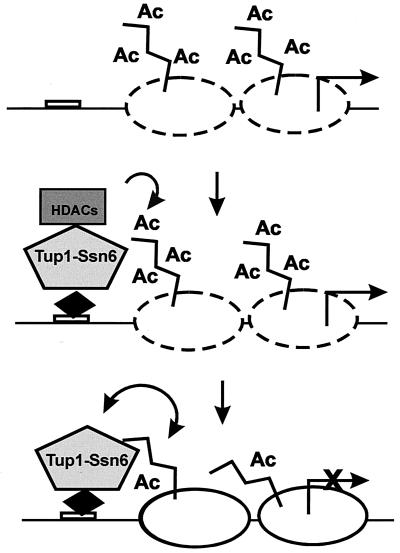
Reciprocal interactions between Tup1-Ssn6 and histones. Upon recruitment of Tup1-Ssn6 by sequence-specific repressor proteins (black diamond), associated histone deacetylase (HDAC) activities reduce the acetylation (Ac) of histones in neighboring nucleosomes, facilitating interactions with Tup1. These interactions convert the active chromatin (dashed nucleosomes) to a repressive structure (solid nucleosomes). Moreover, Tup1-histone interactions are self-reinforcing in that they stabilize the association of the corepressor complex with the target promoter and help to maintain the histones in an underacetylated state.
Corepressor proteins with features similar to Tup1 have been identified in metazoans, including the Groucho protein in flies and the Transducin-like Enhancer of Split (TLE) family in mammalian cells (5). At least two of these proteins, TLE1 and TLE2, interact with proteins related to Ssn6 (12). Like Tup1, Groucho and the TLEs bind to histones and the histone binding correlates with repression (11, 34). Additionally, Groucho interacts with Rpd3, and histone deacetylase activity is required for efficient repression (6). The corepressors SMRT and NcoR, which mediate repression by the thyroid hormone receptor and other nuclear hormone receptors, provide another analogy. These proteins utilize distinct domains to interact with different histone deacetylases, and the specific combination of corepressor and histone deacetylase mediates gene-specific regulation (14, 17, 20, 21, 54). While SMRT and NcoR do not have yeast homologues, SMRT can be found in a complex with TBL1, a WD repeat protein which has functional similarity to Tup1 and Groucho (14, 30). The striking parallels between these proteins and Tup1-Ssn6 suggest that reciprocal interactions between regulatory proteins, histones, and histone-modifying enzymes are a fundamental and conserved mechanism governing gene expression.
Acknowledgments
We thank the following researchers for their generous gifts of reagents: Marian Carlson for ssn3 and ssn8 strains, Stephen Elledge for the Y577 strain and the PMH190 plasmid, M. Mitchell Smith for the MSY590 and MSY577 strains, and David Stillman for the DY150, DY151, and DY4565 strains. We thank Diane Edmondson for many discussions throughout this work, and we thank Diane Edmondson and Michelle Barton for careful reading of the manuscript.
This work was supported by an American Cancer Society postdoctoral fellowship (PF00131) to J.K.D. and by grants from the NIH (GM51189) and the Robert A. Welch Foundation (G1371) to S.Y.R.D.
REFERENCES
- 1.Bannister, A. J., P. Zegerman, J. F. Partridge, E. A. Miska, J. O. Thomas, R. C. Allshire, and T. Kouzarides. 2001. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature 410:120–124. [DOI] [PubMed] [Google Scholar]
- 2.Bone, J. R., and S. Y. Roth. 2001. Recruitment of the yeast Tup1p-Ssn6p repressor is associated with localized decreases in histone acetylation. J. Biol. Chem. 276:1808–1813. [DOI] [PubMed] [Google Scholar]
- 3.Burke, D., D. Dawson, and T. Stearns. 2000. Methods in yeast genetics. A Cold Spring Harbor Laboratory course manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 4.Carlson, M., B. C. Osmond, L. Neigeborn, and D. Botstein. 1984. A suppressor of SNF1 mutations causes constitutive high-level invertase synthesis in yeast. Genetics 107:19–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, G., and A. J. Courey. 2000. Groucho/TLE family proteins and transcriptional repression. Gene 249:1–16. [DOI] [PubMed] [Google Scholar]
- 6.Chen, G., J. Fernandez, S. Mische, and A. J. Courey. 1999. A functional interaction between the histone deacetylase Rpd3 and the corepressor groucho in Drosophila development. Genes Dev. 13:2218–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, S., R. W. West, Jr., S. L. Johnson, H. Gans, B. Kruger, and J. Ma. 1993. TSF3, a global regulatory protein that silences transcription of yeast GAL genes, also mediates repression by alpha 2 repressor and is identical to SIN4. Mol. Cell. Biol. 13:831–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ducker, C. E., and R. T. Simpson. 2000. The organized chromatin domain of the repressed yeast a cell-specific gene STE6 contains two molecules of the corepressor Tup1p per nucleosome. EMBO J. 19:400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edmondson, D. G., M. M. Smith, and S. Y. Roth. 1996. Repression domain of the yeast global repressor Tup1 interacts directly with histones H3 and H4. Genes Dev. 10:1247–1259. [DOI] [PubMed] [Google Scholar]
- 10.Edmondson, D. G., W. Zhang, A. Watson, W. Xu, J. R. Bone, Y. Yu, D. Stillman, and S. Y. Roth. 1998. In vivo functions of histone acetylation/deacetylation in Tup1p repression and Gcn5p activation. Cold Spring Harbor Symp. Quant. Biol. 63:459–468. [DOI] [PubMed] [Google Scholar]
- 11.Flores-Saaib, R. D., and A. J. Courey. 2000. Analysis of Groucho-histone interactions suggests mechanistic similarities between Groucho- and Tup1-mediated repression. Nucleic Acids Res. 28:4189–4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grbavec, D., R. Lo, Y. Liu, A. Greenfield, and S. Stifani. 1999. Groucho/transducin-like enhancer of split (TLE) family members interact with the yeast transcriptional co-repressor SSN6 and mammalian SSN6-related proteins: implications for evolutionary conservation of transcription repression mechanisms. Biochem. J. 337:13–17. [PMC free article] [PubMed] [Google Scholar]
- 13.Gromoller, A., and N. Lehming. 2000. Srb7p is a physical and physiological target of Tup1p. EMBO J. 19:6845–6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guenther, M. G., W. S. Lane, W. Fischle, E. Verdin, M. A. Lazar, and R. Shiekhattar. 2000. A core SMRT corepressor complex containing HDAC3 and TBL1, a WD40-repeat protein linked to deafness. Genes Dev. 14:1048–1057. [PMC free article] [PubMed] [Google Scholar]
- 15.Guldener, U., S. Heck, T. Fielder, J. Beinhauer, and J. H. Hegemann. 1996. A new efficient gene disruption cassette for repeated use in budding yeast. Nucleic Acids Res. 24:2519–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guthrie, C., and G. Fink. 1991. Guide to yeast genetics and molecular biology. Methods Enzymol. 194:1–863. [PubMed] [Google Scholar]
- 17.Huang, E. Y., J. Zhang, E. A. Miska, M. G. Guenther, T. Kouzarides, and M. A. Lazar. 2000. Nuclear receptor corepressors partner with class II histone deacetylases in a Sin3-independent repression pathway. Genes Dev. 14:45–54. [PMC free article] [PubMed] [Google Scholar]
- 18.Huang, L., W. Zhang, and S. Y. Roth. 1997. Amino termini of histones H3 and H4 are required for a1-α2 repression in yeast. Mol. Cell. Biol. 17:6555–6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang, M., Z. Zhou, and S. J. Elledge. 1998. The DNA replication and damage checkpoint pathways induce transcription by inhibition of the Crt1 repressor. Cell 94:595–605. [DOI] [PubMed] [Google Scholar]
- 20.Jepsen, K., O. Hermanson, T. M. Onami, A. S. Gleiberman, V. Lunyak, R. J. McEvilly, R. Kurokawa, V. Kumar, F. Liu, E. Seto, S. M. Hedrick, G. Mandel, C. K. Glass, D. W. Rose, and M. G. Rosenfeld. 2000. Combinatorial roles of the nuclear receptor corepressor in transcription and development. Cell 102:753–763. [DOI] [PubMed] [Google Scholar]
- 21.Kao, H. Y., M. Downes, P. Ordentlich, and R. M. Evans. 2000. Isolation of a novel histone deacetylase reveals that class I and class II deacetylases promote SMRT-mediated repression. Genes Dev. 14:55–66. [PMC free article] [PubMed] [Google Scholar]
- 22.Komachi, K., M. J. Redd, and A. D. Johnson. 1994. The WD repeats of Tup1 interact with the homeo domain protein alpha 2. Genes Dev. 8:2857–2867. [DOI] [PubMed] [Google Scholar]
- 23.Krebs, J. E., and C. L. Peterson. 2000. Understanding “active” chromatin: a historical perspective of chromatin remodeling. Crit. Rev. Eukaryot. Gene Expr. 10:1–12. [PubMed] [Google Scholar]
- 24.Kuchin, S., and M. Carlson. 1998. Functional relationships of Srb10-Srb11 kinase, carboxy-terminal domain kinase CTDK-I, and transcriptional corepressor Ssn6-Tup1. Mol. Cell. Biol. 18:1163–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuchin, S., P. Yeghiayan, and M. Carlson. 1995. Cyclin-dependent protein kinase and cyclin homologs SSN3 and SSN8 contribute to transcriptional control in yeast. Proc. Natl. Acad. Sci. USA 92:4006–4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuo, M. H., and C. D. Allis. 1998. Roles of histone acetyltransferases and deacetylases in gene regulation. Bioessays 20:615–626. [DOI] [PubMed] [Google Scholar]
- 27.Kuras, L., and K. Struhl. 1999. Binding of TBP to promoters in vivo is stimulated by activators and requires Pol II holoenzyme. Nature 399:609–613. [DOI] [PubMed] [Google Scholar]
- 28.Lachner, M., D. O’Carroll, S. Rea, K. Mechtler, and T. Jenuwein. 2001. Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature 410:116–120. [DOI] [PubMed] [Google Scholar]
- 29.Lee, M., S. Chatterjee, and K. Struhl. 2000. Genetic analysis of the role of Pol II holoenzyme components in repression by the Cyc8-Tup1 corepressor in yeast. Genetics 155:1535–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li, J., J. Wang, Z. Nawaz, J. M. Liu, J. Qin, and J. Wong. 2000. Both corepressor proteins SMRT and N-CoR exist in large protein complexes containing HDAC3. EMBO J. 19:4342–4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lipke, P. N., and C. Hull-Pillsbury. 1984. Flocculation of Saccharomyces cerevisiae tup1 mutants. J. Bacteriol. 159:797–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luger, K., A. W. Mader, R. K. Richmond, D. F. Sargent, and T. J. Richmond. 1997. Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature 389:251–260. [DOI] [PubMed] [Google Scholar]
- 33.Nakayama, J., J. C. Rice, B. D. Strahl, C. D. Allis, and S. I. Grewal. 2001. Role of histone H3 lysine 9 methylation in epigenetic control of heterochromatin assembly. Science 292:110–113. [DOI] [PubMed] [Google Scholar]
- 34.Palaparti, A., A. Baratz, and S. Stifani. 1997. The Groucho/transducin-like enhancer of split transcriptional repressors interact with the genetically defined amino-terminal silencing domain of histone H3. J. Biol. Chem. 272:26604–26610. [DOI] [PubMed] [Google Scholar]
- 35.Papamichos-Chronakis, M., R. S. Conlan, N. Gounalaki, T. Copf, and D. Tzamarias. 2000. Hrs1/Med3 is a Cyc8-Tup1 corepressor target in the RNA polymerase II holoenzyme. J. Biol. Chem. 275:8397–8403. [DOI] [PubMed] [Google Scholar]
- 36.Redd, M. J., M. B. Arnaud, and A. D. Johnson. 1997. A complex composed of tup1 and ssn6 represses transcription in vitro. J. Biol. Chem. 272:11193–11197. [DOI] [PubMed] [Google Scholar]
- 37.Roth, S. Y., A. Dean, and R. T. Simpson. 1990. Yeast α2 repressor positions nucleosomes in TRP1/ARS1 chromatin. Mol. Cell. Biol. 10:2247–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schneider, B. L., W. Seufert, B. Steiner, Q. H. Yang, and A. B. Futcher. 1995. Use of polymerase chain reaction epitope tagging for protein tagging in Saccharomyces cerevisiae. Yeast 11:1265–1274. [DOI] [PubMed] [Google Scholar]
- 39.Shimizu, M., S. Y. Roth, C. Szent-Gyorgyi, and R. T. Simpson. 1991. Nucleosomes are positioned with base pair precision adjacent to the alpha 2 operator in Saccharomyces cerevisiae. EMBO J. 10:3033–3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith, R. L., and A. D. Johnson. 2000. Turning genes off by Ssn6-Tup1: a conserved system of transcriptional repression in eukaryotes. Trends Biochem. Sci. 25:325–330. [DOI] [PubMed] [Google Scholar]
- 41.Smith, R. L., M. J. Redd, and A. D. Johnson. 1995. The tetratricopeptide repeats of Ssn6 interact with the homeo domain of alpha 2. Genes Dev. 9:2903–2910. [DOI] [PubMed] [Google Scholar]
- 42.Strahl, B. D., and C. D. Allis. 2000. The language of covalent histone modifications. Nature 403:41–45. [DOI] [PubMed] [Google Scholar]
- 43.Strahl-Bolsinger, S., A. Hecht, K. Luo, and M. Grunstein. 1997. SIR2 and SIR4 interactions differ in core and extended telomeric heterochromatin in yeast. Genes Dev. 11:83–93. [DOI] [PubMed] [Google Scholar]
- 44.Struhl, K. 1998. Histone acetylation and transcriptional regulatory mechanisms. Genes Dev. 12:599–606. [DOI] [PubMed] [Google Scholar]
- 45.Tzamarias, D., and K. Struhl. 1995. Distinct TPR motifs of Cyc8 are involved in recruiting the Cyc8-Tup1 corepressor complex to differentially regulated promoters. Genes Dev. 9:821–831. [DOI] [PubMed] [Google Scholar]
- 46.Tzamarias, D., and K. Struhl. 1994. Functional dissection of the yeast Cyc8-Tup1 transcriptional co-repressor complex. Nature 369:758–761. [DOI] [PubMed] [Google Scholar]
- 47.Wahi, M., and A. D. Johnson. 1995. Identification of genes required for alpha 2 repression in Saccharomyces cerevisiae. Genetics 140:79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wahi, M., K. Komachi, and A. D. Johnson. 1998. Gene regulation by the yeast Ssn6-Tup1 corepressor. Cold Spring Harbor Symp. Quant. Biol. 63:447–457. [DOI] [PubMed] [Google Scholar]
- 49.Watson, A. D., D. G. Edmondson, J. R. Bone, Y. Mukai, Y. Yu, W. Du, D. J. Stillman, and S. Y. Roth. 2000. Ssn6-Tup1 interacts with class I histone deacetylases required for repression. Genes Dev. 14:2737–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Williams, F. E., U. Varanasi, and R. J. Trumbly. 1991. The CYC8 and TUP1 proteins involved in glucose repression in Saccharomyces cerevisiae are associated in a protein complex. Mol. Cell. Biol. 11:3307–3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wolffe, A. 1998. Chromatin: structure and function, 3rd ed. Academic Press, Inc., San Diego, Calif.
- 52.Wolffe, A. P., and J. J. Hayes. 1999. Chromatin disruption and modification. Nucleic Acids Res. 27:711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu, J., N. Suka, M. Carlson, and M. Grunstein. 2001. TUP1 utilizes histone H3/H2B-specific HDA1 deacetylase to repress gene activity in yeast. Mol. Cell 7:117–126. [DOI] [PubMed] [Google Scholar]
- 54.Wu, X., H. Li, E. J. Park, and J. D. Chen. 2001. SMRTe inhibits MEF2C transcriptional activation by targeting HDAC4 and 5 to nuclear domains. J. Biol. Chem. 276:24177–24185. [DOI] [PubMed] [Google Scholar]
- 55.Zaman, Z., A. Z. Ansari, S. S. Koh, R. Young, and M. Ptashne. 2001. Interaction of a transcriptional repressor with the RNA polymerase II holoenzyme plays a crucial role in repression. Proc. Natl. Acad. Sci. USA 98:2550–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]