Abstract
Thymic shared antigen 1 (TSA-1) is a plasma membrane protein of the Ly-6 superfamily expressed on thymocytes, thymic stromal cells, and other cells of the hematopoietic system. TSA-1 is also expressed in other nonhematopoietic tissues, in particular, embryonic and adult adrenal glands. To address the function of TSA-1, we generated mutant mice in which TSA-1 expression was inactivated by gene targeting. Here we show that deletion of both TSA-1 alleles results in abnormal adrenal gland development and midgestational lethality due to cardiac abnormalities. We also report that TSA-1-deficient adrenal glands have significantly reduced levels of the catecholamines noradrenaline and adrenaline. We conclude that TSA-1 is required for normal embryonic development but that deletion of its expression does not obviously impair lymphoid development.
All hematolymphoid cells derive from a multipotential precursor cell present in the fetal liver or adult bone marrow (25). Blood-borne hematopoietic precursors seed the thymus gland and differentiate under the influence of the unique thymic microenvironment to form mature T lymphocytes (10). T-cell development is characterized by regulated changes in expression of cell surface markers which reflect distinct phenotypic stages of maturation (2, 18, 23). Thymic shared antigen 1 (TSA-1) is a cell membrane protein of the Ly-6 superfamily expressed at high levels on immature thymocytes, thymic stromal cells, and other hematopoietic lineages, including B cells and their precursors in bone marrow, dendritic cells, and macrophages (8, 28, 29). TSA-1 is absent from bone marrow hematopoietic stem cells, but it is upregulated as these cells seed the thymus and form the early intrathymic lymphoid-restricted progenitors (31). The ability of anti-TSA-1 monoclonal antibodies (MAbs) to block thymopoiesis in fetal thymic organ culture initially indicated that TSA-1 plays an important role in T-cell development (20). The observation that TSA-1 is markedly upregulated on peripheral T cells following antigen stimulation and that MAb blockade of TSA-1 inhibits activation-induced interleukin 2 (IL-2) release from T cells suggested that TSA-1 also functions in T-cell activation (11, 22). Finally, anti-TSA-1 MAbs can antagonize apoptotic signals in mouse thymocytes in vivo and in vitro which have been induced by engagement of the T-cell receptor (19). Aside from leukocytes, TSA-1 is also expressed in other mouse tissues, including the embryonic adrenal gland and liver and adult kidney and liver, and in various epithelial cells (4, 15), suggesting a more general function for TSA-1 which is not restricted to tissues of the immune system.
Since TSA-1 is expressed at high levels in the embryonic thymus and adrenal gland and from the very earliest stages of thymopoiesis in the adult, we were interested in studying the consequences of deleting TSA-1 expression in these tissues. We produced a null mutation in the TSA-1 gene in mice by gene targeting in embryonic stem (ES) cells. The resulting, homozygous, TSA-1-deficient (TSA-1−/−) mice exhibit abnormal development and impaired function of the embryonic adrenal gland, although development of the embryonic thymus was apparently normal in these animals. TSA-1−/− embryos die at embryonic day 15.5 (E15.5) of gestation of heart failure associated with severe dilated cardiomyopathy. Using a combination of lymphocyte differentiation assays in vitro and in vivo, we also show that TSA-1 is not an obligate requirement for normal differentiation of T or B cells.
MATERIALS AND METHODS
Disruption of TSA-1 gene.
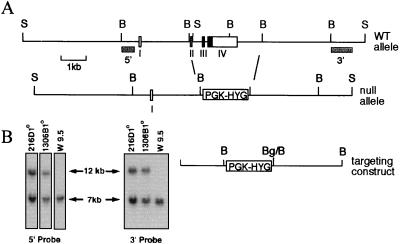
The targeting construct was generated as shown in Fig. 1 and cloned into pBluescript II (Stratagene). The construct was linearized with SalI prior to introduction into W9.5 ES cells by electroporation. Cells were grown in hygromycin, and resistant clones were tested for the homologous recombination event by Southern blotting of genomic DNA. Two independent clones with the correct targeting event were identified, injected into blastocysts, and transferred into pseudopregnant females, to produce chimeric offspring.
FIG. 1.
Disruption of the TSA-1 gene. (A) Exons 2 to 4, encompassing the entire coding region of the TSA-1 protein, were deleted and replaced by the hygromycin phosphotransferase gene cassette (PGK-HYG). WT, wild type. (B) Southern blotting of genomic DNA from primary ES cell clones 216D and 1306B and wild-type W9.5 ES cells. The 3′ probe (A) hybridizes to a 7-kb SacI fragment from the wild-type allele and to a 12-kb SacI fragment from the targeted allele.
Histological analysis, immunohistochemistry, and in situ hybridization.
Tissues or whole embryos were fixed for 8 to 12 h in Bouin’s fixative and then paraffin embedded, and 5-μm-thick sections were stained using hematoxylin and eosin. For immunohistochemical analysis of TSA-1, tissue was frozen in OCT embedding compound (Miles Inc.) and cut at a thickness of 4 to 6 μm. Sections were incubated for 20 min at 4°C with tissue culture supernatant from the anti-TSA-1 MAb hybridoma clone, GR12, diluted 1:100 (gift from A. Kosugi, School of Allied Health Sciences, Osaka University, Osaka, Japan). After washing, bound MAb was detected by incubating sections with anti-rat immunoglobulin G Alexa 488 (Molecular Probes), which was diluted at 1/200 for 20 min at 4°C. For analysis of tyrosine hydroxylase (TH) expression, adrenal glands were fixed in 4% paraformaldehyde/saline, pH 7.2, for 4 h, prior to embedding and cryosectioning as described above. Sections were reacted and stained with a mouse anti-rat TH MAb (Boehringer Mannheim) or an isotype control MAb, which were both diluted to 40 μg/ml, for 1 h at 37°C. Bound MAb was visualized using anti-mouse immunoglobulin G-fluorescein isothiocyanate (Silenius) diluted at 1/200 for 30 min at 4°C. In situ hybridization was carried out with cRNA probes produced by transcription of linearized pBluescript plasmids containing TSA-1 and phenylethanolamine N-methyltransferase (PNMT) 440- and 520-bp inserts, respectively, according to previously published methods (22a).
Catecholamine measurements.
Catecholamines (adrenaline and noradrenaline) in the adrenal gland and whole body (minus adrenal gland) of E14 embryos were quantified by high-performance liquid chromatography with electrochemical detection, as previously described (13).
Fetal thymic organ culture, lymphoid reconstitution of RAG mice, and flow cytometry analysis.
Thymus glands were removed from E14 embryos and cultured for 7 days as previously described (30). Cell suspensions were made by gently crushing the lobes under a glass coverslip in 100 μl of phosphate-buffered saline (PBS) plus 2% fetal calf serum and 0.02% azide and then immediately adjusting the volume to 1 ml. Fetal livers were dissected from E14 embryos, gently crushed with glass slides in sterile PBS, and washed twice in PBS. Approximately 2 × 106 viable cells were injected intravenously via the tail vein into 10-week-old RAG-1−/− mice which had been gamma irradiated with 300 rads 6 h previously. After 7 weeks, recipient mice were assessed for lymphoid reconstitution by analysis of peripheral blood lymphocytes (PBLs) using fluorescence-activated cell sorter analysis. PBLs were obtained from 200 μl of heparinized treated blood after removal of red cells using lysis buffer (Sigma), followed by two washes in the PBS-fetal calf serum-azide mixture. Approximately 106 PBLs or thymocytes from cultured thymus lobes were incubated for 20 min at 4°C with the appropriately diluted MAbs. After washing, cells were analyzed using a FACScan (Becton Dickinson). Lymphocytes were selected on the basis of forward and side scatter characteristics. Typically, data from 3 × 104 cells were collected for analysis. The MAbs used for marker analysis were GR12 (gift from A. Kosugi), M1/70 (CD11b), CD3ε-FITC, CD4-PE, CD8-biotin, B220-PE, αβTCR-FITC, and αβTCR-APC. Biotinylated antibodies were detected with streptavidin-Tricolor. All antibodies were from Pharmingen unless otherwise stated.
Analysis of TSA-1 expression in embryonic hearts by RT-PCR.
For reverse transcriptase PCR (RT-PCR), RNA was prepared using the acid phenol method and reverse transcription was carried out with H− RT (Promega) and priming with oligo(dT). PCR amplification of first-strand cDNA was performed for 23, 27, 30, and 35 cycles using the following primer pairs: TSA-1 (5′-GCAGAGCCAACAAGCTAAG-3′ and 5′-GGCCTCTTCACCCGGAG-3′) and β-actin (5′-ATGGATGACGATATCGCTG-3′ and 5′-ATGAGGTAGTCTGTCAGGT-3′). PCR products were resolved on 1.3% agarose gels and visualized by staining with ethidium bromide.
RESULTS
Disruption of TSA-1 gene by homologous recombination.
A deletion of the TSA-1 gene was introduced in murine W9.5 ES cells by homologous recombination (Fig. 1). The deletion resulted in the replacement of exons 2 to 4 with the hygromycin phosphotransferase gene cassette (PGK-HYG), which results in deletion of the entire coding region of the TSA-1 protein. The targeting vector contained 4.8 kb of homology and was isolated from an isogenic mouse 129/SVJ library. To identify correctly targeted clones, 500 hygromycin-resistant clones were screened by Southern blotting using a 700-bp probe external to the 3′ targeting region. Two independent ES cell clones carrying heterozygous deletions of the TSA-1 gene were obtained (216D and 1306B), which were verified for the correct targeting event by Southern analysis with probes 5′ and 3′ to the integration site and external to the flanking homology regions of the targeting construct (Fig. 1B). The 5′ and 3′ probes hybridize to two adjacent 7-kb genomic SacI fragments from the wild type TSA-1 locus, but both hybridize to the same 12-kb fragment from the TSA-1 locus with a deletion of exons 2 to 4 (Fig. 1). Southern blot analysis with a hygromycin probe demonstrated a single integration site for the targeting vector in ES cell clone 216D but revealed an additional integration event for ES cell clone 1306B (data not shown). Each of the ES clones was injected into blastocysts from C57BL/6 mice, and both transmitted the targeted allele through the germ line.
Analysis of TSA-1-deficient mice.
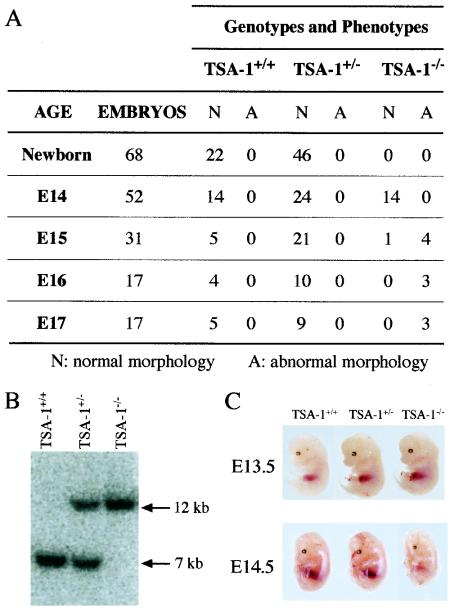
Heterozygous mice carrying the TSA-1 null allele were mated to produce homozygous TSA-1−/− offspring. However, at 3 weeks of age, no viable TSA-1−/− animals were obtained, as determined by Southern blotting, suggesting that this phenotype is embryonically lethal (Fig. 2A). Genotyping of embryos by Southern blot analysis was therefore undertaken to identify TSA-1−/− animals. At E10.5, viable homozygous embryos were seen (Fig. 2B), and up until stage E14 of embryonic development, normal embryos representative of all three genotypes were identified in the expected Mendelian ratios (Fig. 2A). At E13.5, TSA-1−/− mice were not readily distinguishable from wild-type or heterozygous littermate control animals (Fig. 2C, upper panel). However, at E14.5, many TSA-1−/− embryos were pale and smaller than heterozygous and wild-type littermate control animals (Fig. 2C, lower panel). By E16, all TSA-1−/− embryos were necrotic and undergoing resorption. Similar findings were seen for mice created from the 1306B ES cell line, suggesting that the phenotype was unlikely to have derived from an ES cell mutation arising in vitro (data not shown). Mice derived from the 216D ES clone (with a single integration site for the targeting construct) were used in all subsequent analysis.
FIG. 2.
Homozygous TSA-1−/− mice are embryonically lethal. (A) Phenotypes and genotypes of offspring from TSA-1+/− × TSA-1+/− matings, genotyped by Southern blotting of genomic DNA. N, normal morphology; A, abnormal morphology. No live embryos were detected past E15. (B) Southern blotting of genomic DNA from E10 embryos obtained from TSA-1+/− × TSA-1+/− matings. Detection of restriction fragments corresponding to wild-type and targeted alleles was performed as described for Fig. 1. (C) Phenotype of TSA-1−/− embryos. Embryos were dissected from yolk sacs, and tail biopsies were taken for genotyping.
Abnormal adrenal gland phenotype in TSA-1−/− mice.
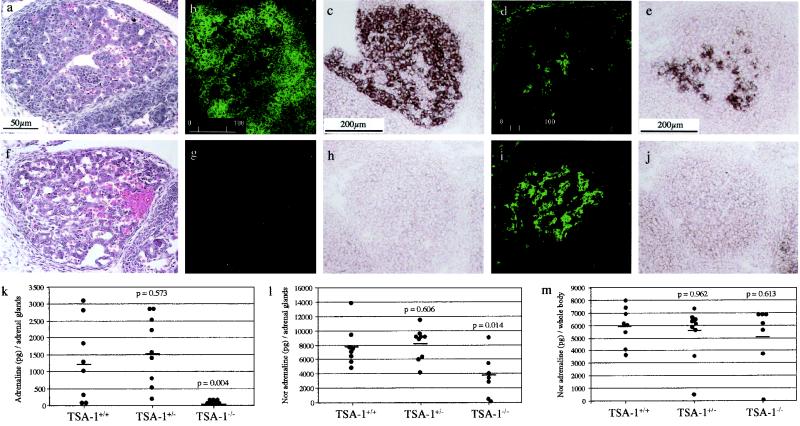
Immunohistochemical and in situ hybridization analysis revealed that TSA-1 is expressed at high levels in the fetal mouse thymus (data not shown) and adrenal gland (Fig. 3b and c). At E13.5, the thymus gland from TSA-1−/− mice appeared histologically normal (data not shown). In contrast, the adrenal gland from TSA-1−/− mice showed a disorganized cellular structure in the cortex (Fig. 3f) compared with those found in wild-type littermate control mice (Fig. 3a). Although the severity of this phenotype was variable, it was consistently observed in all TSA-1−/− embryos examined. TH-positive cells were detected in TSA-1−/− adrenal glands, indicative of medullary chromaffin cells (Fig. 3i) although the adrenal glands of TSA-1−/− mice contained virtually no detectable adrenaline (Fig. 3k) and noradrenaline levels were reduced to approximately 50% of those seen in wild-type control adrenal glands (Fig. 3l). These results were consistent with the lack of detectable levels of PNMT enzyme in TSA-1−/− adrenal glands shown by in situ analysis (Fig. 3j), compared to wild-type controls (Fig. 3e). A comparison of whole-body noradrenaline levels did not reveal any significant differences between TSA-1−/− embryos and wild-type controls (Fig. 3m).
FIG. 3.
Adrenal gland abnormalities in TSA-1−/− mice. A comparison of E14 adrenal gland morphology in wild-type (a) and TSA-1−/− embryos (f). Note the disrupted cellular structure in the TSA-1−/− gland. Analysis of TSA-1 expression by immunostaining (b) and in situ hybridization (c) in E13.5 and E14.5 wild-type embryos, respectively. Immunostaining and in situ analysis reveal the absence of TSA-1 protein and mRNA in TSA-1−/− adrenal glands as expected (g and h, respectively). Medullary chromaffin cells are present in wild-type (d) adrenal glands and TSA-1−/− (i) E14 embryonic adrenal glands, as revealed by immunostaining with an anti-TH antibody. PNMT is present in wild-type (e) but not the TSA-1−/− (j) adrenal gland. Panels k to m show catecholamine analysis of adrenal glands and whole body (minus adrenal gland) from wild-type and TSA-1−/− E13 embryos. Adrenaline is virtually absent (k), and noradrenaline levels are reduced by approximately 50% (l) in the adrenal glands of TSA-1−/− mice. Noradrenaline levels in the whole animal are similar for wild-type and TSA-1−/− mice (m).
TSA-1−/− embryos exhibit an abnormal cardiac phenotype.
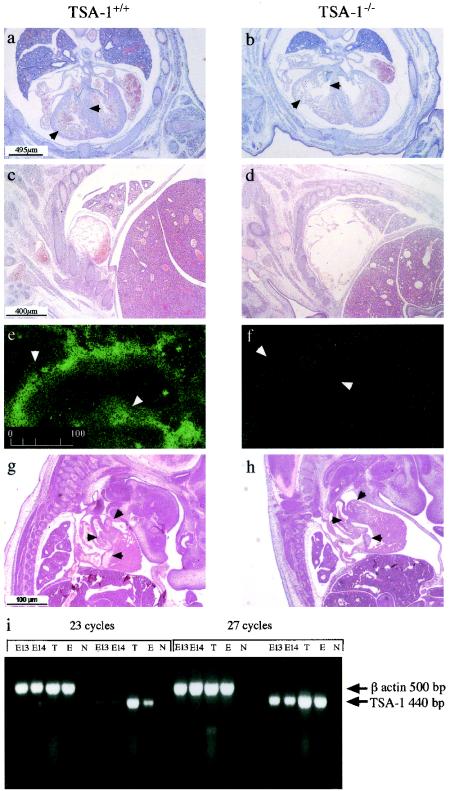
To further investigate the cause of death in TSA-1−/− mice, histological examination of whole-mount E14.5 embryos was performed. By this type of analysis, significant cardiac abnormalities in TSA-1−/− embryos were noted. Both the ventricles and atria were significantly enlarged, compared to those of wild-type control animals (Fig. 4a to d). Severe dilated cardiomyopathy and ventricular trabeculation defects in the hearts of TSA-1−/− mice were also observed (Fig. 4b). In many TSA-1−/− embryos, we observed that the ventricular septum had failed to close by day 14 of gestation (data not shown). At E14.5 to E15.5, ruptured ventricles were frequently seen, suggesting that the cause of death was due to cardiac failure. Histological analysis of embryos between E12.5 and E14.5 of gestation revealed phenotypically normal, patent heart valves in TSA-1−/− mice, similar to those observed in wild-type control animals (data for E13.5 are presented in Fig. 4g and h). We analyzed TSA-1 expression in embryonic mouse heart tissue using immunohistochemistry and mRNA analysis. The results shown in Fig. 4e reveal TSA-1 immunostaining in the aorta at E12.5, which was absent from the TSA-1−/− animals (Fig. 4f), demonstrating effective deletion of TSA-1 expression in TSA-1−/− animals. However, we did not detect significant levels of TSA-1 expression in the aorta or any other compartment of the heart, including the myocardium, at later stages of embryogenesis (E13.5 to E14.5) using immunohistochemical techniques (data not shown), suggesting that cardiac expression of TSA-1 is downregulated between E12.5 and E14.5. However, RT-PCR analysis revealed the presence of TSA-1 mRNA in E13.5 and E14.5 heart tissue after 23 cycles of amplification (Fig. 4i), suggesting that low levels of TSA-1 expression are maintained throughout this stage of embryogenesis.
FIG. 4.
Histological anomalies in the developing hearts of TSA-1−/− mice. A comparison of transverse sections from an E14 wild-type fetus (a) and a TSA-1−/− mutant (b). Ventricle walls and interventricular septa are indicated by arrows. Atria of a wild-type E14 (c) and mutant embryo (d). Expression of TSA-1 by immunohistological staining in the aorta (arrowheads) of wild-type E12 (e) and mutant embryo (f). A comparison of sagittal sections of E13 wild-type (g) and mutant embryo (h) showing normal heart valves (arrowheads) in wild-type and mutant embryos. mRNA analysis of TSA-1 expression by RT-PCR in embryonic E13 and E14 hearts (i). Control samples are thymus (T), an olfactory epithelial cell line (E), and no template DNA (N). The TSA-1 oligonucleotide primer target sequences are separated by a 2,000-bp intron (4) and thus would not be expected to amplify TSA-1 genomic sequences.
T-cell development is apparently normal in TSA-1−/− mice.
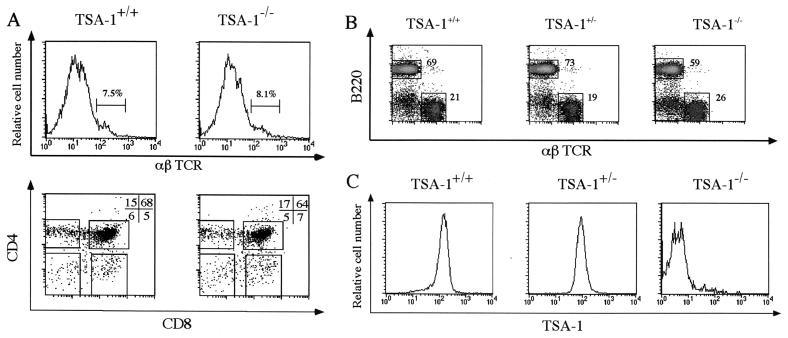
The embryonic lethal phenotype of TSA-1−/− mice precluded a straightforward analysis of T-cell development in TSA-1−/− mice beyond E14.5. Therefore, we used fetal thymic organ culture to assess the capacity of TSA-1−/− intrathymic precursor cells to differentiate in vitro. E14.5 thymic lobes were removed and cultured for 7 days, and the resulting cell populations were analyzed by flow cytometry (Fig. 5). In the embryonic thymus, the majority of thymocytes are of the immature αβTCR− CD4− CD8− phenotype; therefore, acquisition of these markers can be used to follow the appearance of more mature T-cell subsets which derive from intrathymic precursor cells (23). The data shown in Fig. 5A clearly show the presence of αβTCR+ cells in both wild-type and TSA-1−/− cultured thymic lobes, and all thymocyte subsets, defined by the CD4 and CD8 markers, were present in both wild-type and TSA-1−/− cultured lobes (Fig. 5A). Specifically, percentages of the major intermediate CD4+ CD8+ population and the more mature CD4− CD8+ and CD4+ CD8− single positive subsets did not differ significantly between wild-type and TSA-1−/− cultured lobes (Fig. 5A). Analysis of the most immature triple-negative CD3− CD4− CD8− thymocyte subsets, using the CD25 and CD44 markers (9), also revealed no significant differences in T-cell phenotype between wild-type and TSA-1−/− cultured lobes (data not shown). Cell yields at the completion of the culture period were 2.6 × 105 and 2.8 × 105 cells 2per lobe for wild-type and TSA-1−/− samples, respectively. These values are within the normal range for this culture system (20) and reveal that no significant differences in the kinetics of in vitro T-cell differentiation arise as a consequence of deleting TSA-1 expression.
FIG. 5.
TSA-1 is not an obligate requirement for T- or B-lymphoid differentiation. (A) Fetal thymic organ culture of E14 thymic lobes from wild-type (TSA-1+/+) and TSA-1-deficient (TSA-1−/−) embryos. Cells were harvested after culturing thymic lobes for 7 days and were analyzed for αβTCR expression (upper panels) and CD4 and CD8 surface marker expression (lower panels) by flow cytometry. (B and C) Lymphoid reconstitution of RAG-1−/− mice with wild-type (TSA-1+/+), heterozygous (TSA-1+/−), and homozygous (TSA-1−/−) hematopoietic precursors from fetal liver. PBLs were harvested from reconstituted animals 7 weeks after engraftment with fetal liver cells. Flow cytometry analysis was carried out using B220 and αβTCR specific MAbs to label B cells and T cells, respectively (B). Numbers shown indicate the percentage of B and T cells present in reconstituted animals. Donor cell TSA-1 genotypes were verified by immunostaining with the TSA-1 specific MAb GR12 (C).
Lymphoid cell development from TSA-1−/− fetal liver hematopoietic precursors.
To address whether TSA-1 is required for normal development of T and B cells in vivo, we reconstituted lymphoid-deficient RAG-1−/− mice with either TSA-1−/− or wild-type E14.5 fetal liver hematopoietic precursor cells. After 7 weeks, peripheral blood samples were taken from reconstituted animals and analyzed for donor-derived T- and B-cell progeny. The data for Fig. 5B and C show that successful reconstitution of T- and B-cell lineages was achieved in RAG-1−/− mice using either wild-type, TSA-1+/−, or TSA-1−/− precursors, and the cell numbers recovered indicated that the efficiency of reconstitution was comparable for donor cells of all three genotypes (data not shown). As expected, B-cell progeny derived from wild-type fetal liver precursors, but not B cells derived from TSA-1−/− precursors, expressed high levels of TSA-1 at the cell surface (Fig. 5C), thereby confirming the genetic origin of each precursor population. B-cell progeny from heterozygous TSA-1+/− precursors were also surface positive for TSA-1, but these cells exhibited two- to threefold lower levels of expression than did cells derived from wild-type precursors, suggesting a gene dosage effect. Taken together, the fetal thymic organ culture and RAG-1−/− reconstitution data presented in Fig. 5 suggest that TSA-1 is apparently not required for normal T-cell differentiation within the thymus, nor is it necessary for differentiation of T and B cells from fetal liver precursors in vivo.
DISCUSSION
Disruption of the gene encoding the lymphostromal membrane protein, TSA-1, resulted in an embryonically lethal phenotype with 2embryos succumbing to cardiac failure at E15.5 of gestation. TSA-1−/− embryos revealed significant morphological abnormalities accompanied by marked adrenal and cardiac defects. Surprisingly, we were unable to detect an obvious thymus phenotype or significant alterations in the capacity of either TSA-1−/− intrathymic lymphoid precursors or of TSA-1−/− hematopoietic precursors to differentiate into mature T or B lymphocytes, suggesting that TSA-1 is not essential for lymphoid differentiation.
The gene encoding TSA-1 becomes transcriptionally active midgestation, and, in a previous study, high levels of TSA-1 expression were reported in fetal thymus and hematopoietic precursor cells of the fetal liver (1). We report herein that TSA-1 expression is not restricted to cells of the hematopoietic lineage; TSA-1 is expressed at high levels in the embryonic adrenal gland. We conclude that the abnormal adrenal phenotype of TSA-1−/− mice arises directly as a consequence of extinguishing TSA-1 expression in this organ. The absence of detectable levels of PNMT in TSA-1−/− adrenal glands is consistent with the lack of adrenaline and suggests a primary biosynthetic defect in this tissue. Since PNMT expression is partly dependent on glucocorticoids (17), we are currently investigating whether levels of adrenal glucocorticoids are reduced in TSA-1−/− embryos. It is significant to note that TSA-1 is also expressed at high levels in the human fetal adrenal gland (24).
The cardiac phenotype exhibited by TSA-1−/− mice is less easily reconciled with our expression data. While we were able to detect TSA-1 mRNA in the embryonic mouse heart and others have reported expression of TSA-1 in the human embryonic heart (16), we cannot formally exclude the possibility that the cardiac phenotype of TSA-1−/− mice is secondary in nature. It is unlikely, however, that a catecholamine deficiency adequately explains the cardiac phenotype observed in TSA-1−/− mice. For instance, mice deficient for the glucocorticoid receptor also have a complete lack of adrenaline and reduced levels of noradrenaline (approximately 40% of wild-type levels) in their adrenal glands (5), similar to the adrenal catecholamine data presented herein for TSA-1−/− mice. Glucocorticoid receptor-deficient mice exhibit no obvious cardiac phenotype; rather, they die perinatally as a result of lung abnormalities (5). Mice rendered catecholamine deficient as a result of a targeted mutation in the TH gene are also embryonically lethal at E12 to E14 of gestation (32) and exhibit a cardiac phenotype similar to that of TSA-1−/− mice, in that both types of mice have significantly enlarged atrial chambers and disrupted cellular organization in the ventricular myocardium. However, unlike TSA-1−/− mice, TH−/− mice also show a profound lack of noradrenaline, a major catecholamine of embryogenesis (27). Given that TSA-1−/− adrenal glands express 50% of wild-type noradrenaline levels (Fig. 3l) and that whole-body (minus adrenal gland) noradrenaline levels in TSA-1−/− mice are indistinguishable from those of their wild-type counterparts (Fig. 3m), it is unlikely that reduced catecholamine levels adequately account for the cardiac phenotype in TSA-1−/− mice. To further investigate tissue-specific phenotypes in TSA-1−/− mice, we have recently targeted the TSA-1 locus with loxP sites for temporal and spatial ablation of TSA-1 expression (B. J. Classon and D. J. Zammit, unpublished data).
A surprising finding arising from these studies is the apparently normal progression of both T- and B-lymphoid development in the absence of TSA-1, both in vitro and in vivo. This finding was unusual, given previous reports in which anti-TSA-1 MAbs were shown to profoundly inhibit T-cell development (20), thymocyte apoptosis (19), and IL-2 synthesis by activated T cells (22). This discrepancy raises the possibility that binding the TSA-1 molecule at the cell surface with a MAb constitutes receptor engagement with a multivalent ligand of relatively high affinity, thereby transducing an enhanced, nonphysiological signal to the T cell, rather than inducing a receptor blockade effect. This notion is supported by our most recent work, which shows that monovalent F(ab′) fragments of a TSA-1 MAb were unable to block T-cell development in fetal thymic organ culture, whereas F(ab′)2 and whole MAb blocked effectively in parallel experiments (21).
TSA-1 is expressed in a number of embryonic and adult tissues, and there is mounting evidence that TSA-1 and other cell membrane Ly-6 superfamily molecules participate in intercellular adhesion and signaling; however, the molecular basis of their function remains obscure. TSA-1 is structurally related to a number of Ly-6 superfamily molecules known to directly bind and regulate the activity of plasma membrane ion channels, including xenoxin-1 (14), calciseptine (7), and caltrin (6). While there is presently no evidence that TSA-1 acts at the level of plasma membrane ion channels, it is significant that MAb blockade of TSA-1 interferes with Ca2+-dependent cellular responses in T cells, such as IL-2 secretion (11, 22). There is previously reported evidence for a cell surface ligand for TSA-1 expressed on thymocytes (3), and further characterization of this structure is expected to clarify the role of TSA-1 in lymphocytes and other tissues.
It will also be of interest to investigate whether TSA-1−/− lymphocytes, which develop apparently normally in vivo, exhibit subtle functional differences compared to their wild-type counterparts, given the reported role for TSA-1 in signal transduction in T cells (12). In this regard, it is significant that, for the only other member of the Ly-6 superfamily for which a gene-deficient mouse has been described, Ly-6A/E, the mutant animals are apparently normal and fertile but exhibit a hyperproliferative T-cell phenotype, the molecular basis of which has not been extensively characterized (26).
In conclusion, the results presented herein demonstrate that TSA-1 is an obligate requirement for normal development and functional compliance of the embryonic adrenal gland, which may have an additional impact on cardiac function in the embryo, and reveal a more generic function for an Ly-6 superfamily molecule in mammalian development.
Acknowledgments
We thank David Robinson for technical assistance and also thank Kensuke Miyake, Department of Immunology, Saga Medical School, Nabeshima, Japan, and Atsushi Kosugi, School of Allied Health Sciences, Osaka University, Osaka, Japan, for the gift of the GR-12 MAb. The advice of Tim Cole is gratefully acknowledged.
This work was supported by the National Health and Medical Research Council of Australia.
REFERENCES
- 1.Antica, M., L. Wu, and R. Scollay. 1997. Stem cell antigen 2 expression in adult and developing mice. Immunol. Lett. 55:47–51. [DOI] [PubMed] [Google Scholar]
- 2.Boyd, R. L., and P. Hugo. 1991. Towards an integrated view of thymopoiesis. Immunol. Today 12:71–79. [DOI] [PubMed] [Google Scholar]
- 3.Classon, B. J., and R. L. Boyd. 1998. Thymic-shared antigen-1 (TSA-1). A lymphostromal cell membrane Ly-6 superfamily molecule with a putative role in cellular adhesion. Dev. Immunol. 6:149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Classon, B. J., and L. Coverdale. 1996. Genomic organization and expression of mouse thymic shared antigen-1 (TSA-1): evidence for a processed pseudogene. Immunogenetics 44:222–226. [PubMed] [Google Scholar]
- 5.Cole, T. J., J. A. Blendy, A. P. Monaghan, K. Krieglstein, W. Schmid, A. Aguzzi, G. Fantuzzi, E. Hummler, K. Unsicker, and G. Schutz. 1995. Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Dev. 9:1608–1621. [DOI] [PubMed] [Google Scholar]
- 6.Coronel, C. E., D. E. Winnica, M. L. Novella, and H. A. Lardy. 1992. Purification, structure, and characterization of caltrin proteins from seminal vesicle of the rat and mouse. J. Biol. Chem. 267:20909–20915. [PubMed] [Google Scholar]
- 7.de Weille, J. R., H. Schweitz, P. Maes, A. Tartar, and M. Lazdunski. 1991. Calciseptine, a peptide isolated from black mamba venom, is a specific blocker of the L-type calcium channel. Proc. Natl. Acad. Sci. USA 88:2437–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Godfrey, D. I., M. Masciantonio, C. L. Tucek, M. A. Malin, R. L. Boyd, and P. Hugo. 1992. Thymic shared antigen-1. A novel thymocyte marker discriminating immature from mature thymocyte subsets. J. Immunol. 148:2006–2011. [PubMed] [Google Scholar]
- 9.Godfrey, D. I., J. Kennedy, T. Suda, and A. Zlotnik. 1993. A developmental pathway involving four phenotypically and functionally distinct subsets of CD3−CD4−CD8− triple-negative adult mouse thymocytes defined by CD44 and CD25 expression. J. Immunol. 150:4244–4252. [PubMed] [Google Scholar]
- 10.Ikuta, K., N. Uchida, J. Friedman, and I. L. Weissman. 1992. Lymphocyte development from stem cells. Annu. Rev. Immunol. 10:759–783. [DOI] [PubMed] [Google Scholar]
- 11.Kosugi, A., S. Saitoh, S. Narumiya, K. Miyake, and T. Hamaoka. 1994. Activation-induced expression of thymic shared antigen-1 on T lymphocytes and its inhibitory role for TCR-mediated IL-2 production. Int. Immunol. 6:1967–1976. [DOI] [PubMed] [Google Scholar]
- 12.Kosugi, A., S. Saitoh, S. Noda, K. Miyake, Y. Yamashita, M. Kimoto, M. Ogata, and T. Hamaoka. 1998. Physical and functional association between thymic shared antigen-1/stem cell antigen-2 and the T cell receptor complex. J. Biol. Chem. 273:12301–12306. [DOI] [PubMed] [Google Scholar]
- 13.Lambert, G. W., and I. H. Jonsdottir. 1998. Influence of voluntary exercise on hypothalamic norepinephrine. J. Appl. Physiol. 85:962–966. [DOI] [PubMed] [Google Scholar]
- 14.Macleod, R. J., P. Lembessis, S. James, and H. P. Bennett. 1998. Isolation of a member of the neurotoxin/cytotoxin peptide family from Xenopus laevis skin which activates dihydropyridine-sensitive Ca2+ channels in mammalian epithelial cells. J. Biol. Chem. 273:20046–20051. [DOI] [PubMed] [Google Scholar]
- 15.MacNeil, I., J. Kennedy, J., D. I. Godfrey, N. A. Jenkins, M. Masciantonio, C. Mineo, D. J. Gilbert, N. G. Copeland, R. L. Boyd, and A. Zlotnik. 1993. Isolation of a cDNA encoding thymic shared antigen-1. A new member of the Ly6 family with a possible role in T cell development. J. Immunol. 151:6913–6923. [PubMed] [Google Scholar]
- 16.Mao, M., M. Yu, J. H. Tong, J. Ye, J. Zhu, Q. H. Huang, G. Fu, L. Yu, S. Y. Zhao, S. Waxman, M. Lanotte, Z. Y. Wang, J. Z. Tan, S. J. Chan, and Z. Chen. 1996. RIG-E, a human homolog of the murine Ly-6 family, is induced by retinoic acid during the differentiation of acute promyelocytic leukemia cell. Proc. Natl. Acad. Sci. USA 93:5910–5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michelsohn, A. M., and D. J. Anderson. 1992. Changes in competence determine the timing of two sequential glucocorticoid effects on sympathoadrenal progenitors. Neuron 8:589–604. [DOI] [PubMed] [Google Scholar]
- 18.Nikolic-Zugic, J. 1991. Phenotypic and functional stages in the intrathymic development of alpha beta T cells. Immunol. Today 12:65–70. [DOI] [PubMed] [Google Scholar]
- 19.Noda, S., A. Kosugi, S. Saitoh, S. Narumiya, and T. Hamaoka. 1996. Protection from anti-TCR/CD3-induced apoptosis in immature thymocytes by a signal through thymic shared antigen-1/stem cell antigen-2. J. Exp. Med. 183:2355–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Randle, E. S., G. A. Waanders, M. Masciantonio, D. I. Godfrey, and R. L. Boyd. 1993. A lymphostromal molecule, thymic shared Ag-1, regulates early thymocyte development in fetal thymus organ culture. J. Immunol. 151:6027–6035. [PubMed] [Google Scholar]
- 21.Randle-Barrett, E. S., S. P. Berzins, A. Wilson, B. J. Classon, D. I. Godfrey, and R. L. Boyd. TSA-1 ligation prevents early thymocyte development. Dev. Immunol. in press.
- 22.Saitoh, S., A. Kosugi, S. Noda, N. Yamamoto, M. Ogata, Y. Minami, K. Miyake, and T. Hamaoka. 1995. Modulation of TCR-mediated signaling pathway by thymic shared antigen-1 (TSA-1)/stem cell antigen-2 (Sca-2). J. Immunol. 155:5574–5581. [PubMed] [Google Scholar]
- 22a.Schaeren-Wiemers, N., and A. Gerfin-Moser. 1993. A single protocol to detect transcripts of various types and expression levels in neural tissue and cultured cells: in situ hybridization using digoxigenin-labelled cRNA probes. Histochemistry 100:431–440. [DOI] [PubMed] [Google Scholar]
- 23.Scollay, R., P. Bartlett, and K. Shortman. 1984. T cell development in the adult murine thymus: changes in the expression of the surface antigens Ly2, L3T4 and B2A2 during development from early precursor cells to emigrants. Immunol. Rev. 82:79–103. [DOI] [PubMed] [Google Scholar]
- 24.Shan, X., A. Bourdeau, A. Rhoton, D. E. Wells, E. H. Cohen, B. E. Landgraf, and R. G. Palfree. 1998. Characterization and mapping to human chromosome 8q24.3 of Ly-6-related gene 9804 encoding an apparent homologue of mouse TSA-1. J. Immunol. 160:197–208. [PubMed] [Google Scholar]
- 25.Spangrude, G. J., and D. D. Cooper. 2000. Paradigm shifts in stem-cell biology. Semin. Hematol. 37:3–10. [DOI] [PubMed] [Google Scholar]
- 26.Stanford, W. L., S. Haque, R. Alexander, X. Liu, A. M. Latour, H. R. Snodgrass, B. H. Koller, and P. M. Flood. 1997. Altered proliferative response by T lymphocytes of Ly-6A (Sca-1) null mice. J. Exp. Med. 186:705–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas, S. A., A. M. Matsumoto, and R. D. Palmiter. 1995. Noradrenaline is essential for mouse fetal development. Nature 374:643–646. [DOI] [PubMed] [Google Scholar]
- 28.Tucek, C. L., D. I. Godfrey, and R. L. Boyd. 1992. Five novel antigens illustrate shared phenotype between mouse thymic stromal cells, thymocytes, and peripheral lymphocytes. Int. Immunol. 4:1021–1030. [DOI] [PubMed] [Google Scholar]
- 29.Vremec, D., M. Zorbas, R. Scollay, D. J. Saunders, C. F. Ardavin, L. Wu, and K. Shortman. 1992. The surface phenotype of dendritic cells purified from mouse thymus and spleen: investigation of the CD8 expression by a subpopulation of dendritic cells. J. Exp. Med. 176:47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waanders, G. A., and R. Boyd. 1990. The effects of interleukin 2 on early and late thymocyte differentiation in foetal thymus organ culture. Int. Immunol. 2:461–468. [DOI] [PubMed] [Google Scholar]
- 31.Wu, L., M. Antica, G. R. Johnson, R. Scollay, and K. Shortman. 1991. Developmental potential of the earliest precursor cells from the adult mouse thymus. J. Exp. Med. 174:1617–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou, Q. Y., C. J. Quaife, and R. D. Palmiter. 1995. Targeted disruption of the tyrosine hydroxylase gene reveals that catecholamines are required for mouse fetal development. Nature 374:640–643. [DOI] [PubMed] [Google Scholar]